Abstract
The plasma-chemical synthesis of tungsten carbides from multicomponent oxide-containing concentrates is studied. The existing technologies for a high-temperature action of plasma flows on mineral raw materials are analyzed. The processes of extractive metallurgy in the processing of mineral ores are investigated. The experimental dependences of the synthesized amount of tungsten carbides on the plasma flow temperature Tp, action time τ, the charge particle size, the degree of mechanical activation, the amount of introduced graphite, and the type of concentrate are considered. The dependences of the amount of tungsten carbides synthesized from a scheelite concentrate and calcium tungstate on the fraction of tungsten trioxide WO3 contained in them under the same synthesis conditions are compared. The results of spectral and scanning electron microscopy of the plasma-chemical synthesis products and a nanocrystalline tungsten carbide powder are considered. The prospects of plasma-chemical synthesis of tungsten carbides from multicomponent oxide-containing concentrates are considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The production of refractory tungsten-based compounds includes complex high-energy hydrometallurgical operations (at the stage of beneficiation of raw materials) and multistage operations during synthesis [1–3]. In addition, the demand for tungsten carbides for the national economy remains high, and the market environment is unstable and demanding on economic resources. The demand for refractory metal carbides in Russia remains at a fairly high level due to the lack of the necessary capacities for their production. The traditional methods for producing tungsten carbides involve the preparation of raw materials, which is accompanied by significant discharges of chemically active reagents necessary for the decomposition and conversion of the chemical compounds of minerals [4, 5].
Plasma-chemical synthesis of compounds is an advanced trend for research [1, 6]. The development of such technologies has been carried out for a long time in those areas of industry where high purity of the end product and special physical properties (crystal formation, hardness, refractoriness, particle dispersion, etc.) are required, for example, in the nuclear industry or aircraft industry [1]. Tungsten-containing raw materials are difficult to concentrate [7], and the preparation of compounds is carried out using complex vacuum equipment and prolonged high-temperature heating in special-purpose furnaces [2, 8, 9].
For the Far Eastern region, the development of new methods for processing, enrichment, and production of refractory tungsten-based compounds is a challenging problem due to the geological and geographical location of natural sources of tungsten-containing mineral raw materials.
The aim of this work is to develop a method for producing refractory metal carbides from multicomponent tungsten-containing mineral raw materials by plasma-chemical synthesis.
For a more correct estimation of the dependences to be obtained, we used a raw material with a low tungsten content, namely, scheelite concentrate (Lermontov GOK) in which the content of the main tungsten oxide varies in the range 14.7–55.4 wt %, and a raw material with a high tungsten content, namely, calcium tungstate with a tungsten oxide content in the range 69.3–77.98 wt %.
EXPERIMENTAL
The plasma synthesis of tungsten carbides from multicomponent mineral raw materials in a low-temperature plasma flow includes the following main stages: mixture preparation, heating/cooling, and synthesis.
In preparing a mixture, i.e., a charge, we used multicomponent mineral concentrates and a carbon-containing material (carburizing/reducing agent). For experiments, a scheelite concentrate and calcium tungstate were selected as raw materials and graphite was used as a carbon source. The concentrates used in experiments were selected due to the following causes: availability and abundance in the region (Far Eastern region), demand for the resulting compounds (tungsten carbides), and the content of the main metal oxide.
Note that the type of carbon source is not crucial (charcoal, soot, paraffin, etc.) to form chemical bonds with carbon, since a material and a concentrate during plasma synthesis undergo melting, dispersion, evaporation, and thermal decomposition.
The compositions of the scheelite concentrate and the calcium tungstate are given in Table 1.
Because of a nonuniform content of tungsten trioxide WO3 in the concentrates, we solved the problem of determining the optimum experimental conditions for synthesizing tungsten carbides. The amount of carbon in the resulting mixture was determined analytically and experimentally (Table 2).
In addition to changing the carbon-to-concentrate ratio in the charge composition, mechanical activation was carried out using a centrifugal mill to smooth the mixture composition. The particle size in the charge ranged from 0.05 to 1 mm (1-mm particles are the charge particles that have not undergone mechanical activation).
A charge was fed into the heating zone during plasma-chemical synthesis in a protective gas (argon), which is both a plasma-forming and carrier gas, delivering the charge to a synthesis chamber, the mixture. In this zone, particles underwent the following transformations: heating → melting → dispersion → evaporation.
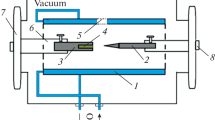
Figure 1 schematically shows the plasma-chemical synthesis setup and the feeding of a charge into the reactor chamber. An indirect-action experimental plasma generator with replaceable cooled nozzle 1 (anode) with an outlet diameter of 1–1.5 mm was used as a plasma source. A copper electrode protected from overheating by cooling system 3 was used as a cathode. The synthesis chamber with a volume of about 11.5 cm3 was made of graphite in the form of a hollow cylinder with windows for gas withdrawal. The output power of the power unit powering the plasma generator was 15.6 kW. A charge was fed into the synthesis chamber in portions of 100–150 g per technological cycle.
Scheme of the main elements of the carbide plasma synthesis setup: (1) nozzle (anode), (2) branch for supplying a plasma-forming gas and a charge (cathode), (3) cooling system, (4) gas removal channel, (5) flow fairing, and (6) synthesis chamber (plasma-chemical reactor). C stands for concentrate; G, graphite.
The stages of charge conversion in the plasma-chemical chamber reactor are schematically shown in Fig. 2 and include the following processes:
(a) transition from a solid phase to a gaseous phase: heating → melting → dispersion → evaporation;
(b) cooling and synthesis of a new phase of future compounds containing carbon (CaC–CaCO3, WC–W2C–W), gas mixture (CO2, CO) and the compounds of associated chemical elements (Si, Al, Fe, Mn, Mg, Na, etc.) with carbon;
(c) condensation, solidification and the formation of dispersed particles of the carbides of the chemical elements that make up a charge.
The chemical elements entering a plasma stream are affected by various factors in addition to high temperature, including the factors that contribute to decomposition, ionization, and acceleration of chemical synthesis of compounds.
These factors include the specific principle of the interaction of particles in a plasma flow [1], which is expressed in both chaotic and ordered motion. When a particle enters a plasma field, it experiences the accelerating forces of a gas stream, the effects of electromagnetic and sound waves, and Alphen waves traveling along the lines of force of the plasma stream. However, in motion particles collide with each other, which leads to a change in the trajectory of their motion, the mass, or the structure.
Thus, the main input controlled indicators of plasma-chemical synthesis include: the amount of carburizing/reducing agent introduced into a charge, the plasma flow temperature, the charge fraction size, the fraction of the main oxide in the charge, and the plasma-chemical synthesis time. The output controlled indicators were as follows: the amount of reduced tungsten carbide (wt %), the stoichiometric carbon content in WxC compound, the of tungsten carbide particle size, the fraction of reduced tungsten, the total amount of slag (wt %), and the amount of material (included in the charge composition) “removed” during sublimation.
Chemical and phase analyzes were carried out on the specialized certified equipment in the laboratories of the Institute of Chemistry, Far East Branch, Russian Academy of Sciences; Pacific State University; the Institute of Tectonics and Geophysics, Far East Branch, Russian Academy of Sciences; and the Far Eastern State Transport University.
Phase analysis of the synthesis products was carried out on a VEGA 3 LMH (TESCAN) scanning electron microscope (SEM) equipped with an X-Max 80 (Oxford Instruments) energy dispersive spectrometer. Photographing samples and searching for microinclusions was mainly performed in the backscattered electron mode (BSE detector).
Phase analysis was also conducted using a ZEISS Libra-120 transmission electron microscope equipped with an HAADF detector and an Ω energy filter. The studies were carried out in the transmission, dark-field, and electron diffraction modes. Samples for transmission microscopy were prepared by electrolytic polishing and ion etching.
The compositions of the slag and the synthesized product were analyzed using a DRON-7 diffractometer and CoKα radiation at a recording speed of two degrees per minute.
The formed plasma flow and its temperature in various chamber zones were studied theoretically with finite element simulation and experimentally with a TN infrared pyrometer and the Toepler schlieren method on a Svil’-80 device at a plasma temperature of 3000–9000 K. The error in measuring the plasma flow temperature was 2–8% (measurement error increased with the plasma temperature).
RESULTS AND DISCUSSION
As a result of experiments on the plasma-chemical synthesis of tungsten carbides from a charge based on scheelite concentrate, calcium tungstate, and graphite, we obtained dependences of the tungsten carbide content in the synthesis products on plasma flow temperature Tp, action time τ, the charge particle size, the degree of mechanical activation, the amount of introduced graphite, and the type of concentrate.
During plasma-chemical synthesis, we formed a mixture of finely dispersed particles of WC–W2C tungsten carbides and reduced tungsten particles, calcium carbides, compounds of associated chemical elements with carbon, and Fe–W–C intermetallic particles.
An analysis of the results obtained showed that, during the action of a high-temperature plasma flow on a charge, the amount of reduced tungsten carbide is mainly affected by the fraction of introduced graphite with respect to the concentrate. Figure 3 shows the dependences of the mass of reduced tungsten carbide (wt %) of the fraction of introduced graphite, the type of concentrate, and the charge fraction size. The content of tungsten carbides was determined using SEM and TEM. As can be seen from Fig. 3, the largest amount of carbides is reached at 35% graphite in a charge. For example, for the scheelite concentrate (Fig. 3a), the content of tungsten carbides in the total amount of the synthesis products is up to 92.5 wt % at 35% graphite in a charge and a fraction size of up to 200 μm. When tungsten carbides are synthesized from a charge based on calcium tungstate (Fig. 3b), their amount was up to 97.6 wt % at 35% graphite in the charge and a fraction size of up to 25 μm.
The amount of the synthesized WC–W2C–WxC carbide mixture was found to increase at a fraction size of 150–250 μm and a lower content of the metal oxide in the concentrate (scheelite concentrate) under the same synthesis conditions (plasma flow temperature, time, graphite concentration in charge).
It is important to note that the fraction of the synthesized carbide mixture presented in the dependences in Fig. 3–5 is related to the material purified from a slag. A mixture of WC–W2C–WxC tungsten carbides was separated from a slag using molar equipment and was washed with distilled water. The product formed after purification from slag and impurities mainly consisted of a mixture of WC–W2C tungsten carbides and reduced tungsten. The mixture composition was determined using a DRON-7 X-ray diffractometer. To determine the amount of carbides relative to the portion of concentrate, leaching process with acids (HNO3, H2SO4, HCl) was carried out. The product formed after leaching was weighed and examined for the presence of reduced tungsten by the methods described above.
The dependence of the tungsten carbide mass on the plasma flow temperature and the fraction of introduced graphite in a charge was studied for the scheelite concentrate and calcium tungstate (Fig. 4). As can be seen from Fig. 4, the optimum plasma flow temperature for producing tungsten carbides from the scheelite concentrate in the near-nozzle region (2–6 mm from the nozzle exit section) is about 6500 K (Fig. 4a). The plasma flow temperature was determined by the Toepler schlieren method, a spectral method, and simulation. The error in determining the temperature by various methods was about 550 K.
For calcium tungstate with a higher fraction of the main metal oxide in a charge (Fig. 4b), the plasma flow temperature should be increased to 7500–8000 K (temperature range was determined theoretically and experimentally by the methods given above). This increase is caused by the fact that, during the evaporation of graphite in the plasma-chemical reactor chamber, atomic carbon forms. It creates a large number of bonds with a refractory metal and a small number of associated chemical elements, mainly oxygen.
When the plasma flow temperature increases further (for the scheelite-concentrate-based charge, to more than 6500 K; for calcium tungstate, to more than 8000 K), the total yield of the main metal carbides decreases. In addition, metallic W is reduced from both the WC carbide phase and tungsten oxides WO–WO2–WO3.
The dependence of the tungsten carbide mass on the time of action of a plasma stream on a charge of tungsten-containing mineral concentrates was studied (Fig. 5).
According to these dependences, plasma stream action time τ is determined by the tungsten oxide volume contained in the concentrate, since the process should lead to complete synthesis of the entire amount of tungsten carbides.
These dependences (Figs. 5a, 5b) demonstrate a change in the fraction of synthesized tungsten carbides for two types of tungsten-containing concentrates at the same plasma flow temperature (6500 K).
The amount of reduced tungsten was found to increase during a long high-temperature action of a plasma stream to the charge melt formed in the reactor chamber. Further boiling and sublimation of the melt leads to a decrease in the total amount of synthesis products and reduced tungsten. The most active sublimation of the charge components occurs at a plasma torch temperature of 7500 K or higher in the near-nozzle region. Studies of the end synthesis products showed that 18–30 wt % of the total amount of the mixture fed into the synthesis chamber is sublimated in the form of a vapor–droplet phase and a gas mixture.
Figure 6 shows photographs of the tungsten carbides formed during plasma-chemical synthesis after slow cooling of the synthesis products (Fig. 6a) and after crushing and washing in distilled water (Fig. 6b). After these procedures, a mixture of tungsten carbides and reduced tungsten WC–W2C–W in the form of a nanocrystalline powder (fractions from 1 to 500 nm) was formed.
The synthesis of a nanocrystalline W–C powder included crushing, the dissolution of calcium carbides with water, and magnetic extraction of Fe–W–C intermetallic compounds.
Figure 7 shows the X-ray diffraction pattern of the nanocrystalline WC–W2C tungsten carbide powder formed from the scheelite concentrate by plasma-chemical synthesis. Phase analysis of samples revealed the presence of the main phases of tungsten carbide, namely, W2C and WC. The carbon content in WC was 6.2–7.1 wt % (57 at % W, 43 at % C), In the W2C phase, carbon reached 3.4 wt % (72 at % W, 28 at % C). The content of reduced tungsten in the nanocrystalline powder (WC–W2C) was found to be 7.5 wt %. Excess tungsten (in at %) in WC and W2C indicates the presence of free tungsten atoms.
Figure 8 shows a spectrogram of the nanocrystalline tungsten carbide powder formed by plasma-chemical synthesis. The purity of the synthesized nanocrystalline WC–W2C–W powder was 99.2 wt %.
SEM results showed the presence of the following crystallographic modifications (Fig. 9):
(i) α-WC with a hexagonal lattice (space group P6m2) and periods a = 0.2906 nm and c = 0.2839 nm;
(ii) β-WC with a cubic face-centered lattice (a = 0.4220 nm, space group Fm3m) and W2C ditungsten carbide, crystals of which form the hexagonal system with cell parameters a = 0.29948 nm, c = 0.47262 nm, and Z = 1.
CONCLUSIONS
(1) WC–W2C tungsten carbides were synthesized from multicomponent oxide-containing concentrates in one step by plasma-chemical synthesis in the specific power range g > 104–105 W/cm2 at a plasma temperature of 3000–9000 K. The synthesized nanocrystalline powder consists of 92.5–97.6 wt % WC–W2C carbides and 2.4–7.5 wt % reduced tungsten. The purity of the synthesized nanocrystalline W–C powder was 99.2 wt %.
(2) Preliminary mechanical activation of the components of a mixture of concentrate and graphite can significantly increase the intensity of plasma-chemical synthesis of WC–W2C tungsten carbides regardless of the fraction of graphite and the main metal oxide in the mixture.
(3) The synthesis of a nanocrystalline W–C powder included crushing, the dissolution of calcium carbides with water, and magnetic extraction of intermetallic compounds.
(4) On the whole, our results demonstrate the prospects of plasma-chemical synthesis of tungsten carbides from tungsten-containing mineral concentrates. The results of the work can be used in the future to replace the hydrometallurgical and chemical methods of processing raw materials and the high-energy long-term methods of producing tungsten carbides.
REFERENCES
Yu. N. Tumanov, Plasma, High-Frequency, Microwave, and Laser Technologies in Chemical–Metallurgical Processes (FIZMALIT, Moscow, 2010).
A. D. Verhoturov, P. S. Gordienko, V. A. Dostovalov, L. A. Konevtsov, E. S. Panin, and D. V. Dostovalov, High-Energy Local Effect on Tungsten-Containing Materials and Metals: A Monograph (Izd. Dal’nevost. Federal. Univ, 2012).
V. L. Butuhanov, A. D. Verhoturov, and T. B. Ershova, “Physicochemical fundamentals of carbothermic reduction of natural tungsten materials,” Khim. Tekhnol., No. 6, 25–30 (2001).
A. D. Verhoturov, “Some methodological approaches to stable development of resource-producing regions,” in Problems of Combined Development of Geological Resources (IGD DVO RAN, Khabarovsk, 2011), pp. 81–92.
Kh. B. Kushkhov, A. L. Kardanov, and M. N. Adamokova, “Electrochemical synthesis of binary molybdenum and tungsten carbides (Mo,W)2C from tungstate–molybdate–carbonate melts,” Rasplavy, No. 4, 65–73 (2012).
G. V. Galevskii, T. V. Kiseleva, and V. V. Rudneva, Study of the Plasma Synthesis of Refractory Compounds Using a Planned Experiment (SibGIU, Novokuznetsk, 2010).
S. S. Bel’skii, “Processing of scheelite concentrate to form tungsten trioxide,” Vestnik IrGTU 107 (12), 204–208 (2015).
S. V. Aleksandrovskii, D. V. Li, and V. M. Sizyakov, “Production of titanium carbide nanopowders by magnesiothermal reduction of a mixture of chlorides,” Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 5, 60–65 (2004).
A. S. Kurlov and A. I. Gusev, Physics and Chemistry of Tungsten Carbides: A Monograph (FIZMALIT, Moscow, 2013).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Balakhonov, D.I., Makarov, I.A. Plasma-Chemical Synthesis of Tungsten Carbides from Multicomponent Oxide-Containing Concentrates. Russ. Metall. 2020, 870–876 (2020). https://doi.org/10.1134/S0036029520080029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520080029