Features of cassiterite concentrate carbothermic reduction in melt of the salt system Na2CO3–NaNO3 (1:0.3) are studied. It is shown that cassiterite (SnO2) in molten salt (900–950°C) is converted into the form of metastannate (Na2SnO3) and is reduced to metal, mainly by CO gas, with a high rate and completeness (97 wt.%). Conditions are considered for preparing tungsten powder by aluminothermic reduction of scheelite concentrate in molten salts of alkali metals (NaCl–Na2CO3, NaCl–Na2CO3–NaF, NaCl–Na2CO3–Na3AlF6). It is established that powder yield is ~ 94%, and tungsten purity is 97%. It is shown that powder specific surface reaches 12.96·105 m−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Contemporary engineering requires use of material exhibiting not only increased strength, but also a number of special properties (wear-, heat-, and corrosion-resistance, etc.) providing prolonged and reliable equipment operation in very different operating conditions. In order to prepare these materials from ferrous alloys (steels, cast irons, and others) it is necessary to alloy them with such elements as W, Mo. V, Ti, etc.
A very promising area is development of methods for synthesizing tin and its alloys (bronzes, babbit, tin solders).
The Far East region exhibits considerable mineral raw material reserves containing these elements, including tin and tungsten. In this connection an important problem is effective use of metal resources for production of metallic materials, and also the problem of creating new technology for preparing them with processing of tin and tungsten mineral concentrates in the areas of their recovery.
An electric-arc method developed previously for obtaining tin and copper from mineral concentrates has a high energy content, prolonged preparatory processes, and other disadvantages [1]. These disadvantages may be overcome as a result of using a new method for obtaining tin.
Obtaining tin by carbothermic reduction of cassiterite concentrate in molten alkali metal salts, i.e., traditional technology for producing tin metal, is a multistage process including processing ore concentrate with the aim of breaking it down, purification from impurities, and subsequent reduction melting. Firing is used as preliminary treatment in order to remove sulfur and arsenic, magnetic separation is used for separating iron-containing fractions from the main mineral, and leaching is used with the aim of purification from impurity elements. The concluding stage of the technology is carbothermic reduction of cassiterite, which is performed at 1200–1300°C with addition of fluxes (CaCO3, SiO2). As a result of this alloys are obtained within whose composition there is 50–91% Sn depending on its content in the concentrate [2]. On the whole traditional technology for obtaining tin from mineral concentrates is a complex scheme, highly expensive, and does not provide sufficiently complete tin extraction.
A very advanced area is use of alkali metal molten salts as a medium for reducing metal oxides. These melts answer to a considerable extent specifications for a medium within which there is reaction of tin oxide reduction: they exhibit good dissolving capacity with respect to original substances, and provide a high reduction reaction rate without marked loss of valuable product.
Physicochemical bases of carbothermic reduction of metal oxides is described in sufficient detail in publications [3]. It is well known that reduction of cassiterite is realized through a series of oxidation-reduction reactions, and in summary is expressed by an equation
Equilibrium reactions move in the direction of forming valuable product, and this trend is reinforced in the temperature range t = 800–1200°C [4]. In addition, the rate of solid phase reduction is limited in the stage of reducing agent (C, CO) diffusion in solid cassiterite phase. With t > 850°C, there is charge sintering, as a result of which the reduction rate is reduced.
Melting of charges with a flux (CaCO3, SiO2) in the range 1100–1200°C increases reaction rate, although selectivity of tin reduction is lost [5]. These disadvantages are overcome with reduction in alkali metal molten salts [6, 7]. The object studied was cassiterite concentrate whose composition is given in Table 1.
In our case, carbothermic reduction of cassiterite is performed in a melt Na2CO3 – NaNO3 (weight fraction 1:0.3) with t = 850–950°C. A study of thermal transformations in the SnO2–Na2CO3–NaNO3–C in the range 300–900°C (Fig. 1) showed that reaction of cassiterite with melt provides SnO2 transfer in the form of sodium metastannate (Na2SnO3), and this is confirmed by x-radiographic analysis data for the reaction product. By exhibiting greater reaction capacity than SnO2, sodium metastannate is reduced in the liquid phase of a melt with a high rate and completeness. This is indicated by an increase in the content of tin metal in product of reduction system SnO2–Na2CO3–NaNO3–C even with t = 600°C (Table 2).
Sodium nitrate (NaNO3) within the composition of a salt system breaks down with t = 380°C with release of oxygen and formation of NaNO2, which in turn is broken down to Na2O. The oxygen released activates combustion of carbon with t = 450°C, which on a DTA curve is reflected by an increase in effect intensity (see Fig. 1). It is not excluded that the process is accompanied by iron (II) oxidation, and this is indicated by the reduction in its content in black alloy (Table 3).
Presence of Na2O in a salt system provides complete transfer of tin oxide into its metastannate in accordance with the reaction
The reducing agent used was low-sulfur carbon. Probably reduction of cassiterite proceeds by a combined mechanism, when both solid carbon and gas (CO) within the composition participate. Under molten salt conditions reduction of cassiterite by gas and melt bubbling predominates, which is observed in experiments for obtaining tin. The effect of an increase in the degree of cassiterite reduction is confirmed in the course of carrying out test melts: yield of tin in black alloy comprises 95–97%.
A dependence has been revealed by experiment for metal yield on carbon concentration in a charge. It is shown in Fig. 2 that the greatest metal extraction (≈97%) is achieved with a content in the charge of the order of 14 wt.% carbon, and this corresponds to about 20% excess with respect to calculated amount. The amount of sodium carbonate in a charge is close to that determined for the stoichiometric reaction of the latter with cassiterite.
Thus, for the concentrates indicated the following charge composition is required: for one weight fraction of concentrate there are weight fractions: 0.2–0.22 carbon, 0.08 sodium nitrate, and 0.3–0.35 sodium carbonate. A prepared charge is melted at 850–950°C for 1.5–2 h.
As a result of this ingots of black tin are obtained, whose elemental composition is given in Table 3. Also given is the composition of slag taking account of which it is recommended to treat it by traditional technology.
Preparation of tungsten powder by metallothermic treatment of scheelite concentrate in alkali metal molten salts. Methods for obtaining tungsten metal powder are very varied, they are separated into mechanical, physical, and they differ both in nature of processes and also the original raw material composition. However, the most widespread method in domestic practice is chemical reduction of oxide [6].
Traditional technology for producing tungsten metal is complicated, and it is a multistage process providing processing of ore concentrate with extraction of useful component in the form of oxide or other compounds, their purification, and reduction by hydrogen at 800–1200°C. The technology has relatively low productivity with considerable expenditure and does not answer entirely the specifications for contemporary technology [7]. A common disadvantage of the majority of methods known for preparing metal powder materials, including tungsten, is the high cost of end product, caused by use of expensive starting raw material and complex equipment [8, 9].
In spite of success achieved in the field of obtaining powder materials, the problem of creating economic processes remains, and is particularly important under conditions of the Far East region where tungsten concentrates are produced in large quantities.
In the first stage, conditions for metallothermic reduction tungsten oxide and obtaining metal powder were studied. High-temperature dissolution of tungsten oxide in alkali metal molten salts, for example in carbonates, corresponds to reactions
Thermodynamic analysis of reactions showed that the reaction capacity for molten salts with respect to tungsten oxide increases in the series, lithium, sodium, and potassium salts.
The probability of tungsten formation reactions from its higher oxide with reduction by aluminum in a medium of molten carbonates with specific fractions is described by equations
It is apparent that reduction proceeds by a more complex scheme in view of the specific features of ionic melt properties.
From results of thermodynamic analysis of reactions it follows that reduction is accompanied by significant decrease in the value of isobaric potential and it is feasible over a wide temperature range. Reactions proceed with very high values of equilibrium constant K e. They are exothermic, and correspondingly absolute values of ΔG and K e decrease with an increase in temperature (Figs. 3 and 4).
The tendency of a shift in reaction equilibrium in the direction of original products is hardly observed, since the process occurs at a high rate, tungsten in the form of solid phase is removed from the reaction medium, and this is typical for irreversible reactions occurring to complete reaction of original substances.
Metallothermic reduction of tungsten oxide in air and in a molten medium is specified by differential thermal analysis (DTA) and thermogravimetric (TG) methods. Temperature ranges have been determined for transformations in the systems WO3–Al, WO3–Al–Na2CO3, WO3–Al–NaCl (Fig. 5). On the DTA curve for the WO3–Al system an endothermic effect is noted at 600°C, accompanying melting of aluminum, then on the curve an axisymmetric exothermic effect is observed at 700–950°C. This indicates intense development of reaction of molten aluminum with tungsten oxide. Analysis of the TG curve shows that the weight increase in the range 800–1000°C corresponds to complete oxidation of aluminum (see Fig. 5a ). Reaction of tungsten oxide with aluminum in a molten salt medium (Na2CO3, NaCl) proceeds less intensely, and the temperature for it to commence is reduced by 100–150°C (see Fig. 5b ).
The process of obtaining powders includes two stages. High-temperature dissolution of the original tungsten compound with oxygen (for example WO3) in alkali metal molten slats, and reduction of tungsten compounds by introduction of aluminum powder into molten salt. In the course of experimental determination of parameters for tungsten powder preparation it has been established that reduction of the original tungsten compounds in relatively dilute solutions (WO3–Na2CO3, WO3–NaCl – 1:10) with a stoichiometric WO3–Al ratio provides a yield of about 85 wt.% tungsten powder. The tungsten powder yield increases as a result of introduction into the melt of excess aluminum with respect to the calculated amount, and reaches 97–98 wt.% with 25–25% excess reducing agent (Table 4).
A requirement for excess aluminum is probably due to occurrence of subsidiary reactions in molten salts. Alongside this the degree of reduction of original tungsten compounds depends on melt temperature. The degree of sodium tungstate reduction in molten NaCl, determined from the weight of tungsten powder yielded from melts, equals 0.82 at 1100 K, and a WO3–Al stoichiometric ratio. With an increase in melt temperature there is a tendency towards an increase in the degree of reduction. With an NaCl melt temperature of 1273 K the degree of reduction increases to 0.91, and with 1373 K it increases to 0.93 (Fig. 6). Fine tungsten metal powder is obtained from carbonate melts, sodium chloride, and mixtures of them. Conditions for powder preparation are provided in Table 4.
A method has been developed for obtaining tungsten metal powder in the stage of pyrometallurgical processing of scheelite concentrate having the composition, wt.%: WO3 55, Fe2O3 5, TiO2 0.23, MnO 0.2, CaO 19, MgO 2.4, Al2O3 0.8, SiO2 8. The tungsten preparation process consists of high-temperature decomposition of concentrate in molten salt of the system and reduction of the tungsten oxide compound by aluminum within a melt.
Use of two- or three-component salt systems, for example, NaCl–Na2CO3 or NaCl–NaF–Na2CO3 provides 93–96% tungsten extraction from concentrate in melt in the form of sodium tungstate. With concentrate melting in a ternary salt system at 1223–1273 K during one hour tungstate is transferred into the molten salt, and part of the concentrate (≈20%) remains undissolved, and it mainly contains SiO2, CaO, and Fe2O3. The upper salt layer of tungstate melt is poured off with solid residue. Tungsten is obtained by reducing sodium tungstate with aluminum in a molten medium. Results of obtaining tungsten powder for scheelite concentrate are given in Table 5.
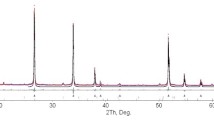
x-Ray phase analysis showed that reduction products are single-phase tungsten powder. According to results of elements analysis, the impurity element content depends on the original raw material. Tungsten powder obtained from oxide has the following composition, wt.%: W 98–99, including impurities Fe 0.2–0.3, Cu 0.3–0.4; powder from scheelite concentrate: W 96–97, Fe 0.5–0.6%, Cu 0.4–0.5; nonmetallic impurities 1.2–1.5.
The grain size composition of tungsten powder obtained from oxide in carbonate and chloride alkali metal melts, and also tungsten obtained from scheelite concentrate in ternary salt system melts (Table 6, Fig. 7) have been established by experiment. It follows from the data obtained that tungsten powder with the greatest specific surface is formed in alkali metal chloride melts (for example sodium chloride, S = 29·105 m−1, Table 6).
Conclusion. Thus, on the basis of experimental data it may be concluded that use of ionic melts makes it possible within the scope of a single-stage process to provide mineral concentrate decomposition with extraction of useful components (tin, tungsten, in the form of oxide compounds) in a melt followed by reduction to metals in a melt. A high degree of extraction of valuable products is provided in the stage of pyrometallurgical processing of mineral concentrates.
References
Ri Khosen, E. M. Baranov, E. V. Shchukin, and A. S. Grebennikov, Patent 2224037 RF, "Electric-arc method for preparing tin from cassiterite concentrate," publ. 02.20.2004.
O. M. Katkov, Tin Concentrate Processing, Metallurgiya, Moscow (1993).
V. P. Elyutin, Yu. A. Pavlov, P. V. Polyakov, and S. B. Sheboldaev, Reaction of Metal Oxides with Carbon, Metallurgiya, Moscow (1976).
A. S. Lebedev, V. E. D’yakov, and A. N. Terebenin, Complex Tin Metallurgy, ID Novosibirskii Pisatel, Novosibirsk (2004).
D. V. Belyaev, Tin Metallurgy, Metallurgiya, Moscow (1960).
V. S. Panov and A. M. Chuvilin, Technology and Properties of Hard Alloys and Objects Made from Them, MISiS, Moscow (2001).
R. U. Kalamazov, Yu. V. Tsvetkov, and A. A. Kalkov, Very Fine Tungsten and Molybdenum Powders, Metallurgiya, Moscow (1988).
Liao Ji-gino, Huang Zhi–Jeng, and Lu Hai-bo, “Obtaining tungsten powder recovery oxides by hydrogen,” J. Zhongnan Goagye Daxue Xuebao, 31, No. 1, 51–55 (2000).
L. V. M. Antony and R. G. Reddy, "Processes for production of high-purity metal powder,” J. Mineral Mater. Soc., 55, No. 3, 14–18 (2003).
This study was supported by the Russian Foundation for Basic Research (Grant No. 12–03–98506).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 6, pp. 77–83, June, 2015.
Rights and permissions
About this article
Cite this article
Gostishchev, V.V., Ri, K., Ri, E.K. et al. Preparation of Tin and Tungsten from Mineral Concentrates in Ionic Melts. Metallurgist 59, 526–534 (2015). https://doi.org/10.1007/s11015-015-0135-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-015-0135-0