Abstract
Artemisinin is widely used as an antimalarial drug, and the regulation of artemisinin metabolism is at the forefront of artemisinin research. A WRKY transcription factor, named as AaWRKY4, was cloned from high artemisinin-yielding Artemisia annua, which has similar expression pattern with the key enzymes in artemisinin biosynthetic pathway. AaWRKY4 was preferentially expressed in glandular secretory trichomes (GSTs) of young leaves and flower buds, but weakly expressed in other tissues. To further study the function of AaWRKY4, plant expression vector pHB-AaWRKY4 containing AaWRKY4 driven by CaMV 35S promoter was constructed and introduced into A. annua via Agrobacterium tumafeciens-mediated transformation. Expression analysis showed that the expression of AaWRKY4 was increased in transgenic plants. Four independent transgenic plants overexpressing AaWRKY4 were selected for further analysis. The expression levels of artemisinin biosynthetic pathway genes ADS, CYP71AV1, DBR2 and ALDH1 were dramatically increased in AaWRKY4-overexpressing A. annua plants. Furthermore, the artemisinin yield was increased by 35–50% in AaWRKY4-overexpressing A. annua plants. These results indicate AaWRKY4 can upregulate artemisinin content through regulating artemisinin metabolism.
Key message
A WRKY transcription factor, named as AaWRKY4, was cloned from high artemisinin-yielding Artemisia annua. AaWRKY4 was preferentially expressed in glandular secretory trichomes (GSTs) of young leaves and flower buds. The expression levels of artemisinin biosynthetic pathway genes ADS, CYP71AV1, DBR2 and ALDH1 were dramatically increased in AaWRKY4-overexpressing A. annua plants. Furthermore, the artemisinin yield was increased by 35–50% in AaWRKY4- overexpressing A. annua plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisia annua, also named Huang Hua Hao, is an important Chinese medicinal plant according to the Chinese materia medica (Hsu 2006; Miller et al. 2011). Artemisinin, extracted from A. annua, was discovered by Youyou Tu and used in the treatment of malaria, which has saved millions of lives all over the world (Miller et al. 2011). In addition, some other functions of artemisinin such as inducing apoptosis in human cancer cells were reported (Singh et al. 2004; Romero et al. 2005). As the genome of A. annua was sequenced recently, the A. annua genome and transcriptome data are quite valuable for fundamental biological research and applied breeding programs (Shen et al. 2018).
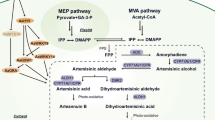
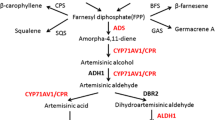
The artemisinin biosynthetic pathway in A. annua was studied for many years and it was almost completed (Covello 2008). The precursors of artemisinin biosynthesis dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) were formed from both mevalonate pathway (MVA pathway) and nonmevalonate pathway (MEP pathway). IPP and DMAPP form farnesyl diphosphate (FPP) by farnesyl diphosphate synthase, which is the common precursor of sesquiterpenes (Towler 2007). Amorpha-4, 11-diene synthase (ADS), the first key enzyme in the artemisinin biosynthetic pathway, can convert FPP to amorpha-4, 11-diene (Bouwmeester et al. 1999; Wallaart et al. 2001; Picaud et al. 2005). Through cytochrome P450 enzyme CYP71AV1 (CYP), amorpha-4, 11-diene is oxidized to artemisinic alcohol, and is further oxidized to artemisinic aldehyde (Teoh et al. 2006). Futhermore, through artemisinic aldehyde ∆11 (13) reductase (DBR2) encoding a member of the enoate reductase family with similarity to plant 2-oxophytodienoate reductases, artemisinic aldehyde is reduced to dihydroartemisinic aldehyde (Zhang et al. 2008). Through an aldehyde dehydrogenase (ALDH1), dihydroartemisinic acid (DHAA) is formed (Teoh et al. 2009). The process from DHAA to artemisinin is still not clear now. It is considered to be a spontaneous non-enzymatic reaction (Sy et al. 2002; Covello 2008). However, it was also reported that peroxidase enzymes or an alternative series of oxidations might catalyze the last reaction (Bryant et al. 2015).
Transcription factors play an important role in the regulation of plant secondary metabolism. Transcriptional regulation of key enzymes in plant secondary metabolism is an important regulatory process, and regulation of biosynthetic pathways is often achieved through specific transcription factors. These transcription factors include bHLHs, MYBs, WRKYs, ERFs and other transcription factor families, which are widely involved in many secondary metabolic processes, including the synthesis of pigments, terpenoids, and alkaloids (Broun 2004). Artemisinin is an important sesquiterpene extracted from A. annua, and several transcription factors have been cloned and proved to have functions on secondary metabolism, especially artemisinin metabolism in A. annua. Ma et al. cloned a transcription factor AaWRKY1, which was preferentially expressed in GSTs. Biochemical tests showed that AaWRKY1 could activate the expression of ADS (Ma et al. 2009). Han et al. transformed AaWRKY1 into A. annua, and the results showed that trichome-specific overexpression of AaWRKY1 can significantly increase content of the artemisinin in the transgenic plants, up to about 1.8 times (Han et al. 2014). A bHLH transcription factor AabHLH1 can regulate the expression of key enzyme genes in the artemisinin biosynthetic pathway of A. annua, thus regulating the synthesis of artemisinin (Ji et al. 2014). AaORA transcription factor belongs to the AP2/ERF transcription factor family. Overexpression of AaORA resulted in significantly increased expression of key enzyme genes in the artemisinin biosynthetic pathway, as well as the artemisinin content in transgenic plants, while artemisinin content decreased with RNAi interference of AaORA (Lu et al. 2013). Shen et al. cloned AaMYC2 in A. annua, an important transcription factor in Jasmonic acid signal transduction pathway. AaMYC2 can bind to the promoter of key enzymes CYP71AV1 and DBR2. Excessive expression of AaMYC2 can increase the content of artemisinin (Shen et al. 2016).
AaMYB1 was also cloned by our group, which positively affects artemisinin biosynthesis and other related processes (Matias-Hernandez et al. 2017). The molecular mechanisms how AaMBY1 control artemisinin metabolism is still unclear. In this study, a transcription factor AaWRKY4 was isolated from AaMYB1 overexpressed transgenis plants, in which the expression level of AaWRKY4 was obviously increased compared with the control. The results show that AaWRKY4 regulates artemisinin metabolism by regulating the expression of key enzyme genes in the artemisinin biosynthesis pathway. It can aslo further reveal the molecular mechanisms how AaMBY1 control artemisinin biosynthesis.
Materials and methods
Plant materials and growth conditions
The seeds of A. annua were obtained from Southwest University, Chongqing, China. Seeds were sterilized and germinated on Murashige and Skoog (MS) medium under a 16 h light/8 h dark photoperiod, providing 40–60 µmol m−2 s−1 light intensity, at 23 ± 1 °C. The seedling was transferred to greenhouse in Shanghai Jiao Tong University after 10 days.
Reverse transcription PCR
Total RNA was extracted from different tissues of A. annua using RNAprep pure Plant Kit (Tiangen Biotech, China) according to instructions. The total RNA samples acquired above were employed in first-strand synthesis of cDNA by PrimeScript RT Master Mix (TaKaRa, Japan) according to the instructions. The expression of genes was carried out by quantitative reverse transcription PCR (qPCR) using the fluorescent intercalating dye SYBR Green (TaKaRa, Japan). AaActin1 was used as the control in the qPCR analysis.
Bioinformatics analysis
Nucleotide acid sequences and protein sequences in this study were analyzed using Vector NTI software (Invitrogen, USA). Bioinformatics analysis of AaWRKY4 and other genes was performed online at the NCBI database (http://www.ncbi.nlm.nih.gov/) and EBI database (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
A. annua transformation and PCR analysis
pHB-AaWRKY4 was constructed by amplifying the gene with the specific primers AaWRKY4-up and AaWRKY4-down (Table 1), and then excising the amplified sequence with BamH I and Sac I. pHB vector was digested with the same enzymes. The construct was introduced into A. tumefaciens strain EHA105. The A. annua plant transformation was carried out as previously described [21]. Hygromycin B-resistant plants were regenerated by A. tumefaciens-mediated transformation. After genomic DNAs were extracted by CTAB method, AaWRKY4-overexpressing A. annua plants were identified from regenerated A. annua plants by PCR analysis.
Protein subcellular localization
The AaWRKY4 cDNAs were recombined into pEarly-Gateway104 vector (pEG104) to generate pEG104-AaWRKY4. The AaWRKY4-YFP fusion constructs were used for transient expression in tobacco as described previously [22]. After 2–5 days, the fluorescence was imaged by confocal microscope.
HPLC measurement
For each sample, 20 μL of filtrate was injected into the HPLC sample injector. The samples were determined using a Waters Alliance 2695 high-performance liquid chromatography system in series with a 2420 ELSD detector (Milford, USA). 60% methanol was used as a mobile phase. Filtrate passed through 5 μm C18 column at a flow rate of 1 mL/min. The content of the measured substance in the sample was calculated according to the concentration and peak area of the standard substance. Artemisinin content was indicated as mg/g dry weight. Three biological replicates were measured for each sample.
Results
Cloning of AaWRKY4 transcription factor
Using qPCR method to analyze the expression of transcription factors WRKYs, ERFs and bHLHs which were co-expressed with the key enzyme genes in the artemisinin synthetic pathway, a transcription factor AaWRKY4 from the WRKY transcription factor family was obtained by analyzing A. annua genome and transcriptome data already acquired in the transgenic plants overexpressing AaMYB1 [20].
By comparing the transcription factors AaWRKY4 and Arabidopsis WRKY transcription factor family, AaWRKY4 and AtWRKY15 were found to have the closest evolutionary relationship (Fig. 1). AtWRKY15 is a member of group IId subfamily of WRKY transcription factors. AaWRKY4 may also belong to the same group.
Sequence analysis of AaWRKY4
The protein structure of AaWRKY4 was analyzed. The ORF of AaWRKY4 contains 316 amino acids. AaWRKY4 contains only one WRKY structural domain, and the zinc finger structure is C2H2, belonging to the WRKY Group II subfamily. Through protein structure analysis, it was further confirmed that AaWRKY4 belongs to WRKY Group IId subfamily (Fig. 2).
The protein sequence alignment of AaWRKY4 with other similar WRKY transcription factors in other species. AaWRKY4 (from this research), CmWRKY14, TcWRKY7 and PtWRKY15. The sequences marked with thick black line are conserved WRKYGQK motif, and the sequences marked with * were the cystein and histidine amino acid residues characteristic of the zinc finger motif. The sequences with black background indicate the completely identical residues and the sequences with gray background indicate the similar residues
Expression analysis of AaWRKY4 in different tissues
The expression level of AaWRKY4 was detected by qPCR. AaWRKY4 was preferentially expressed in buds and young leaves, weakly expressed in older leaves and fully developed flowers, and had a quite low expression in roots and stems. The expression level of AaWRKY4 in the bud was about 15.4 times higher than that in the root. This expression pattern is similar to the expression of key enzymes including ADS, CYP71AV1, DBR2 and ALDH1 in the artemisinin biosynthetic pathway (Fig. 3). At the same time, some transcription factors regulating artemisinin synthesis, such as AaORA, are also highly expressed in flower buds. The expression pattern of AaWRKY4 suggests that AaWRKY4 may be involved in the metabolic regulation of artemisinin in A. annua.
Expression levels of AaWRKY4 and key enzyme genes in artemisinin biosynthetic pathway in various tissues of A. annua Plants. Expression levels of AaWRKY4, ADS, CYP71AV1 and DBR2 by qPCR in roots (R), stems (S), young leaves (YL), old leaves (OL), flower buds (FB) and fully developed flowers (FDF). Values indicate the mean fold compared with sample R. Three biological repeats were measured for each sample. Error bars are SE (n = 3)
Subcellular localization
In this study, subcellular localization of AaWRKY4 was studied by transient transformation of tobacco. The C-terminal of YFP on the pEG104 vector was fused with AaWRKY4 to form YFP-AaWRKY4 fusion protein. The subcellular localization of AaWRKY4 in tobacco was observed by microscope after transient transformation of tobacco. The results showed that AaWRKY4 was located in the nucleus (Fig. 4).
Identification and qPCR analysis of transgenic A. annua plants overexpressing AaWRKY4
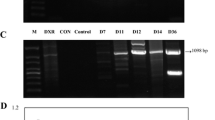
A. annua was transformed with A. tumefaciens containing pHB-AaWRKY4 construct, and ninety-five plants were obtained by Hygromycin B screening. Using AaWRKY4-up as the upstream primer and rbc-48A as the downstream primer for PCR amplification, the band size of about 1 kb could be amplified. In this study, DNA was extracted from the leaves of transgenic plants 6 weeks after transferred to the soil. Through PCR identification, 47 plants were confirmed as positive transgenic plants. Four independent lines AaWRKY4-6, AaWRKY4-22, AaWRKY4-24 and AaWRKY4-28 were randomly selected from these positive plants for further analysis (Fig. 5).
PCR analysis of transgenic plants overexpressing AaWRKY4. Forward primer AaWRKY4-up and reverse primer rbc-48A were used in PCR analysis. M: DNA size marker DL2000; 6, 22, 24, and 28: independent lines of transgenic plants overexpressing AaWRKY4; N: non-transgenic A. annua control plant; P: positive plasmid control
The four independent transgenic lines were used for expression analysis. The leaves of the same part of transgenic plants were selected as materials for qPCR analysis. The expression analysis of these four independent transgenic lines showed that the expression level of AaWRKY4 in these transgenic lines was significantly increased compared with that of the control. The expression level of AaWRKY4 in these transgenic lines was increased by about 19-fold, 22-fold, 21-fold and 15-fold respectively (Fig. 6).
Expression analysis of AaWRKY4 in transgenic plants overexpressing AaWRKY4. Expression analysis of AaWRKY4 in transgenic plants overexpressing AaWRKY4. AaWRKY4-6, AaWRKY4-22, AaWRKY4-24 and AaWRKY4-28: independent lines of transgenic plants overexpressing AaWRKY4. Three biological repeats were measured for each sample. Statistical significance was determined by Student’s t-test (**P < 0.01)
Overexpression of AaWRKY4 resulted in increased expression of key enzymes in the artemisinin biosynthetic pathway
The expression levels of key enzyme genes in the artemisinin biosynthetic pathway was analyzed. Compared with control plants, the expression levels of ADS in transgenic A. annua plants is significantly improved, 6.0- to 9.0-fold higher. The expression levels of CYP71AV1, DBR2 and ALDH1 in transgenic A. annua plants were also detected. Compared with that in the control plants, the expression of CYP71AV1 was increased by 6.1- to 9.8-fold, the expression of DBR2 was increased by 5.2- to 6.6-fold, and the expression of ALDH1 was increased by 2.0- to 2.7-fold (Fig. 7). AaWRKY4 may regulate expression of key enzymes in the artemisinin biosynthetic pathway, thus regulating the content of artemisinin in plants.
Expression analysis of key enzyme genes in artemisinin biosynthetic pathway in transgenic plants overexpressing AaWRKY4. Expression analysis of the key enzyme genes in artemisinin biosynthetic pathway by qPCR in transgenic plants overexpressing AaWRKY4. AaWRKY4-6, AaWRKY4-22, AaWRKY4-24 and AaWRKY4-28: independent lines of transgenic plants overexpressing AaWRKY4. Three biological repeats were measured for each sample. Statistical significance was determined by Student’s t-test (**P < 0.01)
Overexpression of AaWRKY4 gene resulted in increased artemisinin content
In this study, artemisinin content of A. annua was measured by HPLC. Compared with non-transgenic A. annua control plants, the content of artemisinin in transgenic plants overexpressing AaWRKY4 was significantly increased. The content of artemisinin in the four independent transgenic lines AaWRKY4-6, AaWRKY4-22, AaWRKY4-24 and AaWRKY4-28 were increased by 48%, 50%, 48% and 35% respectively (Fig. 8).
The content of artemisinin measured in transgenic plants overexpressing AaWRKY4. The content of artemisinin measured by HPLC in transgenic plants overexpressing AaWRKY4. AaWRKY4-6, AaWRKY4-22, AaWRKY4-24 and AaWRKY4-28: independent lines of transgenic plants overexpressing AaWRKY4. Three biological repeats were measured for each sample. Statistical significance was determined by Student’s t-test (**P < 0.01)
Discussion
Transcription factors play an important role in the regulation of artemisinin metabolism. Transcription factors can regulate the expressions of key enzymes in artemisinin biosynthetic pathway (Ma et al. 2009; Yu et al. 2012; Lu et al. 2013; Ji et al. 2014; Zhang et al. 2015; Shen et al. 2016; Chen et al. 2016; Jiang et al. 2016). Transcription factors can also regulate trichome development (Tan et al. 2015; Yan et al. 2016, 2018; Shi et al. 2017; Wang et al. 2019). AaMYB1 can regulate the artemisinin biosynthetic pathway by regulating the expressions of key enzymes in artemisinin biosynthetic pathway, and also regulate trichome development [20]. Although AaMYB1 has been found to regulate the artemisinin biosynthesis, the mechanism is not well understood. In this study, based on transgenic plants overexpressing AaMYB1, a WRKY transcription factor, AaWRKY4, which has similar expression patterns to key enzyme genes in the artemisinin biosynthetic pathway, was identified. AaWRKY4 was preferentially expressed in buds and young leaves, and had low or no expression in other tissues, which was the same as the expression pattern of key enzyme genes in the artemisinin biosynthetic pathway. According to previous studies, the key enzyme genes in the artemisinin biosynthetic pathway are mainly expressed in the GSTs of flower buds and young leaves. The results above indicate that AaWRKY4 may be involved in artemisinin metabolism regulation.
The results showed that the expression levels of key enzymes in the artemisinin biosynthetic pathway in transgenic plants overexpressing AaWRKY4, were significantly increased. At the same time. Compared with A. annua control plants, artemisinin content in transgenic plants overexpressing AaWRKY4 was also significantly increased. Therefore, AaWRKY4 might be involved in the transcriptional regulation network of artemisinin metabolism in A. annua. However, the mechanism AaWRKY4 is involved in the regulation of artemisinin metabolism requires further investigation.
In conclusion, AaWRKY4 might be involved in the transcriptional regulation network of artemisinin metabolism in A. annua, which has important application value for artemisinin metabolic engineering.
Data availability
The Gene Bank number for AaWRKY4 is MW169031. Sequence data from this article can be found in the Arabidopsis Genome Initiative or the GenBank databases.
Abbreviations
- GSTs:
-
Glandular secretory trichomes
- DMAPP:
-
Dimethylallyl diphosphate
- IPP:
-
Isopentenyl diphosphate
- MVA pathway:
-
Mevalonate pathway
- MEP pathway:
-
Nonmevalonate pathway
- ADS:
-
Amorpha-4, 11-diene synthase
- CYP:
-
Cytochrome P450 enzyme CYP71AV1
- DBR2:
-
Artemisinic aldehyde ∆11 (13) reductase
- ALDH1:
-
Aldehyde dehydrogenase
References
Bouwmeester HJ, Wallaart TE, Janssen MHA, Van Loo B, Jansen BJM, Posthumus MA, Schmidt CO, De Kraker JW, König WA, Franssen MCR (1999) Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry 52(5):843–854. https://doi.org/10.1016/S0031-9422(99)00206-X
Broun P (2004) Transcription factors as tools for metabolic engineering in plants. Curr Opin Plant Biol 7:202–209. https://doi.org/10.1016/j.pbi.2004.01.013
Bryant L, Flatley B, Patole C, Brown G, Cramer R (2015) Proteomic analysis of Artemisia annua – towards elucidating the biosynthetic pathways of the antimalarial pro-drug artemisinin. BMC Plant Biol 15(1):175. https://doi.org/10.1186/s12870-015-0565-7
Chen M, Yan T, Shen Q, Lu X, Pan Q, Huang Y, Tang Y, Fu X, Liu M, Jiang W, Zongyou L, Shi P, Ma Y-N, Hao X, Zhang L, Li L, Tang K (2016) Glandular trichome-specific WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol 214(1):304–316. https://doi.org/10.1111/nph.14373
Covello PS (2008) Making artemisinin. Phytochemistry 69(17):2881–2885. https://doi.org/10.1016/j.phytochem.2008.10.001
Han J, Wang H, Lundgren A, Brodelius PE (2014) Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry 102:89–96. https://doi.org/10.1016/j.phytochem.2014.02.011
Hsu E (2006) The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg 100(6):505–508. https://doi.org/10.1016/j.trstmh.2005.09.020
Ji Y, Xiao J, Shen Y, Ma D, Li Z, Pu G, Li X, Huang L, Liu B, Ye H, Wang H (2014) Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua. Plant Cell Physiol 55(9):1592–1604. https://doi.org/10.1093/pcp/pcu090
Jiang W, Lu X, Qiu B, Zhang F, Shen Q, Lv Z, Fu X, Yan T, Gao E, Zhu M, Chen L, Zhang L, Wang G, Sun X, Tang K (2014) Molecular cloning and characterization of a trichome-specific promoter of artemisinic aldehyde δ11(13) reductase (DBR2) in Artemisia annua. Plant Mol Biol Rep 32(1):82–91. https://doi.org/10.1007/s11105-013-0603-2
Jiang W, Fu X, Pan Q, Tang Y, Shen Q, Lv Z, Yan T, Shi P, Li L, Zhang L, Wang G, Sun X, Tang K (2016) Overexpression of AaWRKY1 leads to an enhanced content of artemisinin in Artemisia annua. Biomed Res Int 2016:1–9. https://doi.org/10.1155/2016/7314971
Lu X, Jiang W, Zhang L, Zhang F, Shen Q, Wang T, Chen Y, Wu S, Lv Z, Gao E, Qiu B, Tang K (2012) Characterization of a novel ERF transcription factor in Artemisia annua and its induction kinetics after hormones and stress treatments. Mol Biol Rep 39(10):9521–9527. https://doi.org/10.1007/s11033-012-1816-4
Lu X, Zhang L, Zhang F, Jiang W, Shen Q, Lv Z, Wang G, Tang K (2013) AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. New Phytol 198(4):1191–1202. https://doi.org/10.1111/nph.12207
Ma D, Pu G, Lei C, Ma L, Wang H, Guo Y, Chen J, Du Z, Li G, Ye H, Liu B (2009) Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol 50(12):2146–2161. https://doi.org/10.1093/pcp/pcp149
Matias-Hernandez L, Jiang W, Yang K, Tang K, Brodelius P, Pelaz S (2017) AaMYB1, and its orthologue AtMYB61, affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana. Plant J 90(3):520–534. https://doi.org/10.1111/tpj.13509
Miller LH, Su X (2011) Artemisinin: discovery from the Chinese herbal garden. Cell 146(6):855–858. https://doi.org/10.1016/j.cell.2011.08.024
Picaud S, Olofsson L, Brodelius M, Brodelius PE (2005) Expression, purification, and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch Biochem Biophys 436(2):215–226. https://doi.org/10.1016/j.abb.2005.02.012
Romero MR, Efferth T, Serrano MA, Castaño B, MacIas RIR, Briz O, Marin JJG (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res 68(2):75–83. https://doi.org/10.1016/j.antiviral.2005.07.005
Shen Q, Lu X, Yan T, Fu X, Zongyou L, Zhang F, Pan Q, Wang G, Sun X, Tang K (2016) The jasmonate-responsive AaMYC2 transcription factor positively regulates artemisinin biosynthesis in Artemisia annua. New Phytol 210(4):1269–1281. https://doi.org/10.1111/nph.13874
Shen Q, Zhang L, Liao Z, Wang S, Yan T, Shi P, Liu M, Fu X, Pan Q, Wang Y, Zongyou L, Lu X, Zhang F, Jiang W, Ma Y, Chen M, Hao X, Li L, Tang Y, Tang K (2018) The genome of Artemisia annua provides insight into the evolution of asteraceae family and artemisinin biosynthesis. Mol Plant 11(6):776–788. https://doi.org/10.1016/j.molp.2018.03.015
Shi P, Fu X, Shen Q, Liu M, Pan Q, Tang Y, Jiang W, Zongyou L, Yan T, Ma Y, Chen M, Hao X, Liu P, Li L, Sun X, Tang K (2017) The roles of AaMIXTA1 in regulating the initiation of glandular trichomes and cuticle biosynthesis in Artemisia annua. New Phytol 217(1):261–276. https://doi.org/10.1111/nph.14789
Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24(4):2277–2280
Sy LK, Brown GD (2002) The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron 58(5):897–908. https://doi.org/10.1016/S0040-4020(01)01193-0
Tan H, Xiao L, Gao S, Li Q, Chen J, Xiao Y, Ji Q, Chen R, Chen W, Zhang L (2015) Trichome and artemisinin regulator 1 is required for trichome development and artemisinin biosynthesis in Artemisia annua. Mol Plant 8(9):1396–1411. https://doi.org/10.1016/j.molp.2015.04.002
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580(5):1411–1416. https://doi.org/10.1016/j.febslet.2006.01.065
Teoh KH, Polichuk DR, Reed DW, Covello PS (2009) Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 87(6):635–642. https://doi.org/10.1139/B09-032
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep 26(12):2129–2136. https://doi.org/10.1007/s00299-007-0420-x
Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NCA (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212(3):460–465. https://doi.org/10.1007/s004250000428
Wang Y, Fu X, Xie L, Qin W, Li L, Sun X, Xing SH, Tang K (2019) Stress associated protein 1 regulates the development of glandular trichomes in Artemisia annua. Plant Cell Tissue Org Cult 139(2):249–259. https://doi.org/10.1007/s11240-019-01677-5
Yan T, Chen M, Shen Q, Li L, Fu X, Pan Q, Tang Y, Shi P, Zongyou L, Jiang W, Ma Y-N, Hao X, Sun X, Tang K (2016) HOMEODOMAIN PROTEIN 1 is required for jasmonate-mediated glandular trichome initiation in Artemisia annua. New Phytol 213(3):1145–1155. https://doi.org/10.1111/nph.14205
Yan T, Li L, Xie L, Chen M, Shen Q, Pan Q, Fu X, Shi P, Tang Y, Huang H, Huang Y, Huang Y, Tang K (2018) A novel HD-ZIP IV/MIXTA complex promotes glandular trichome initiation and cuticle development in Artemisia annua. New Phytol 218(2):567–578. https://doi.org/10.1111/nph.15005
Yu ZX, Li JX, Yang CQ, Hu WL, Wang LJ, Chen XY (2012) The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol Plant 5(2):353–365. https://doi.org/10.1093/mp/ssr087
Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS (2008) The molecular cloning of artemisinic aldehyde Δ11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283(31):21501–21508. https://doi.org/10.1074/jbc.M803090200
Zhang F, Fu X, Lv Z, Lu X, Shen Q, Zhang L, Zhu M, Wang G, Sun X, Liao Z, Tang K (2015) A basic leucine zipper transcription factor, aabzip1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol Plant 8(1):163–175. https://doi.org/10.1016/j.molp.2014.12.004
Acknowledgements
This work was supported by Hunan Provincial Natural Science Foundation of China (Grant No. 2018JJ3008), NSF of Anhui Province (Grant No. 1908085MH268), SJTU Trans-med Awards Research (Grant No. 20190104), the Open Fund Project of Key Laboratory in Hunan Universities (Grant No. NY20K04), Science Foundation of Hengyang Normal University (Grant No. 17D14), and Science Foundation of Hengyang Normal University (Grant No. 17A03).
Author information
Authors and Affiliations
Contributions
WJ and KT conceived and designed the project. HH and XH conducted the experiments and analyzed the data. HH and WJ drafted the paper. WJ and KT reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Written informed consent was obtained from individual or guardian participants.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Communicated by Maria Margarida Oliveira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, H., Xing, S., Tang, K. et al. AaWRKY4 upregulates artemisinin content through boosting the expressions of key enzymes in artemisinin biosynthetic pathway. Plant Cell Tiss Organ Cult 146, 97–105 (2021). https://doi.org/10.1007/s11240-021-02049-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02049-8