Abstract
Artemisinin has attracted interest due to its medicinal value in treating malaria and its potential for use against certain cancers and viral diseases. Trichome density and capacity determine artemisinin content in Artemisia annua plants. Thus, the ATP-binding cassette transporter G (ABCG) subfamily involved in trichome cuticle development may also influence artemisinin accumulation. In this study, putative A. annua ABC transporter unigenes were identified and classified from the unigene sequences up to date in the National Center for Biotechnology Information database, and nine putative A. annua ABCG transporter unigenes that may be involved in cuticle development were selected for expression analyses. Two of them, AaABCG6 and AaABCG7, showed parallel expression pattern as two artemisinin biosynthesis-specific genes (amorpha-4, 11-diene synthase and a cytochrome P450-dependent hydroxylase, CYP71AV1) in different tissues and different leaf development stages and also showed similar induction in the plants after methyl jasmonate or abscisic acid treatments. Identification of these putative A. annua ABCG transporter unigenes could provide the basis for cloning of the full-length genes and further functional investigation to find the artemisinin relevant transporters, which could be used for improving artemisinin yield in both A. annua plants and heterologous systems using transgenic technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisinin is a sesquiterpenoid isolated from Artemisia annua L. plants. The therapies based on artemisinin are right now the most effective treatment against malaria, which causes 300–500 million cases and at least one million deaths annually (Graham et al. 2010; Milhous and Weina 2010). Artemisinin also showed potential as treatment for certain cancers and viral diseases (Efferth 2007; Efferth et al. 2008). Many works of fermentative production of artemisinin precursors through microbes have been developed (Arsenault et al. 2008; Chang et al. 2007; Ro et al. 2006; Zeng et al. 2008), but the traditional resource from A. annua plants would still be valuable for improving the yield of artemisinin (Covello 2008; Graham et al. 2010; Maes et al. 2010; Milhous and Weina 2010).
According to the researches of many groups, it is well accepted that artemisinin is synthesized in ten-cell glandular trichomes and finally accumulated in the subcuticular space at the top of the trichomes, which is between the cell wall of secretory cells and the cuticle (Duke et al. 1994; Maes et al. 2010; Schramek et al. 2010). The precursor for artemisinin biosynthesis, isopentenyl phosphate (IPP), could be obtained from both plastid (MEP) and cytosol (MVA) pathway, then IPP is condensed to farnesyl diphosphate (FPP) by farnesyl diphosphate synthase (FPS) (Schramek et al. 2010). The first artemisinin-specific biosynthesis step is the conversion from FPP to amorpha-4, 11-diene, which is catalyzed by amorpha-4, 11-diene synthase (ADS) (Mercke et al. 2000; Wallaart et al. 2001). Amorpha-4, 11-diene is further hydroxylated to artemisinic alcohol and then oxidized to artemisinic aldehyde by a cytochrome P450-dependent hydroxylase, CYP71AV1 (CYP) (Teoh et al. 2006). Subsequently, the most important direct precursor for artemisinin, dihydroartemisinic acid (DHAA), is converted from artemisinic aldehyde via dihydroartemisinic aldehyde by two enzymes, artemisinic aldehyde Delta 11(13) reductase (DBR2) (Zhang et al. 2008) and aldehyde dehydrogenase 1 (ALDH1) (Teoh et al. 2009), respectively. At the same time, artemisinic acid (AA) is also synthesized from artemisinic aldehyde with the help of CYP and ALDH1 (Teoh et al. 2006, 2009). It has been suggested that the last steps of artemisinin biosynthesis, conversion from DHAA to artemisinin, are enzyme independent, and AA is converted to arteannuin B (AB) in a similar way (Brown and Sy 2004, 2007; Lommen et al. 2006, 2007). Olsson et al. found that most artimisinin-specific biosynthetic enzymes, such as ADS, CYP, ALDH1, and DBR2, are expressed exclusively in the two outer apical cells, while FPS transcripts could be found through the whole trichome cells, and the 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR), which is in the MEP pathway for IPP production, is not expressed in the two outer apical cells but in the subapical cells, where chloroplasts exist (Olsson et al. 2009).
One of the recent focuses is placed on searching genes influencing artemisinin yield (Graham et al. 2010; Maes et al. 2010), and since engineering transporters is a very important strategy for manipulation of secondary metabolites (Oksman-Caldentey and Inzé 2004), identification of artemisinin yield relevant transporters would be very useful in engineering A. annua plants or the heterologous host to improve artemisinin production.
Plant ATP-binding cassette (ABC) transporters play very important roles in the transport of secondary metabolites, such as alkaloids, phenol, terpenoids, and wax (Rea 2007; Verrier et al. 2008; Yazaki 2005, 2006). ABCG subfamily transporters include WBC and PDR transporters. It was proven that some ABCG transporters are involved in the transport of terpenoids (Campbell et al. 2003; Jasiński et al. 2001) or cuticle development (Bird et al. 2007; Panikashvili and Aharoni 2008; Panikashvili et al. 2010, 2011; Pighin 2004) in plants, and some are induced in the engineered yeast that produced more artemisinin precursors (Ro et al. 2008). It is possible that some ABCG transporters are involved in A. annua glandular trichome cuticle development and/or play roles in the transport relevant to artemisinin biosynthesis and accumulation, so it would be promising to screen artemisinin yield relevant transporters from the ABCG subfamily.
There is a large amount of A. annua cDNA and EST sequences from different libraries up to date, and the National Center for Biotechnology Information (NCBI) has integrated these data to a relative complete inventory of A. annua unigene sequences. There are many successful bioinformatic data mining works on A. annua (Dai et al. 2010; Zhang et al. 2011b) and other plants (Kim et al. 2011; Wang et al. 2011; Zhang et al. 2011a). Part of A. annua unigene sequences from trichome-specific or non-trichome library has been classified in the TrichOME database (Dai et al. 2010). In this study, the unigene sequences from the NCBI and TrichOME database were compared and integrated, and 340 putative ABC transporter sequences were selected. After classification, nine putative ABCG transporter sequences that may be involved in cuticle development were picked, and their expression in different tissues and in methyl jasmonate (MJ) or abscisic acid (ABA)-treated plants was analyzed and discussed for their relevance to artemisinin yield and transport.
Materials and Methods
Transporters Identification and Classification
A. annua unigene and EST sequences were obtained from NCBI and TrichOME database (http://www.planttrichome.org/), and then they were compared with TCDB ABC transporter proteins, which were retrieved from the TCDB database (http://www.tcdb.org/), using BLASTX with e-values of less than 1e − 04. The sequences with significant similarity to TCDB ABC transporter proteins were selected for further analysis.
The selected sequences were assembled with Vector NTI (Invitrogen) and then annotated and classified with the best match compared against UniprotKB ABC transporter proteins, which were retrieved from UniprotKB database (http://www.uniprot.org/), through BLASTX with e-values of less than 1e − 04. The alignment of protein sequences of selected A. annua unigenes and ABCG transporters from other species was carried out with CLUSTAL X (1.81) using default parameters.
Plant Materials
The seeds of A. annua were obtained from the School of Life Sciences, Southwest University in Chongqing, People’s Republic of China. Plants of A. annua were grown in soil in a growth room at 25°C under 16 h of light and 8 h of dark per day, and some of them were transferred to a green house and field after 4 to 6 weeks.
A. annua plants at the beginning of florescence after grown in the field for 5 months were selected for RNA extraction and expression analyses. The plants’ young buds (Bud0), mature leaves (Leaf), and stems and roots (Stem and Root) were collected from five plants and pooled separately. The buds not fully blossomed (Bud1) and the fully florescent flowers (Flower) were collected and mixed after 7 and 14 days, respectively, from the same plants. On the other hand, A. annua plants grown in the green house for 1 month, 35 to 45 cm height, were chosen for collecting leaves from different positions of the plants. The meristem and the first, second, third, fifth, seventh, and ninth leaves counted from the apical top (Meristem, Leaf1, Leaf2, Leaf3, Leaf5, Leaf7, Leaf9) were collected separately from the main stem of five different plants. Leaves were then pooled respectively for each sample. Half of each leaf was cut and mixed for RNA extraction and expression analyses, and the rest of the part of the same leaf was for metabolite measurement.
MJ and ABA Treatment
A. annua plants grown in the growth room for 8 weeks were treated with solutions of 100 μM ABA, 100 μM MeJA (Sigma Aldrich, USA), and distilled water as mock control. Five plants were used for each treatment, respectively. Leaves were collected randomly from the pooled plants before treatment (0 h) and at different time points after treatment (1, 3, 6, 12, and 24 h) for the gene expression analyses and artemisinin quantification.
Real-Time qPCR for Expression Analysis
RNA was extracted using TIANGEN RNA prep pure plant kit (TIANGEN Biotech, Beijing, China) following the manufacturer’s instructions. cDNA was prepared with PrimeScript RT reagent kit (Perfect Real Time) (Takara, Shiga, Japan). Real-time qPCR was performed on Peltier Thermal Cycler PTC-200 PCR Machines with Chromo4 continuous fluorescence detector (Bio-Rad, Watford, UK) with SYBR Premix Ex Taq (Perfect Real Time) (Takara) and gene-specific primers (Table 1). ACTIN was used as the control gene to normalize the expression level. Procedures of qPCR were performed as described in our previous research (Lu et al. 2011). Relative gene expression was calculated based on 2−ΔΔCT comparative method (Livak and Schmittgen 2001).
Metabolite Quantification
Leaves of A. annua plants were pooled and freshly weighed, then frozen in liquid nitrogen and kept at −70°C up to 1 month. Metabolites were extracted by immerging the fresh or frozen samples in chloroform for 2 min, dried in fume hood, and then re-dissolved in methanol, or by ultrasonic treatment of 100 mg of ground dry sample with 1 mL of methanol directly. The following treatments and artemisinin measurement were performed with HPLC-ELSD as mentioned in our previous works (Lu et al. 2011; Zhang et al. 2009). Furthermore, the DHAA was measured with GC–MS (AutoSystem XL GC/TurboMass MS, Perkin Elmer, USA), equipped with a DB-5MS capillary column (30 m long × 0.25 mm internal diameter; 0.25 μm particle size) under the following conditions: injection port temperature, 250°C; carrier gas, He (99.999%) 1.0 mL/min; split ratio, 5:1; initial column temperature, 80°C (raised at 10°C/min to 300°C and then maintained for 14 min); and ionization voltage, 70 eV. The DHAA was identified according to the mass spectrum described by Wallaart et al. (1999), and relative DHAA content was calculated from the peak areas.
Results
Identification and Classification of Putative ABC Transporter Unigenes
A. annua unigenes, consisting of 93,672 sequences, from the NCBI database were compared with TCDB ABC transporter proteins through BLASTX, thus resulting 459 sequences being found with significant similarity to ABC transporter proteins. Since some unigene sequences were already classified in the TrichOME database, 67 A. annua sequences that were annotated as ABC transporters were retrieved from this database. The sequences from these two databases were integrated, and 340 putative ABC transporter sequences were selected and then classified by comparing with Uniprot database ABC transporter proteins through BLASTX (Table 2).
Since ABCG transporters involved in trichome cuticle development might also determine artemisinin production, 129 sequences that have the highest similarity to the ABCG subfamily were selected for screening artemisinin yield relative transporters. As we know in Arabidopsis, ABCG11, ABCG12, and ABCG13 are involved in cuticle development for wax and/or cutin transport (Bird et al. 2007; Panikashvili and Aharoni 2008; Panikashvili et al. 2010, 2011; Pighin 2004). So all the ABCG transporters close to them according to the phylogenetic tree described in previous researches (Sánchez-Fernández et al. 2001; Yazaki 2005), including ABCG11, ABCG12, ABCG13, ABCG15, and ABCG3, were targeted for screening artemisinin relative transporters. Nine putative A. annua ABCG transporter unigenes (AaABCG1 ~ 9) were selected for the following analyses due to their highest similarity to the five Arabidopsis ABCG transporters. The protein sequences of these unigenes were aligned with selected ABCG transporters from other species (Fig. 1), and significant similarity could be found between them.
The protein sequence alignment of selected AaABCG transporter unigenes with similar ABCG transporter from other species. The deduced amino acid sequence of AaABCG1 (Aan.97672, Aan.7512), AaABCG2 (Aan.29133), AaABCG3 (Aan.20798, Aan.21342), AaABCG4 (Aan.30350, Aan.32607, Aan.103542), AAABCG5 (Aan.67011), AaABCG6 (Aan.67737), AaABCG7 (Aan.68336), AaABCG8 (Aan.83267), AaABCG9 (Aan.70089, Aan.54903), and the protein sequence of AtABCG3 (Q9ZUU9), AtABCG11 (Q8RXN0), AtABCG12 (Q9C8K2), AtABCG13 (Q9C8J8), AtABCG15 (Q8RWI9), ZmCER5 (ACG26663), ZmABCG2 (ACG25567), PpABCG6 (XP_001782413), PpABCG7 (EDQ59314), PpABCG8 (XP_001782003), and PpABCG28 (EDQ52515) were aligned with Clustal X program. The sequences marked with the black bars are the typical ABC transporter sequences. The sequences shown as white color with black and dark gray background indicate the completely identical and conservative residues, respectively, and the sequences shown as black color with light gray background indicate the similar residues
Expression Analyses of Different Tissues and Leaf Development Stages
Expression of nine selected ABCG unigenes in different tissues was investigated through real-time qPCR (Fig. 2). The artemisinin biosynthesis-specific gene ADS and CYP were highly expressed in young buds (Bud0), lower in non-fully flowering buds (Bud1) and fully blossomed flowers (Flower), poorly expressed in mature leaves (Leaf) and stems (Stem), and hardly expressed in roots (Root). AaABCG6 and AaABCG7 got very similar expression pattern like ADS and CYP, except that they were slightly expressed in the roots. AaABCG1 was also highly expressed in Bud0, Bud1, and Flower samples, but still kept a certain expression level in mature leaves and stems. AaABCG4 was just highly expressed in Bud0, Bud1, and Flower samples as ADS and CYP, but its expression increased as the flower matures. AaABCG2 and AaABCG9 were also hardly expressed in the roots, while AaABCG5 was almost expressed at the same level in all the tissues.
Expression of selected unigenes in different tissues of A. annua plants. Expression of AaADS, AaCYP, AaABCG1 ~ AaABCG9 in different tissues, including young buds (Bud0), buds not fully blossomed (Bud1), fully florescent flowers (Flower), mature leaves (Leaf), and stems and roots (Stem and Root), was measured through real-time qPCR. Values indicate the mean fold relative to sample Bud0. Error bars present SD (standard deviation) of three biological repeated samples
To dissect the expression dynamics of artemisinin biosynthesis genes and the selected ABCG transporter unigenes during leaf development, the expression of these genes in the meristem and the leaves at different nodal positions of the plant (first, second, third, fifth, seventh, and ninth leaves) was also analyzed through real-time qPCR. AaABCG6, AaABCG7, and AaABCG4 were just highly expressed in meristems and very young leaves, and then their expression reduced quickly during leaf development, which was the same as ADS and CYP (Fig. 3a). The other six candidates show some different patterns (Fig. 3b), or even a reverse pattern as ADS and CYP (Fig. 3c). Artemisinin and DHAA content in these leaves was also measured, respectively (Fig. 3d, e), suggesting that DHAA was accumulated rapidly during the early stage of leaf development (Meristem, Leaf1, Leaf2, Leaf3), when biosynthesis genes ADS and CYP, as well as the putative ABCG transporter unigenes AaABCG6, AaABCG7, and AaABCG4, were highly expressed, and then DHAA declined as the leaves aged, while the expression of ADS, CYP, AaABCG6, AaABCG7, and AaABCG4 reduced simultaneously. On the other hand, from the beginning of leaf initiation, artemisinin content increased continuously up to a platform in the seventh and ninth leaves.
Expression of selected unigenes and metabolite analyses in different leaves of A. annua. Expression of AaADS, AaCYP, and AaABCG1 ~ AaABCG9 in different leaves (Meristem, Leaf1, Leaf2, Leaf3, Leaf5, Leaf7, Leaf9) was measured through real-time qPCR (a–c). Values indicate the mean relative ratio of expression levels compared to sample Meristem. Error bars present SD (standard deviation) of three biological repeated samples. Artemisinin and dihydroartemisinic acid (DHAA) content per fresh weight (d) and per leaf (e) in different leaves of A. annua plants was measured by HPLC-ELSD and GC–MS, respectively. Columns show the mean values of artemisinin content. Lines with square dots show the relative content of DHAA. FW fresh weight. Error bars present SD (standard deviation) of three biological repeated samples
Expression Analyses After MJ and ABA Treatment
MJ and ABA have great influence on plant growth (Demetriou et al. 2010; Tripathy et al. 2011; Zambounis et al. 2011), and since it was proven that MJ and ABA could induce artemisinin accumulation in A. annua plants (Lu et al. 2011; Maes et al. 2010), expression of the nine selected ABCG transporter unigenes as well as ADS and CYP was also analyzed in MJ- and ABA-treated samples, which were compared to water-treated mock samples, and the artemisinin levels were also measured in these samples (Fig. 4).
Artemisinin content and expression of selected genes after different treatments. Expression of AaADS, AaCYP, and AaABCG1 ~ AaABCG9 in the leaves of plants before (0 h) and 1, 3, 6, 12, and 24 h after treated by mock, ABA, and MJ was measured through real-time qPCR. Values indicate the mean relative ratio of expression levels compared to sample 0 h. The artemisinin content of leaves from the same plants before (0 h) and 6, 12, 24, and 48 h after treated by mock, ABA, and MJ was measured by HPLC-ELSD method. Error bars present SD (standard deviation) of three biological repeated samples. DW dry weight
In a short time period (1 to 6 h), expression of ADS and CYP was inhibited significantly in mock samples, but maintained or induced slightly in ABA- and MJ-treated samples. Twelve hours after treatment, the expression of these two genes increased vigorously in all MJ-, ABA-, and mock-treated samples, up to about six- to tenfold of 0-h samples, except that the expression of ADS was stimulated up to 30-fold in mock samples.
The expression of AaABCG6 and AaABCG7 was similar compared to ADS and CYP. Their expression was induced slightly (two- to threefold) after treated with ABA and MJ in a short time (3 to 6 h). At 12 h after treatments, expression of both of them was induced to six- to tenfold than 0-h samples in all the ABA-, MJ-, and mock-treated samples, except that AaABCG7 expression was induced to around 18-fold in ABA-treated samples. AaABCG4 got a similar pattern, though its expression was induced sharply up to 29-fold at 12 h after MJ treatment and was induced continuously up to 17-fold at 24 h after ABA treatment. All the other selected ABCG transporter unigenes got significant difference in induction pattern after treatments.
The artemisinin level was reduced in a short time (6 h) after mock treatment, but maintained at the same level or even higher in the ABA- or MJ-treated samples. Of all the treatment, the artemisinin content rose up significantly after 12 h (12, 24, and 48 h), which complied with the vigorous increase of expression of ADS and CYP at 12 h.
Discussion
In this work, the young trichome-specific pattern of artimisinin biosynthesis and the conversion process from DHAA to artemisinin in A. annua leaves were verified again. The meristem and the first, second, third, fifth, seventh, and ninth leaves on the main stem of A. annua plants are of the same type (not the leaves from leafy branches), so metabolites and gene expression analyses of these leaves could reflect the situation during leaf development. The expression pattern of ADS and CYP that was just highly expressed in the early stage of leaf development (Fig. 3) and that was highly expressed in young trichome abundant tissues, such as young buds (Fig. 2), could verify the conclusions in previous works that most artemisinin biosynthesis genes are highly expressed during the early stage of trichome development (Graham et al. 2010; Olofsson et al. 2011). Lommen et al. showed that DHAA increases very fast after leaf appearance, then starts to convert to artemisinin later when the leaves are still rapidly expanding, the conversion continuing through the whole process of leaf development, even after the leaf starts senescence (Lommen et al. 2006). We got the same scenario in this study (Fig. 3d, e).
This work also verified that the expression of ADS and CYP would be inhibited by the release of artemisinin due to collapse of trichomes. Arsenault et al. proved that the expression of ADS and CYP would be inhibited by spraying the artemisinin or AA on the leaves of A. annua plants (Arsenault et al. 2010). They believed that a negative feedback of artemisinin and AA might exist on ADS and CYP genes. In this study, we also found that the expression of ADS and CYP was inhibited significantly in a short time in the water-sprayed samples (Fig. 4). It could be caused by artemisinin or AA released due to trichome collapse during water treatment and sample collection. Trichome collapse could also explain why artemisinin level declined rapidly in the mock-treated samples (Fig. 4).
MJ and ABA could induce artemisinin accumulation in both short and long periods after hormonal treatment. In the short time period, trichome collapse caused by water stress may lead to the loss of artemisinin and its precursors in all mock-, MJ-, and ABA-treated samples, but in MJ- and ABA-treated samples, expression of biosynthesis genes and relative transporters was induced almost immediately, thus complementing the loss of precursors and artemisinin. This may explain why artemisinin content was maintained at the same level or even increased a little in the MJ- and ABA-treated samples. On the other hand, interestingly, the expression of ADS and CYP increased vigorously at 12 h in all mock-, ABA-, and MJ-treated samples, suggesting that this induction may not only be caused by ABA or MJ, but may also be due to the process of treatment and sample collection. We supposed that this sharp increase is stimulated by new trichome initiation. These results could explain why artemisinin content started to increase steadily after 12 h in all mock-, ABA-, and MJ-treated samples (Fig. 4).
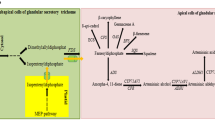
There are some evidences which could support the existence of artemisinin biosynthesis relevant transporters. Firstly, artemisinin-specific biosynthesis genes ADS, CYP, DBR2, and ALDH1 are expressed exclusively in the two outer apical cells, while the DXR in the MEP pathway is expressed in the subapical cells (Olsson et al. 2009). Based on these results and a 13CO2 feeding experiment, Schramek et al. suggested that mixed IPP origin from both cytosol and plastid is condensed to a geranyl diphosphate (GPP) in subapical cells, then GPP is transported to apical cells and condensed to FPP with another IPP from the cytosol of apical cells (Schramek et al. 2010). It is reasonable that some transporters exist between apical and subapical cells of the glandular trichomes to transport the precursors to the location where artemisinin is synthesized. Secondly, since conversion from DHAA to artemisinin, as well as the process from AA to AB, is enzyme independent, while artemisinin is accumulated in the subcuticular space, it could also suggest that transporters are needed for transporting DHAA or AA to the final storage before their conversion to artemisinin or AB slowly. Besides the transporters involved in artemisinin biosynthesis, the transporters involved in trichome and cuticle development could also be relevant to artemisinin yield. Glandular trichome density and capacity were proven to be associated with artemisinin production (Lommen et al. 2006; Maes et al. 2010), so the transporters that influence trichome development may also determine artemisinin yield. Figure 5 showed the summary of the hypothetic artemisinin yield relevant transporters mentioned above.
Putative transporters relevant to artemisinin yield. Transporters involved in artemisinin biosynthesis and cuticle development (illustrated as green cylinders) are shown as artemisinin yield relevant transporters. IPP isopentenyl phosphate, GPP geranyl diphosphate, FPP farnesyl diphosphate, DHAA dihydroartemisinic acid, AA artemisinic acid, AB arteannuin B, DXR 1-deoxy-d-xylulose-5-phosphate reductoisomerase, FPS farnesyl diphosphate synthase, ADS amorpha-4, 11-diene synthase, CYP amorphadiene-12-hydroxylase CYP71AV1, DBR2 artemisinic aldehyde Delta 11(13) reductase, ALDH1 aldehyde dehydrogenase 1
In this study, all the A. annua ABC transporter unigenes were classified, and nine putative ABCG transporter sequences that may be involved in cuticle development were selected for analyses. The results could provide primary support for the hypothesis of artemisinin yield relevant transporters mentioned in Fig. 5. Maes et al. cloned TFAR1, a trichome-specific fatty acyl-CoA reductase, and postulated that it is involved in cuticular wax formation during trichome development. This gene also has a similar trichome and young leaf-specific expression pattern as ADS and CYP, so they pointed out that a common mechanism for regulating metabolites and structure development of trichomes may exist (Maes et al. 2010). Similarly, in this work, AaABCG6 and AaABCG7 got parallel expression pattern as ADS and CYP in the samples from different tissues (Fig. 2) and different leaf development stages (Fig. 3) and also got similar induction as ADS and CYP in the samples after water, ABA, and MJ treatments (Fig. 4). It could suggest that these two putative transporter unigenes may also share the same regulatory mechanism with artemisinin biosynthesis genes, thus are highly possible to be correlated to artemisinin yield. Though these two ABCG transporter unigene sequences are not the full-length cDNA, both of them got high similarity to Arabidopsis ABCG3 transporter, whose function has not been identified. It is likely that AtABCG3 is also involved in secondary metabolite accumulation or trichome development. Besides AaABCG6 and AaABCG7, some kind of similarity of expression pattern between AaABCG1, AaABCG4, and those two artemisinin-specific genes suggests that these putative transporters may also be relevant to artemisinin yield.
In summary, we identified and classified putative A. annua ABC transporter unigenes, and nine candidate ABCG transporter unigenes were selected for expression and metabolite analyses in different tissues and leaves from different development stages, as well as the samples after ABA and MJ treatment. Two of them, AaABCG6 and AaABCG7, have parallel expression patterns and may share the same regulatory system with artemisinin biosynthesis genes ADS and CYP and may have high relevance to artemisinin yield. Identification of these putative A. annua ABCG transporter unigenes could provide the foundation for cloning the full-length genes and the further functional characterization to find artemisinin relevant transporters and then improving artemisinin yield in both A. annua plants and heterologous systems through transgenic technology.
References
Arsenault PR, Vail D, Wobbe KK, Erickson K, Weathers PJ (2010) Reproductive development modulates gene expression and metabolite levels with possible feedback inhibition of artemisinin in Artemisia annua. Plant Physiol 154:958–968
Arsenault PR, Wobbe KK, Weathers PJ (2008) Recent advances in artemisinin production through heterologous expression. Curr Med Chem 15:2886–2896
Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52:485–498
Brown GD, Sy L-K (2004) In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 60:1139–1159
Brown GD, Sy L-K (2007) In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron 63:9548–9566
Campbell EJ, Schenk PM, Kazan K, Penninckx IAMA, Anderson JP, Maclean DJ, Cammue BPA, Ebert PR, Manners JM (2003) Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 133:1272–1284
Chang MCY, Eachus RA, Trieu W, Ro D-K, Keasling JD (2007) Engineering Escherichia coli for production of functionalized terpenoids using plant p450s. Nat Chem Biol 3:274–277
Covello PS (2008) Making artemisinin. Phytochemistry 69:2881–2885
Dai X, Wang G, Yang DS, Tang Y, Broun P, Marks MD, Sumner LW, Dixon RA, Zhao PX (2010) Trichome: a comparative omics database for plant trichomes. Plant Physiol 152:44–54
Demetriou K, Kapazoglou A, Bladenopoulos K, Tsaftaris AS (2010) Epigenetic chromatin modifiers in barley: II. Characterization and expression analysis of the HDA1 family of barley histone deacetylases during development and in response to jasmonic acid. Plant Mol Biol Rep 28:9–21
Duke M, Paul R, Elsohly H, Sturtz G, Duke S (1994) Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci 155:365–372
Efferth T (2007) Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Med 73:299–309
Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJG, Marschall M (2008) The antiviral activities of artemisinin and artesunate. Clin Infect Dis 47:804–811
Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, Larson TR, Li Y, Pawson T, Penfield T, Rae AM, Rathbone DA, Reid S, Ross J, Smallwood MF, Segura V, Townsend T, Vyas D, Winzer T, Bowles D (2010) The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science 327:328–331
Jasiński M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13:1095–1107
Kim D-W, Kim RN, Choi S-H, Kim D-W, Nam S-H, Choi H-S, Koh HD, Kim A, Chae S-H, Ahn JC, Kang A, Park H-S (2011) EST analysis predicts putatively causative genes underlying the pharmaceutical application of Glycyrrhiza uralensis Fisch. Plant Mol Biol Rep 29:814–824
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(t)) method. Methods 25:402–408
Lommen WJM, Elzinga S, Verstappen FWA, Bouwmeester HJ (2007) Artemisinin and sesquiterpene precursors in dead and green leaves of Artemisia annua L. crops. Planta Med 73:1133–1139
Lommen WJM, Schenk E, Bouwmeester HJ, Verstappen FWA (2006) Trichome dynamics and artemisinin accumulation during development and senescence of Artemisia annua leaves. Planta Med 72:336–345
Lu X, Lin X, Shen Q, Zhang F, Wang Y, Chen Y, Wang T, Wu S, Tang K (2011) Characterization of the jasmonate biosynthetic gene allene oxide cyclase in Artemisia annua L., source of the antimalarial drug artemisinin. Plant Mol Biol Rep 29:489–497
Maes L, Van Nieuwerburgh FCW, Zhang Y, Reed DW, Pollier J, Vande Casteele SRF, Inzé D, Covello PS, Deforce DLD, Goossens A (2010) Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol 189:176–189
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE (2000) Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Milhous WK, Weina PJ (2010) Plant science. The botanical solution for malaria Science 327:279–280
Oksman-Caldentey K-M, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440
Olofsson L, Engström A, Lundgren A, Brodelius PE (2011) Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol 11:45. doi:10.1186/1471-2229-11-45
Olsson ME, Olofsson LM, Lindahl A-L, Lundgren A, Brodelius M, Brodelius PE (2009) Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 70:1123–1128
Panikashvili D, Aharoni A (2008) ABC-type transporters and cuticle assembly: linking function to polarity in epidermis cells. Plant Signal Behav 3:806–809
Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, Aharoni A (2010) The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol Plant 3:563–575
Panikashvili D, Shi JX, Schreiber L, Aharoni A (2011) The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis. New Phytol 190(1):113–124. doi:10.1111/j.1469-8137.2010.03608.x
Pighin JA (2004) Plant cuticular lipid export requires an ABC transporter. Science 306:702–704
Rea PA (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58:347–375
Ro D-K, Ouellet M, Paradise EM, Burd H, Eng D, Paddon CJ, Newman JD, Keasling JD (2008) Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol 8:83. doi:10.1186/1472-6750-8-83
Ro D-K, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Sánchez-Fernández R, Davies TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276:30231–44
Schramek N, Wang H, Römisch-Margl W, Keil B, Radykewicz T, Winzenhörlein B, Beerhues L, Bacher A, Rohdich F, Gershenzon J, Liu B, Eisenreich W (2010) Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochemistry 71:179–187
Teoh KH, Polichuk DR, Reed DW, Covello PS (2009) Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 87:635–642
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome p450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Tripathy MK, Tyagi W, Goswami M, Kaul T, Singla-Pareek SL, Deswal R, Reddy MK, Sopory SK (2011) Characterization and functional validation of tobacco PLC Delta for abiotic stress tolerance. Plant Mol Biol Rep. doi:10.1007/s11105-011-0360-z
Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL (2008) Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci 13:151–159
Wallaart T, van Uden W, Lubberink H, Woerdenbag H, Pras N, Quax W (1999) Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod 62:430–433
Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NC (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wang W-K, Liu C-C, Chiang T-Y, Chen M-T, Chou C-H, Yeh C-H (2011) Characterization of expressed sequence tags from flower buds of alpine Lilium formosanum using a subtractive cdna library. Plant Mol Biol Rep 29:88–97
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8:301–307
Yazaki K (2006) ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett 580:1183–1191
Zambounis AG, Kalamaki MS, Tani EE, Paplomatas EJ, Tsaftaris AS (2011) Expression analysis of defense-related genes in cotton (Gossypium hirsutum) after Fusarium oxysporum f. sp. vasinfectum infection and following chemical elicitation using a salicylic acid analog and methyl jasmonate. Plant Mol Biol Rep doi:. doi:10.1007/s11105-011-0335-0
Zeng Q, Qiu F, Yuan L (2008) Production of artemisinin by genetically-modified microbes. Biotechnol Lett 30:581–592
Zhang D, Hu C, Ouyang Y, Yao J (2011a) Construction of a full-length cDNA library and analysis of expressed sequence tags from inflorescence of apomictic sabaigrass (Eulaliopsis binata). Plant Mol Biol Rep. doi:10.1007/s11105-011-0316-3
Zhang H, Jin J, Tang L, Zhao Y, Gu X, Gao G, Luo J (2011b) Planttfdb 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res 39:D1114–1117
Zhang L, Jing F, Li F, Li M, Wang Y, Wang G, Sun X, Tang K (2009) Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol Appl Biochem 52:199–207
Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS (2008) The molecular cloning of artemisinic aldehyde delta11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–21508
Acknowledgments
This work was funded by China National High-Tech “863” Program (2011AA100605), Shanghai Science and Technology Committee (08391911800), and Shanghai Leading Academic Discipline Project (B209). We also thank the Instrumental Analysis Center of the Shanghai Jiao Tong University for assistance with the GC–MS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Lu, X., Shen, Q. et al. Identification of Putative Artemisia annua ABCG Transporter Unigenes Related to Artemisinin Yield Following Expression Analysis in Different Plant Tissues and in Response to Methyl Jasmonate and Abscisic Acid Treatments. Plant Mol Biol Rep 30, 838–847 (2012). https://doi.org/10.1007/s11105-011-0400-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-011-0400-8