Abstract
Aims
Ecological restoration of mine tailing ponds plays a crucial role in managing heavy metal pollution and enhancing biodiversity. This study aimed to investigate the ecological restoration potential of pioneer plants under high manganese metal pollution, as well as the microbial community composition and functional characteristics of their rhizosphere soil.
Methods
Sampling and analysis were conducted on seven plants of Neyraudia reynaudiana (LL), Pueraria montana (GT), Bidens pilosa (GZC), Buddleja asiatica (BBF), Pogonatherum crinitum (JSC), Crotalaria albida (XND), Thysanolaena maxima (ZYL) and their rhizosphere soil in Daxin manganese tailing ponds of Guangxi Province.
Results
Pioneer plants improved the physicochemical properties of rhizosphere soil, and increase soil enzyme activity and the abundance and diversity of microbial communities, and reduce soil available metal content. The BTF values of GZC, GT, BBF, and XND are close to or greater than 1 for Mn, Cd and Cu, indicating their strong ability to absorb and transfer heavy metals from underground to above-ground. Proteobacteria, Actinobacteria, and Bacteroidetes are the bacterial communities with the dominant abundance, showing a significant positive correlation with the BTF value and promoting the absorption of heavy metals by plants. FAPROTAX function prediction revealed that chemoheterotrophy, aerobic chemoheterotrophy, and chloroplasts were the main metabolic modes.
Conclusions
GZC, GT, BBF, and XND can be utilized as habitat improvement plants in the preliminary ecological restoration of manganese tailing ponds. Rhizosphere soil microorganisms of pioneer plants respond to heavy metal pollution by regulating community structure and influencing metabolic function.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese resources belong to the major strategic needs of the country and play an indispensable role in the development of metallurgy, chemical industry, iron and steel industry, aerospace, and other important fields in the construction of the national economy (Liu et al. 2019). Due to the low grade of manganese ore resources and poor technical processing performance, there is a contradiction between supply and demand, making China the world's largest consumer and importer of manganese resources (Shao 2017). The domestic distribution of manganese mineral resources is wide, with Guangxi being recognized as the hometown of non-ferrous metals due to its superior metallogenic geological conditions (Niu et al. 2021). It holds 55.8% of the total national manganese mineral reserves (Hou et al. 2019). However, the mining activities have led to serious geological disasters such as landslides, ground vibrations, explosions, as well as pollution of water environment, atmospheric environment, and surface vegetation through mining wastes like slag and tailings (Chen et al. 2023; Onifade et al. 2023).
Heavy metals in the surface soil of mining areas are mainly transported horizontally through rain-driven surface runoff and sediment transport (Qiao et al. 2023). These heavy metals accumulate in the soil through the food chain, causing harm to crops and posing a threat to human health (Worlanyo and Jiangfeng 2021). For example, prolonged exposure to excessive manganese can lead to symptoms like yellowing and necrotic leaf spots in plants (Schmidt and Husted 2019). Furthermore, excessive manganese, an essential trace metal for humans, can also exhibit neurotoxic effects (Bakulski et al. 2020). Long-term exposure to cadmium is linked to various cancers, including skin and lung cancer (Kubier et al. 2019; Okereafor et al. 2020), while excessive copper exposure can disrupt the normal function of multiple human systems, such as the respiratory, immune, and nervous systems (Taylor et al. 2020). Mining activities are recognized as a significant source of heavy metal pollution (Li et al. 2014), with the pollution caused by mining posing substantial potential harm to the environment (Wu et al. 2023). Ecological restoration of lands devastated by the mining industry has become an urgent environmental issue that needs to be addressed.
Vegetation restoration is a fundamental requirement for the recovery of degraded ecosystems and forms the basis for the development and succession of soil ecosystems in mining areas (Sarker et al. 2023; Barroso et al. 2023; Han et al. 2023). Over time, certain naturally occurring plants in mining areas have developed unique tolerance and absorption mechanisms for heavy metals under poor nutritional and harsh environmental conditions (Song et al. 2022; Mardonova and Han 2023), becoming pioneer plants in the restoration of vegetation in tailing ponds. These plants facilitate the creation of a conducive environment for the survival of diverse microorganisms within the rhizosphere and engage in interactions such as competition and cooperation between plants and microorganisms (Chepsergon and Moleleki 2023). Through root secretions and plant litter deposition, these pioneer plants directly improve the biogeochemical conditions in the root zone, providing favorable conditions for the growth of other plants and establishing a solid foundation for the succession of plant communities (Munford et al. 2022).
The restoration of soil microbial communities is the key to vegetation restoration and plays a positive role in realizing soil health, driving ecosystem versatility and sustainable use (Cai et al. 2022; Wang et al. 2023a; Matthews et al. 2023). Therefore, it is of great significance for the restoration of heavy metal pollution and the improvement of biodiversity to study the natural settlement pioneer plants and soil microbial communities and functional characteristics of manganese tailing ponds.
In recent years, scholars have continued to study the pioneer dominant plants in manganese mining areas. For instance, Nong et al. studied six pioneer plants, such as herbaceous plants S. viridis and P. massonian in Hunan Xiangtan manganese mine (Nong et al. 2023), and the soil quality and plant community diversity were promoted under the growth of the plants, which alleviated the heavy metal pollution. Luo Yang et al. studied 29 dominant plants (Luo et al. 2022), such as Miscanthus floridulus and Buddleja lindleyana, in manganese residue disposal sites located in eastern Guizhou Province and found that the nutrient composition of manganese slag was improved and the diversity of bacterial community was significantly increased by plant growth.
Currently, there is a limited amount of research on pioneer plants in manganese tailing ponds specifically in the Guangxi region. This study took seven pioneer plants of Neyraudia reynaudiana, Pueraria montana, Bidens pilosa, Buddleja asiatica, Pogonatherum crinitum, Crotalaria albida, and Thysanolaena maxima grown naturally in the restoration area of Daxin manganese tailing ponds in Guangxi and analyzed the characteristics of manganese absorption, translocation, and tolerance mechanisms of pioneer plants, so as to clarify the ecological adaptability and ecological restoration potentials of the pioneer plants in manganese tailing ponds. Additionally, high-throughput sequencing was employed to explore the characteristics of soil microbial communities and their functions in the pioneer plants rhizosphere soil under conditions of high manganese metal pollution, in order to provide a theoretical basis for the restoration and management of manganese tailing ponds in Guangxi and the selection of vegetation species, and to lay a foundation for the formulation of tailing environmental management policies.
Materials and methods
Overview of the study area
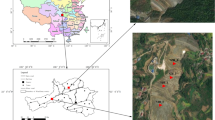
Daxin manganese mine (Fig. 1), renamed as Xialei manganese mine, belongs to the state-run large-scale open-air manganese mine and is the largest manganese mine with the largest proven manganese ore reserves, with a total manganese ore reserve of 125 million tons, including 120 million tons of manganese carbonate. The manganese tailing pond restoration area belongs to the area to be restored artificially that no mining activities have been carried out within ten kilometers, and no amendment has been applied or any restoration has been carried out.
Geographic location of the Daxin manganese mine sampling site. The sampling site is located in Xialei Town, Daxin County, Chongzuo City, Guangxi Province, China (latitude: 22°54ʹ58ʺ N, longitude: 106°45ʹ18ʺ E), with an elevation of 241~845 m above sea level. It is a subtropical monsoon climate area with an annual rainfall of 135cm ~ 192cm
Sample collection and processing
The 50 m × 50 m area with no obvious height difference was artificially divided to reduce the influence of the uneven spatial distribution of soil. The main plants visible in the area were collected and recorded (Table 1), and the withered leaves, roots, gravel, biological debris, and other debris on the surface of the soil were removed. All samples were packed in clean polyethylene plastic sample bags, labeled with plant species, and preserved in ice packs for transport back to the laboratory. Wash the whole plant with running water and rinse with deionized water. The roots, stems, and leaves were individually excised, subjected to heat treatment at 100 ℃ for 30 min, and subsequently dried in an oven at 60 ℃ until a constant weight was achieved. The dry weight of each plant part was determined. The resultant dried plant samples were finely ground into a powder using a stainless steel grinder. This powdered material was then sieved through a 200 mesh (standard sieve) nylon sieve, homogenized, and stored in a desiccator for further analysis.
The S-shaped sampling method was applied to gather bare soil from a vegetation-free area (1 m × 1 m), located at least 5 m away from any pioneer plants (Zhao et al. 2019). Five duplicate soil samples, with weights ranging from 500 to 1000 g, were collected. This particular plant-free location was designated as the blank sampling point CK. For rhizosphere samples, soil loosely adhered to the roots of excavated plants was delicately brushed off using a sterile brush. Rhizosphere soil samples obtained from the same plants were then combined into composite samples. These composite samples were gathered using the quartering method, carefully stored in ice bags, and transported back to the laboratory. Soil samples were frozen and stored for measurement of soil enzymes and microorganisms, while other samples were dried naturally, further removed from debris, crushed, ground, blended, passed through a 20-mesh (standard sieve) and 200-mesh (standard sieve) nylon sieve, homogenized and placed in a cool, dry place.
Sample measurement methods
The physicochemical properties and enzyme activities of soil samples were determined according to the corresponding national standards (Table S1). Each sample was analyzed thrice for accurate determination. The total amount of heavy metals in soil and plant roots, stems, and leaves was determined by the microwave digestion method (GB HJ 832–2017) using the EXPEC 790 automatic microwave digestion instrument (Hangzhou Puyu Technology Development Co., Ltd.). Soil and plant samples were subjected to digestion using hydrochloric acid, nitric acid, hydrofluoric acid, hydrogen peroxide, and hydrogen peroxide-nitric acid methods, respectively. The available concentration of heavy metals was extracted by 0.1 mol/L hydrochloric acid solution. The concentrations of heavy metals were measured using an AA-6300 atomic absorption meter (Shimadzu Company, Japan).
Soil microbial community composition was sequenced using the 16S rDNA amplicon to detect microbial diversity. Soil samples were subjected to DNA extraction using the E.Z.N.A™ Mag-Bind Soil DNA Kit. The amplification library was generated with primers 341f (5ʹ-C CTA CGG GNG GCW GCA G-3ʹ) and 805r (5ʹ-GAC TAC HVG GGT ATC TAA TCC-3ʹ) targeting the V3-V4 hypervariant region of the bacterial 16S rRNA gene. Sequencing and data analysis were performed using the Illumina Miseq platform of Sangon Biotech (Shanghai) Co., Ltd., and PCR amplification, purification, and quantification of PCR products were performed according to its standard protocol.
Data processing
The experimental data from three repeated samples were subjected to statistical analysis and processing using Microsoft Excel 2020, SPSS 26.0, and Origin 17.0 software. One-way analysis of variance (ANOVA) was employed to determine significant analysis of variance on the measured results. The Pearson correlation coefficient was utilized to evaluate the correlation between various factors. Canoco 5 software was applied to analyze and visually represent the association between community diversity and environmental factors.
Bio Enrichment Coefficient (BEC) refers to the ratio of heavy metal concentrations in plants to heavy metal concentrations in soil. A higher value indicates a stronger ability of the plant to accumulate heavy metal, while a lower value indicates a weaker ability (Torbati et al. 2021). The calculation formula is as follows:
Bio Translocation Factor (BTF) refers to the ratio of heavy metal concentrations in the above-ground part of the plant to the heavy metal concentrations in the roots. A higher BTF value indicates a stronger ability of the plant to transfer heavy metal from the underground part to the above-ground part (Wu et al. 2022). BTF is calculated using the following formula:
Bio Accumulation Factor (BAF) is the ratio of heavy metal concentrations in the roots to the heavy metal concentrations in the soil. It is used to evaluate the potential of soil phytoremediation and to analyze the plant's tolerance to heavy metals (Aliyu et al. 2023). BAF is calculated using the following formula:
Note: (1) (2) (3) where \({\text{C}}_{\text{plant }}\) is the heavy metal concentrations in the body of each plant (mg/kg); \({\text{C}}_{\text{aboveground}}\) is the heavy metal concentrations of plant above-ground parts (mg/kg); \({\text{C}}_{\text{root}}\) is the heavy metal concentrations of plant roots; \({\text{C}}_{\text{soil}}\) is the heavy metal concentrations of the soil; \({\text{C}}_{\text{root}}\), \({\text{C}}_{\text{stem}}\), \({\text{C}}_{\text{leaf}}\) is the heavy metal concentrations of the roots, stems and leaves of each plant, respectively (mg/kg); \({\text{M}}_{\text{root}}\), \({\text{M}}_{\text{stem}}\), \({\text{M}}_{\text{leaf}}\) is the dry weight of the roots, stems and leaves of each plant, respectively (kg).
Results
Basic physicochemical properties and enzyme activities of soil
The study of soil condition is the primary task of ecological restoration, and the physicochemical properties of soil are an important reference for the study of soil health and fertility (Hao et al. 2023). The physicochemical properties of pioneer rhizosphere soil improved significantly relative to CK, with significant increases in pH, CEC, and AK (Fig. 2A, B). In general, among the seven pioneer plants, the rhizosphere soils of Pueraria montana (GT) and Bidens pilosa (GZC) had higher contents of CEC, OC, AP, and AK (Fig. 2A, B), and the contents of ammonium nitrogen were much less than those of CK (Fig. 2B), indicating that the colonization of these two pioneer plants was more conducive to the healthy development of soil. As shown in Fig. 2C, the CAT range of each rhizosphere soil was 3.33–3.84 mg·g−1·h−1, which got 55%-79% enhancement compared with CK soil, and S-SC range was 1.32–5.91 mg·g−1·d−1, and S-UE range was 4.20–11.72 mg·g−1·d−1. The enzyme activity of the rhizosphere soil of pioneer plants was generally increased, thus improving the ecological environment and soil fertility.
Basic physicochemical properties and enzyme activities of different soil, A of the pH, EC, and CEC values for different soils, B of the values of the corresponding indicators OC, NH3-N, AP, and AK for different soils with carbon, nitrogen, phosphorus, and potassium, C of the different soil enzyme activities. Potassium permanganate titration was used for soil CAT, while the colorimetric method was utilized for soil S-SC and S-UE. Error lines are standard errors, and different lowercase letters on the columns indicate significant differences between treatments (P < 0.05)
In order to further analyze the relationship between basic soil physicochemical properties and enzyme activities, Pearson correlation analysis was done as shown in Fig. 3. Soil pH had a greater influence on all environmental factors, and the positive correlation between soil pH, AK, and all other indexes except NH3-N was shown to varying degrees. There was a highly significant positive correlation between pH and AK (P < 0.01, R = 0.61). pH was significantly positively correlated with the three enzymes (respectively, R = 0.92, R = 0.41, and R = 0.60). AK was significantly positively correlated with CAT (R = 0.59) and S-UE (R = 0.45), and highly significantly positively correlated with OC (P < 0.01, R = 0.63). Soil NH3-N showed different degrees of negative correlation with all indicators except S-UE, and a highly significant negative correlation with CEC (P < 0.01, R = -0.84) and CAT (P < 0.01, R = -0.61). CEC and CAT were highly significant positively correlated with each other (P < 0.01, R = 0.73). Soil AP was significantly positively correlated with S-SC (R = 0.53) and S-UE (R = 0.45). S-SC and S-UE exhibited a strong and statistically significant positive correlation (P < 0.01, R = 0.63).
Pearson correlation analysis between basic soil physicochemical properties and enzyme activity. Significant differences were analyzed by t-test at the 0.05 level (*), 0.01 level (**), and 0.001 level (***). Red indicates positive correlation, while blue indicates negative correlation; the flatter and darker the circle, the greater the absolute value of the correlation coefficient
Soil available and total heavy metal content
In the tailing pond soil, heavy metal concentrations were found to be highest for Mn, followed by Cu and Cd (Fig. 4). The total heavy metal concentrations in the rhizosphere soil of pioneer plants in the tailings were measured to be 8210–14755 mg/kg for Mn, 1.25–4.58 mg/kg for Cd, and 61.9–77.8 mg/kg for Cu, respectively (Fig. 4B). These values exceeded the soil background values in Guangxi by 46.6–83.8 times for Mn, 17.1–62.7 times for Cd, and 2.8–3.5 times for Cu (Li et al. 2007). Specifically, the rhizosphere soil of Pueraria montana (GT) had the highest levels of all three heavy metals, while Bidens pilosa (GZC) had lower levels of Cd compared to the other plant rhizosphere soils. CK soil showed a higher proportion of the three heavy metals in an effective state compared to the other plant rhizosphere soils (Fig. 4A), with manganese making up 18.7% of the total amount, Cd 33.8%, and Cu 12.0%. Within the rhizosphere soils of the plants, the available state of Mn ranged from 3.9% to 6.5% of the total amount, Cd from 3.8% to 13.8%, and Cu from 2.6% to 5.5%. The growth of pioneer plants was found to decrease the available states of heavy metals in the rhizosphere soil, thereby reducing the biotoxicity of the heavy metals (Li et al. 2023).
Manganese (Mn), cadmium (Cd), and copper (Cu) content in different soils: available state content (A) and total content (B). The content of Mn (color yellow) is plotted on the left side, while the content of Cd (color blue) and Cu (color red) is plotted on the right side, consistent with the color. Error lines are standard errors, and different lowercase letters on the columns indicate significant differences between treatments (P < 0.05). Among them, the content of lead (Pb) and zinc (Zn) has not been detected and has not been analyzed and compared
Pioneer plant uptake, transfer, and tolerance of heavy metals
The concentrations of heavy metals in the pioneer plant species varied across different parts of the plants (Fig. 5). In the body of the plants, the concentrations ranged from 245.4 to 594.7 mg/kg for Mn, 0.06 to 0.42 mg/kg for Cd, and 3.6 to 8.9 mg/kg for Cu. In the aboveground parts of the plants, the concentrations were between 146.5 and 490.3 mg/kg for Mn, 0.06 and 0.41 mg/kg for Cd, and 3.6 to 9.2 mg/kg for Cu. The underground parts of the plants had the highest concentrations, with values ranging from 125.0 to 1541.9 mg/kg for Mn, 0.08 to 0.46 mg/kg for Cd, and 3.5 to 17.6 mg/kg for Cu.
Corresponding indicators of absorption, transfer, and tolerance to heavy metal in different pioneer plants: BEC for Mn (A), Cd (D), and Cu (J); BTF for Mn (B), Cd (E), and Cu (H); and BAF for Mn (C), Cd (F), and Cu (I). Error lines are standard errors. The auxiliary line in (A) is the total Mn content of CK soil 10,420.6 mg·kg−1, and different lowercase letters on the columns indicate that the differences between the Mn contents in the rhizosphere soil and plant body of different plants are significant (P < 0.05); B Different lowercase letters on the middle column indicated significant differences in Mn content between above-ground and underground parts of different plants (P < 0.05), and different uppercase letters indicated significant differences in Mn content between above-ground and underground parts of the same plants (P < 0.05). (C) Different lowercase letters in the middle column indicate significant differences among treatments (P < 0.05). The graphical plotting of the relevant indicators for Cd and Cu is consistent with that for Mn
Diversity analysis of soil microbial community structure
The average number of effective sequences obtained from different plant rhizosphere soil samples by Illumina Miseq sequencing ranged from 51,725.0 to 69,386.0 in size order, and the sequence coverage was above 99% (Table 2). In the dilution curve analysis (data not shown), the curves tended to be stable after 10,000 sequences, and the amount of sequencing data was asymptotically reasonable, with a similarity degree of 97%. Additional sequencing did not affect species diversity. Among them, the number of soil microorganisms in CK was small, and the number of amplifications did not meet the requirements for on-line sequencing. Combined with each diversity index in Table 2, the Shannoneven index was around 0.80, and species evenness within the community was basically the same in different samples. The sequence with the highest diversity of bacterial communities was Pueraria montana (GT), Thysanolaena maxima (ZYL), Buddleja asiatica (BBF). The order of higher bacterial community richness was Thysanolaena maxima (ZYL), Crotalaria albida (XND), Pueraria montana (GT), Bidens pilosa (GZC).
Based on the 97% similarity clustering level, the size order of the total OTUs (data not shown) for each sample was ZYL (3007), GT (2876), XND (2856), BBF (2726), GZC (2725), LL (2557) and JSC (2530). The number of OTUs common to the seven samples was 899, and the number of OTUs specific to each sample ranged from 223 to 473 (Fig. S1), with the ranking of the proportion of total OTUs being ZYL (15.7%), BBF (14.7%), GT (14.7%), JSC (13.7%), GZC (13.7%), XND (9.4%), and LL (8.7%).
Soil bacterial community composition
Sequencing readings sorted by phylum level from different rhizosphere soil samples were associated with 12 bacterial phyla, as shown in Fig. 6A. The dominant phyla were mainly Proteobacteria (relative abundance ranging from 26.9% to 38.53%), Actinobacteria (17.8% to 35.3%), Acidobacteria (5.10% to 9.97%), Cyanobacteria Chloroplast (1.10% to 15.50%) and Bacteroidetes (2.49% to 5.63%). As shown in Fig. 6B, in the study based on class level, Actinobacteria (17.44% to 34.44%) had the highest percentage of the seven rhizosphere soil samples in the phylum Actinobacteria. Other dominant classes included Alphaproteobacteria (13.77% to 24.45%), Betaproteobacteria (5.61% to 9.04%), Gammaproteobacteria (1.84% to 7.56%) in the phylum Proteobacteria, and Chloroplast (From 0.92% to 13.4%) in the phylum Cyanobacteria Chloroplast.
Bacterial community composition in different rhizosphere soils. A shows the relative abundance of each bacterial community at the phylum level. At least one sample had a relative abundance greater than 0.01, and bacterial phylum species with a relative abundance less than 0.01 were combined into other. B shows the relative abundance of each bacterial community at the class level. The larger the circle, the higher the relative abundance of bacterial communities at the corresponding class level
Correlation between soil bacterial communities and environmental factors
To further investigate the correlation between soil microorganisms and environmental factors, as well as to analyze the effect of rhizosphere soil microbial communities on plant uptake of heavy metals, 12 dominant phyla with high abundance were used as microbial species data, and the basic physicochemical properties of the soil (pH, SOC, NH3-N, AP, and AK) as well as the accumulation capacity of heavy metals in pioneer plants (BTF-Mn, BTF-Cd, BTF-Cu, BAF-Cd, and BEC-Cu) as environmental variables were plotted with Canoco 5 software for redundancy analysis, as shown in Fig. 7.
Redundancy Analysis (RDA) of soil bacteria with basic physical and chemical properties (A) and the ability of plants to absorb heavy metals (B) based on phylum level. Blue arrows represent soil bacteria at the phylum level, and red arrows represent environmental factors. The cosine of the angle between two blue arrows represents the correlation between two microbial groups, and the cosine of the angle between a blue arrow and a red arrow represents the relationship between the microbial and environmental factors
The eigenvalues of axes 1, 2, 3, and 4 in Fig. 7A were 0.53, 0.41, 0.02, and 0.02, respectively, as shown in Table S2. The cumulative interpretation amount of all microbial community data changes was 98.29%, and the cumulative interpretation amount of microbial fraction changes—soil environmental factors was 99.78%. As shown in Fig. 7A, the correlations between Candidate division WPS-1 and Chloroflexi showed a highly significant positive correlation. Verrucomicrobia showed a significant negative correlation with the abundance of Gemmatimonadetes, and Cyanobacteria Chloroplast showed a significant negative correlation with the abundance of Proteobacteria. Soil bacteria showed a significant correlation with basic physicochemical properties, and the abundance of Candidate division WPS-1 showed a significant positive correlation with soil pH, SOC, and AK. The abundance of Candidatus Saccharibacteria showed a significant positive correlation with the content of soil NH3-N. The abundance of Planctomycetes showed a significant positive correlation with soil AP content. The abundance of Parcubacteria showed a significant negative correlation with soil pH and OC content. Similar to the data in this study, Vargas et al. found that Acidobacteria showed a negative correlation with AK as well as with pH, and soil pH is an important feature in shaping the microbial community (Vargas et al. 2023).
The eigenvalues of axes 1, 2, 3, and 4 in Fig. 7B were 0.53, 0.41, 0.02, and 0.02, respectively, as shown in Table S2. The cumulative interpretation amount of all microbial community data changes was 98.18%, and the cumulative interpretation of microbial component changes—soil environmental factors was 99.39%. As shown in Fig. 7B, it is evident that among the nine indexes reflecting the absorption of Mn, Cd, and Cu by plants, five indexes displayed a clear correlation with the root soil bacterial community. Specifically, the BTF indexes of the three heavy metals were involved. The abundance of Proteobacteria exhibited a significant positive correlation with BTF-Mn and BTF-Cu, while the abundance of Actinobacteria was significantly positively correlated with BEC-Cu and BTF-Cd. Additionally, the abundance of Bacteroidetes showed a significant positive correlation with all four indicators mentioned above. On the other hand, the abundance of Acidobacteria and Parcubacteria demonstrated a significant positive correlation with the abundance of BAF-Cd.
Prediction of soil bacterial community function
K03088 (rpoE; RNA polymerase sigma-70 factor, ECF subfamily) is the dominant gene at higher Mn concentrations in this repair region in Fig. 8A, and K03088 is also dominant at high Al concentrations (Zhang et al. 2023), verifying the role of K03088 in inducing gene transcription in response to environmental stress (Guo et al. 2023). Dominant genes K01692 and K00059 were associated with carbon metabolism, glycolysis, and methane metabolism (Cao et al. 2022), suggesting that higher Mn concentrations have an effect on microbial carbon metabolism. There is a close relationship between the iron complex outer membrane receptor protein K02014 and iron carriers, which can be synthesized by microorganisms to obtain iron from insoluble iron oxides and promote microbial metabolism (Lu et al. 2022). ABC transporter proteins can be strictly involved in plant metal transport, and most of the functional gene in other dominant genes, such as the hypothetical ABC transporter system osmolytic enzyme protein K02004, the antibiotic transporter system ATP-binding protein K09687, the Peptide/Nickel transporter system substrate binding protein K02033, K02034, and K02035, and polysaccharide transporter system permease proteins K02025 and K02026, etc., belong to the membrane transport pathway in environmental information processing (Fajardo et al. 2019; Sami et al. 2021; Wang et al. 2023b; Yu et al. 2023). As shown in Fig. 8B, the main functional models of rhizosphere soil bacteria of pioneer plants were analyzed based on FAPROTAX. Chemoheterotrophy, aerobic chemoheterotrophy, chloroplasts synthesis, aromatic compound degradation, and aromatic compound degradation were the main metabolic modes.
Discussion
The positive impact of pioneer plant growth on soil in manganese mine areas
The soil in the Daxin Mn tailing ponds in Guangxi was found to be acidic and nutrient-poor (Fig. 2). However, the natural growth of pioneer plants has been found to be effective in enhancing the soil's physical and chemical properties (Fig. 2), as well as microbial activity (Fig. 6). These pioneer plants, belonging to 7 genera and 4 families, are mostly perennial herbs (Table 1), which are often selected as starting plants for ecosystem restoration due to their fast growth, erosion prevention, and facilitation of early topsoil formation (Doyama et al. 2024).
The pioneer plants that thrive in such extreme environments demonstrate strong environmental adaptability and pollution resistance (Li et al. 2024). The rhizosphere microbial community leverages the plant litter, decayed above-ground parts, roots, stems, and root secretions to promote the reproduction of microbial masses and release extracellular enzymes into the surrounding environment (Canarini et al. 2019). Additionally, the release of intracellular enzymes through cellular autolysis after the plants' demise further promotes the increase of enzyme activity and available nutrients (Santoyo et al. 2021). These processes impact the nutrient content required for plant growth and the diversity of the community in the tailing ponds, as they are influenced by the long-term effects of natural factors (Chaudhary et al. 2023). Notably, there was no significant correlation found between rhizosphere soil enzyme activity and microbial community, suggesting that changes in soil enzyme activity caused by heavy metals might be primarily attributed to the biological toxicity of heavy metals and soil nutrient content (Tang et al. 2019). It's important to note that the positive effect of nutrient stimulation may not completely offset the inhibitory effect caused by the increase of heavy metals (Pinto-Poblete et al. 2022).
Soil pH plays a crucial role in influencing various environmental factors (Figs. 3 and 7A). It has been observed that vegetation restoration tends to have a significant neutralizing effect on soil pH (Goulding 2016). The presence of negatively charged groups (such as carboxyl and phenol groups) in the growth of pioneer plants provides additional adsorption sites for cations (Yang et al. 2024), resulting in an increase in rhizosphere soil CEC (Fig. 2A). Plants can impact processes such as nutrient cycling, capture of acid deposition, and litter input by absorbing exchangeable cations, thereby affecting the production and consumption of soil hydrogen ions (Raza et al. 2020). pH is also a critical factor in shaping the soil microbial community (Fig. 7A), which greatly influences microbial activity. The process of microbial metabolic activity is very susceptible to the influence of a high or low pH value (Hong et al. 2018). Pioneer plants regulate the acidity of the soil to maintain the soil in a pH environment suitable for microbial growth, which directly or indirectly enhances microbial activity (Naz et al. 2022). Furthermore, soil pH can significantly affect the solubility and availability of heavy metal ions (Meng et al. 2023). In this study (Fig. S3), the pH value was found to be negatively correlated with the content of three exchangeable heavy metals (RMn = -0.95, RCd = -0.85, and RCu = -0.90). Soil acidification leads to a significant increase in the mobility of metals in the soil, and the rise of pH value due to the growth of pioneer plants is shown to have a certain activating and inhibitory effect on heavy metals (Kicińska et al. 2022). This reduces the competition between hydrogen ions and metals for surface adsorption sites. In addition, electrical conductivity and organic matter content were found to be positively correlated with the contents of the three exchangeable heavy metals (Fig. S3). The increase in rhizosphere soil electrical conductivity enhances the competitiveness of water-soluble salts, such as Na+, K+, Ca2+, and Mg2+, and other strong electrolytes (Huang et al. 2023), to compete with heavy metal ions for adsorption sites, thereby reducing the specific adsorption of heavy metals (Zhong et al. 2020). Furthermore, functional groups in organic matter can form organometallic complexes with heavy metals through chelation or complexation (Zeng et al. 2011), thereby improving the solubility of heavy metals (Wang et al. 2021).
Pioneer plant root interactions can enhance soil physicochemical and microbiological properties through processes such as respiration, secretion, and absorption (Wang et al. 2017). The abundant microorganisms proliferating in the rhizosphere can facilitate the adsorption of heavy metals in the soil through mechanisms such as extracellular precipitation, complexation, and intracellular binding (Ma et al. 2016). Additionally, the bacterial communities of Bacteroidetes, Proteobacteria, and Actinobacteria were found to have a significant positive correlation with the BTF values in this study (Fig. 7B). This correlation promotes the transport of heavy metals from the underground parts of the plant to the aboveground parts. Plants have evolved specific tolerance mechanisms to cope with heavy metal stress in their environment (Fig. 5). Plants that are adapted to heavy metal stress can enhance their ability to compete for limited nutrient resources, consequently increasing their uptake of heavy metals (Feng et al. 2021). This establishes a beneficial cycle among pioneer plants, microorganisms, and soil quality under heavy metal pollution conditions. This cycle promotes the development of the soil environment in a positive direction, fostering soil health and sustainability.
Analysis of heavy metal absorption, transfer, and tolerance in pioneer plants
In this restoration area, the content of heavy metals in pioneer plants is Mn, Cu and Cd in order (Fig. 5). These characteristics are consistent with the heavy metal content in the soil (Fig. 4). Although the proportion of available Cd in the soil of mining areas is relatively high, and the BEC for Cd in the pioneer plant is also higher than that for Cu and Mn, the proportion of available Cu and Mn content does not align with the BEC value of the pioneer plant (Fig. 5). Additionally, the levels of the three heavy metals in the pioneer plants do not follow the same trend as the total amount of heavy metals in the soil, indicating that the absorption of heavy metals by plants is influenced by both the total amount of heavy metals in the soil and the form in which the heavy metals occur (Zakaria et al. 2021).
Currently, based on different mechanisms of heavy metal tolerance, plants can be classified into accumulator, indicator and exclusion types (Vaculík et al. 2012). The BEC values of the seven pioneer plants were all less than 1 (Fig. 5), which is inconsistent with the characteristics of accumulator-type plants that actively absorb and accumulate heavy metals from the soil and transfer them to above-ground parts (Malayeri et al. 2013). While there were no hyperaccumulators with strong phytoextract abilities identified in the restoration area (Kumar et al. 2021), the growth of the seven pioneer plants did not exhibit obvious toxic symptoms such as leaf chlorosis or necrotic leaf spots (Santos et al. 2017; Dey et al. 2023). This could be attributed to the BTF of Mn, Cd, and Cu being greater than 1 for Bidens pilosa (GZC), Buddleja asiatica (BBF) and Crotalaria albida (XND) (Fig. 5). Additionally, The BTF-Mn and BTF-Cu of Pueraria montana (GT) are greater than 1, with the BTF-Cd is close to 1. The BTF-Mn of Thysanolaena maxima (ZYL) is greater than 1, indicating some phytoextraction potential (Sheoran et al. 2016). These pioneer plants are capable of transferring heavy metals from their underground parts to the above-ground parts, sequestering the heavy metals to cell wall or vacuole compartmentization to avoid physiological toxicity (Parrotta et al. 2015). Conversely, Neyraudia reynaudiana (LL) and Pogonatherum crinitum (JSC) exhibit low absorption capacities for Mn, Cd, and Cu (Fig. 5), making them suitable for thriving in heavily metal-polluted environments. This ability of pioneer plants to tolerate and manage heavy metal contamination is crucial for enhancing ecosystem diversity and promoting ecosystem stability in polluted mining areas (Li et al. 2014).
Rhizosphere microorganisms play a crucial role in promoting plant growth and health (Hakim et al. 2021), with some possessing the ability to resist heavy metals and support plant growth (Shen et al. 2022). In the study, it was found that Cyanobacteria_Chloroplast in the rhizosphere soil of Neyraudia reynaudiana (LL) and Pogonatherum crinitum (JSC), which have the lowest heavy metal absorption capacity (Fig. 5), exhibited significantly higher levels (13.4% and 15.5%, respectively) compared to other pioneer plants (1.1%-7.7%). This suggests that Cyanobacteria_Chloroplast may play a key role in protecting plants from heavy metal toxicity. Furthermore, rhizosphere microorganisms can enhance the absorption of heavy metals by plants and improve the efficiency of phytoremediation in heavy metal-contaminated soil (Qin et al. 2024). The study also revealed a positive correlation between the abundance of Bacteroidetes and the BEC value of the three heavy metals. Additionally, the root microorganisms of Bidens pilosa (GZC) and Pueraria montana (GT), which have high total Mn content in plant rhizosphere soil, also demonstrated higher levels of Bacteroidetes, indicating their potential tolerance to Mn. This finding is consistent with other studies that have shown Bacteroidetes to display higher resistance to Cd (Fu et al. 2023; Pan et al. 2023). At the same time, Proteobacteria and Actinobacteria, with the higher abundance, exhibited a significant positive correlation with the BEC values of Mn and Cu (Fig. 7B). This suggests that Proteobacteria, Actinobacteria and Bacteroidetes play a crucial role in promoting the absorption of heavy metals by the pioneer plants in the restoration area.
Pueraria montana (GT) can tolerate high concentrations of heavy metal due to its well-developed vines and symbiosis with rhizobia (Mensah et al. 2021), thereby improving soil quality (Fig. 2). Similarly, Bidens pilosa (GZC) has been shown to effectively enhance soil fertility (Fig. 2) and is known as a Cd superaccumulator plant (Dai et al. 2021). It is worth noting that there are limited studies on the phytoremediation of mine metals by Crotalaria albida (XND), possibly due to its smaller biomass and a lesser role in the remediation of heavy metal soils in mining areas. However, the exceptional capacity of Bidens pilosa (GZC), Pueraria montana (GT), Buddleja asiatica (BBF), and Crotalaria albida (XND) to absorb heavy metals (Fig. 5) and enrich soil microbial communities (Fig. 6) makes them highly suitable choices for ecological restoration in mining areas.
Bacterial communities and functional characteristics of rhizosphere soil in pioneer plants
Proteobacteria, Actinobacteria, and Acidobacteria are the bacterial communities with the highest abundance in plant rhizosphere soil (Fig. 6A). In the Mn mining area of Xiangtan, Hunan Province studied by Nong (Nong et al. 2023), Proteobacteria, Actinobacteria and Acidobacteria were also the three most abundant bacterial communities, and the average abundance of Proteobacteria was also over 30%. The results indicated that Proteobacteria had strong tolerance to Mn metal. Actinobacteria has the ability to bioremediate heavy metals through the secretion of biosurfactants, iron carriers, and organic acids (Behera and Das 2023). The high tolerance shown by its metabolic diversity and high content ratio makes Actinobacteria a potential microorganism for bioremediation process in this restoration area. The dominant bacteria Alphaproteobacteria class (Fig. 6B), particularly the orders Sphingomonadales and Rhizobiales, significantly influence nitrogen fixation (Veerasamy et al. 2023). Certain bacteria belonging to Betaproteobacteria actively participate in the Mn redox cycle, influencing the mobilization and fixation of arsenic in groundwater (Chakraborty et al. 2020), and also play a certain role in the biological filtration of Mn (McCormick et al. 2023). The dominance of Gammaproteobacteria may be attributed to its adaptability to arid environments with low humidity (Radeva et al. 2013). Additionally, some members of this group have the capability to form biofilms on stone surfaces, providing bacterial colonies with potential resistance against the toxic effects of heavy metals (Zuo et al. 2023). The phylum Proteobacteria, Actinobacteria and Acidobacteria were found to be prevalent in the rhizosphere soil as major bacterial groups (Dai et al. 2018). Previous studies have demonstrated that Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes are key endophytic plant growth-promoting rhizosphere bacteria. These bacteria stimulate plant growth and development, enhance resistance to biotic and abiotic stress, and perform various functions such as internal environmental protection and carbon compound metabolism (Upadhyay et al. 2022). These factors may contribute to their high abundance within the bacterial community in plant rhizosphere soil.
According to Fierer et al., microorganisms can be classified into copiotrophic and oligotrophic phyla (Fierer et al. 2007), with the copiotrophic organisms exhibiting high growth rates in nutrient-rich environments, while oligotrophic organisms dominate in conditions of low nutrient availability. Fierer et al. observed that Betaproteobacteria and Bacteroidetes were more inclined to display copiotrophic characteristics, whereas Acidobacteria showed a tendency towards oligotrophic properties. Additionally, studies indicated that Actinobacteria also tended to exhibit copiotrophic traits (Dopheide et al. 2023). Figure 7A, depicting indicators of soil bacteria and fertility status, reveals a positive correlation between higher abundance of Proteobacteria, Actinobacteria, and Acidobacteria showed a certain positive correlation with soil fertility, indicating copiotrophic attributes. Simultaneously, Acidobacteria with the same high abundance exhibited a negative correlation with pH (Fig. 7A). The pH of rhizosphere soil increased during the growth of pioneer plants (Fig. 2A), resulting in a decrease in the abundance of Acidobacteria, which tend to manifest oligotrophic properties. These findings collectively demonstrate that pioneer plant growth directly or indirectly influences microbial activity, enhancing soil health and increasing the richness and diversity of soil microbial communities.
Most of the functional gene (Fig. 8) have an impact on the rate of heavy metal enrichment and translocation to the plant roots (Sheoran et al. 2016; Steingräber et al. 2022; Feng et al. 2023), and participate in the process of controlling heavy metals in Mn tail mining area. The region exhibited a high abundance of genes involved in metabolic function, reflecting the adaptability of soil microorganisms to the stress of heavy metal pollution (Harindintwali et al. 2020; Qureshi et al. 2024). Among the highly abundant bacteria phyla, Proteobacteria, Actinobacteria, and Acidobacteria have chemoheterotrophic functions, which affect the metabolism modes of chemoheterotrophic and aerobic chemoheterotrophy (Fig. 8B). Excessive Mn(II) can disrupt chloroplast structure, leading to decreased chlorophyll synthesis and photosynthetic rate (Kobayashi et al. 2016). It also affects the abundance of genes related to the metabolism of chemoheterotrophy, aerobic chemoheterotrophy, and chloroplasts. Additionally, functions such as urea catabolism and lignin decomposition were found to be associated with the soil nitrogen cycle (Bani et al. 2018; Zhang et al. 2019), and their functional abundance showed a positive correlation with the phylum Actinobacteria (Elframawy et al. 2022). In this study, the abundance of urea decomposition functional groups in GZC (2.04%) and XND (1.32%) were higher than other pioneer plants (0.30%-0.77%), and the abundance of cellulose hydrolysis functional groups in GZC (0.11%) and XND (0.10%) were also higher than other plants (0.004%-0.04%). The abundance of GZC (35.3%) and XND (33.9%) was also higher than that of other pioneer plants (17.8% ~ 27.9%), which confirmed the correlation between the two and demonstrated the role of soil microorganisms in the biogeochemical cycle of nutrients (Yang et al. 2022).
Conclusion
The soil in Daxin Mn tailing ponds in Guangxi was acidic and nutrient poor. However, the natural settlement growth of pioneer plants has been found to increase soil pH, positively impacting a variety of environmental factors and microbial activities. Additionally, it leads to improvements in the physical and chemical properties of the manganese tailings pond soil, enhanced soil enzyme activity, and a reduction in the content of available metals in the soil. Notably, the BTF values of Bidens pilosa (GZC), Pueraria montana (GT), Buddleja asiatica (BBF), and Crotalaria albida (XND) are close to or greater than 1 for the three heavy metals, indicating their strong ability to absorb and transfer heavy metals from underground to above-ground. Furthermore, these pioneer plants have been shown to effectively increase the richness and diversity of bacterial communities, making them suitable for use as habitat improvement plants during the initial ecological restoration of manganese tailings ponds. At the phylum level, Proteobacteria, Actinobacteria, and Bacteroidetes were found to be the dominant bacterial communities that are positively correlated with soil nutrient levels and significantly associated with the BTF values of Mn, Cd, and Cu, thereby promoting the absorption of heavy metals by plants. Rhizosphere soil microorganisms of pioneer plants respond to heavy metal pollution by regulating community structure and influencing metabolic function. This community participated in the process of heavy metal control by regulating environmental information processing in membrane transport metabolism. Additionally, it induced gene transcription to cope with the stress of Mn metal pollution. Functional prediction analysis using FAPROTAX revealed that chemoenergetic chemoheterotrophy, aerobic chemoheterotrophy, and chloroplast synthesis were the main metabolic modes observed.
Data availability
Data are contained within the article.
References
Aliyu AA, Mudansiru A, Obadiah CD, Dharmendra S (2023) Accumulation of heavy metals in autochthonous plants around Bagega Artisanal Gold Mining Village and the remediation potential of selected plants. Acta Ecol Sin 43:1007–1018. https://doi.org/10.1016/j.chnaes.2023.02.005
Bakulski KM, Seo YA, Hickman RC et al (2020) Heavy metals exposure and Alzheimer’s disease and related dementias. J Alzheimers Dis 76:1215–1242. https://doi.org/10.3233/JAD-200282
Bani A, Pioli S, Ventura M et al (2018) The role of microbial community in the decomposition of leaf litter and deadwood. Appl Soil Ecol 126:75–84. https://doi.org/10.1016/j.apsoil.2018.02.017
Barroso GM, dos Santos EA, Pires FR et al (2023) Phytoremediation: a green and low-cost technology to remediate herbicides in the environment. Chemosphere 334:138943. https://doi.org/10.1016/j.chemosphere.2023.138943
Behera S, Das S (2023) Potential and prospects of Actinobacteria in the bioremediation of environmental pollutants: cellular mechanisms and genetic regulations. Microbiol Res 273:127399. https://doi.org/10.1016/j.micres.2023.127399
Cai X, Zhang D, Wang Y et al (2022) Shift in soil microbial communities along ~160 years of natural vegetation restoration on the Loess Plateau of China. Appl Soil Ecol 173:104394. https://doi.org/10.1016/j.apsoil.2022.104394
Canarini A, Kaiser C, Merchant A et al (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157. https://doi.org/10.3389/fpls.2019.00157
Cao L, Su C, Wu J et al (2022) Impact of perfluorooctanoic acid on treatment wastewater by a tandem AnSBR-ASBR system: performance, microbial community and metabolism pathway. Process Saf Environ Prot 164:373–383. https://doi.org/10.1016/j.psep.2022.06.013
Chakraborty A, DasGupta CK, Bhadury P (2020) Diversity of Betaproteobacteria revealed by novel primers suggests their role in arsenic cycling. Heliyon 6:e03089. https://doi.org/10.1016/j.heliyon.2019.e03089
Chaudhary S, Sindhu SS, Dhanker R, Kumari A (2023) Microbes-mediated sulphur cycling in soil: impact on soil fertility, crop production and environmental sustainability. Microbiol Res 271:127340. https://doi.org/10.1016/j.micres.2023.127340
Chen M, Wu J, Qiu X et al (2023) The important role of the interaction between manganese minerals and metals in environmental remediation: a review. Environ Sci Pollut Res 30:39319–39337. https://doi.org/10.1007/s11356-023-25575-8
Chepsergon J, Moleleki LN (2023) Rhizosphere bacterial interactions and impact on plant health. Curr Opin Microbiol 73:102297. https://doi.org/10.1016/j.mib.2023.102297
Dai H, Wei S, Skuza L, Zhang Q (2021) Phytoremediation of two ecotypes cadmium hyperaccumulator Bidens pilosa L. sourced from clean soils. Chemosphere 273:129652. https://doi.org/10.1016/j.chemosphere.2021.129652
Dai Z, Su W, Chen H et al (2018) Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Change Biol 24:3452–3461. https://doi.org/10.1111/gcb.14163
Dey S, Tripathy B, Kumar MS, Das AP (2023) Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environ Chem Ecotoxicol 5:55–61. https://doi.org/10.1016/j.enceco.2023.01.001
Dopheide A, Davis C, Wakelin SA et al (2023) Labile carbon inputs support the recovery of bacterial communities, but not fungal communities, from a simulated bovine urine event. Biol Fertil Soils 59:333–349. https://doi.org/10.1007/s00374-023-01710-y
Doyama K, Yamaji K, Haruma T et al (2024) Vegetation at the former open-pit Ningyo-toge mine, 36 years after closure treatment: Impact of soil cover on woody plant establishment and dominance of the perennial herb Miscanthus sinensis. J Environ Manage 362:121292. https://doi.org/10.1016/j.jenvman.2024.121292
Elframawy A, El-Hanafy A, Sharamant M, Ghozlan H (2022) Molecular identification of native Egyptian Actinobacteria: screening for lignin utilization and degradation of lignin model compounds. Biocatal Agric Biotechnol 40:102289. https://doi.org/10.1016/j.bcab.2022.102289
Fajardo C, Costa G, Nande M et al (2019) Pb, Cd, and Zn soil contamination: monitoring functional and structural impacts on the microbiome. Appl Soil Ecol 135:56–64. https://doi.org/10.1016/j.apsoil.2018.10.022
Feng D, Wang R, Sun X et al (2023) Heavy metal stress in plants: ways to alleviate with exogenous substances. Sci Total Environ 897:165397. https://doi.org/10.1016/j.scitotenv.2023.165397
Feng R, Wang L, Yang J et al (2021) Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J Hazard Mater 402:123570. https://doi.org/10.1016/j.jhazmat.2020.123570
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.1890/05-1839
Fu Y, Zhu Y, Dong H et al (2023) Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf Environ Prot 169:418–427. https://doi.org/10.1016/j.psep.2022.11.020
Goulding KWT (2016) Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag 32:390–399. https://doi.org/10.1111/sum.12270
Guo Y, Li D, Gao Y et al (2023) The knock-on effects of different wastewater feeding modes: change in microbial communities versus resistance genes in pilot-scale aerobic sludge granulation reactors. Sci Total Environ 892:164500. https://doi.org/10.1016/j.scitotenv.2023.164500
Hakim S, Naqqash T, Nawaz MS et al (2021) Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front Sustain Food Syst 5:617157. https://doi.org/10.3389/fsufs.2021.617157
Han H, Yao H, Wei C, Ma J (2023) Responses of soil biochemical and microbial properties to vegetation combinations of quarry restoration strategies in mountainous areas in China. Ecol Eng 195:107061. https://doi.org/10.1016/j.ecoleng.2023.107061
Hao X, Abou Najm M, Steenwerth KL et al (2023) Are there universal soil responses to cover cropping? A systematic review. Sci Total Environ 861:160600. https://doi.org/10.1016/j.scitotenv.2022.160600
Harindintwali JD, Zhou J, Yang W et al (2020) Biochar-bacteria-plant partnerships: eco-solutions for tackling heavy metal pollution. Ecotoxicol Environ Saf 204:111020. https://doi.org/10.1016/j.ecoenv.2020.111020
Hong S, Piao S, Chen A et al (2018) Afforestation neutralizes soil pH. Nat Commun 9:520. https://doi.org/10.1038/s41467-018-02970-1
Hou X, Liu S, Zhao S et al (2019) Selection of suitable species as a key factor for vegetation restoration of degraded areas in an open-pit manganese-ore mine in Southern China using multivariate-analysis methods. Land Degrad Dev 30:942–950. https://doi.org/10.1002/ldr.3281
Huang X-W, Guo J, Li K-Q et al (2023) Predicting the thermal conductivity of unsaturated soils considering wetting behavior: a meso-scale study. Int J Heat Mass Transf 204:123853. https://doi.org/10.1016/j.ijheatmasstransfer.2023.123853
Kicińska A, Pomykała R, Izquierdo-Diaz M (2022) Changes in soil pH and mobility of heavy metals in contaminated soils. European J Soil Science 73:e13203. https://doi.org/10.1111/ejss.13203
Kobayashi K, Endo K, Wada H (2016) Multiple impacts of loss of plastidic phosphatidylglycerol biosynthesis on photosynthesis during seedling growth of Arabidopsis. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.00336
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388. https://doi.org/10.1016/j.apgeochem.2019.104388
Kumar V, Ferreira LFR, Sonkar M, Singh J (2021) Phytoextraction of heavy metals and ultrastructural changes of Ricinus communis L. grown on complex organometallic sludge discharged from alcohol distillery. Environ Technol Innov 22:101382. https://doi.org/10.1016/j.eti.2021.101382
Li MS, Luo YP, Su ZY (2007) Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ Pollut 147:168–175. https://doi.org/10.1016/j.envpol.2006.08.006
Li W, He E, Van Gestel CAM et al (2024) Pioneer plants enhance soil multifunctionality by reshaping underground multitrophic community during natural succession of an abandoned rare earth mine tailing. J Hazard Mater 472:134450. https://doi.org/10.1016/j.jhazmat.2024.134450
Li Y, Rahman SU, Qiu Z et al (2023) Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: a review. Environ Pollut 325:121433. https://doi.org/10.1016/j.envpol.2023.121433
Li Z, Ma Z, van der Kuijp TJ et al (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090
Liu B, Zhang Y, Lu M et al (2019) Extraction and separation of manganese and iron from ferruginous manganese ores: a review. Miner Eng 131:286–303. https://doi.org/10.1016/j.mineng.2018.11.016
Lu J, Guo Z, He M et al (2022) Highly enhanced removal of nutrients and benzo[a]pyrene in a siphon constructed wetland with magnetite: performance and mechanisms. Chem Eng J 446:136895. https://doi.org/10.1016/j.cej.2022.136895
Luo Y, Liu F, Ren J et al (2022) Effects of dominant plant growth on the nutrient composition and bacterial community structure of manganese residues. Int J Phytorem 24:525–535. https://doi.org/10.1080/15226514.2021.1957769
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918. https://doi.org/10.3389/fpls.2016.00918
Malayeri BE, Chehregani A, Mohsenzadeh F et al (2013) Plants growing in a mining area: screening for metal accumulator plants possibly useful for bioremediation. Toxicol Environ Chem 95:434–444. https://doi.org/10.1080/02772248.2013.788701
Mardonova M, Han Y-S (2023) Environmental, hydrological, and social impacts of coal and nonmetal minerals mining operations. J Environ Manag 332:117387. https://doi.org/10.1016/j.jenvman.2023.117387
Matthews KE, Facelli JM, Cavagnaro TR (2023) Response of soil microbial community structure, carbon and nitrogen cycling to drying and rewetting. Appl Soil Ecol 192:105099. https://doi.org/10.1016/j.apsoil.2023.105099
McCormick NE, Earle M, Kent A et al (2023) Betaproteobacteria are a key component of surface water biofilters that maintain sustained manganese removal in response to fluctuations in influent water temperature. Water Res 244:120515. https://doi.org/10.1016/j.watres.2023.120515
Meng J, Li W, Diao C et al (2023) Microplastics drive microbial assembly, their interactions, and metagenomic functions in two soils with distinct pH and heavy metal availability. J Hazard Mater 458:131973. https://doi.org/10.1016/j.jhazmat.2023.131973
Mensah AK, Marschner B, Antoniadis V et al (2021) Human health risk via soil ingestion of potentially toxic elements and remediation potential of native plants near an abandoned mine spoil in Ghana. Sci Total Environ 798:149272. https://doi.org/10.1016/j.scitotenv.2021.149272
Munford KE, Asemaninejad A, Basiliko N et al (2022) Native plants facilitate vegetation succession on amended and unamended mine tailings. Int J Phytorem 24:963–974. https://doi.org/10.1080/15226514.2021.1987382
Naz M, Dai Z, Hussain S et al (2022) The soil pH and heavy metals revealed their impact on soil microbial community. J Environ Manage 321:115770. https://doi.org/10.1016/j.jenvman.2022.115770
Niu S, Zhao L, Lin X et al (2021) Mineralogical characterization of manganese oxide minerals of the Devonian Xialei manganese deposit. Minerals 11:1243. https://doi.org/10.3390/min11111243
Nong H, Liu J, Chen J et al (2023) Woody plants have the advantages in the phytoremediation process of manganese ore with the help of microorganisms. Sci Total Environ 863:160995. https://doi.org/10.1016/j.scitotenv.2022.160995
Okereafor U, Makhatha M, Mekuto L et al (2020) Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int J Environ Res Public Health 17:2204. https://doi.org/10.3390/ijerph17072204
Onifade M, Said KO, Shivute AP (2023) Safe mining operations through technological advancement. Process Saf Environ Prot 175:251–258. https://doi.org/10.1016/j.psep.2023.05.052
Pan X, Raaijmakers JM, Carrión VJ (2023) Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol 31:959–971. https://doi.org/10.1016/j.tim.2023.03.018
Parrotta L, Guerriero G, Sergeant K et al (2015) Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:133. https://doi.org/10.3389/fpls.2015.00133
Pinto-Poblete A, Retamal-Salgado J, Lopez MD et al (2022) Combined effect of microplastics and Cd alters the enzymatic activity of soil and the productivity of strawberry plants. Plants-Basel 11:536. https://doi.org/10.3390/plants11040536
Qiao P, Wang S, Li J et al (2023) Process, influencing factors, and simulation of the lateral transport of heavy metals in surface runoff in a mining area driven by rainfall: a review. Sci Total Environ 857:159119. https://doi.org/10.1016/j.scitotenv.2022.159119
Qin H, Wang Z, Sha W et al (2024) Role of plant-growth-promoting Rhizobacteria in plant machinery for soil heavy metal detoxification. Microorganisms 12:700. https://doi.org/10.3390/microorganisms12040700
Qureshi FF, Ashraf MA, Rasheed R et al (2024) Microbial-assisted alleviation of chromium toxicity in plants: a critical review. Plant Stress 11:100394. https://doi.org/10.1016/j.stress.2024.100394
Radeva G, Kenarova A, Bachvarova V et al (2013) Bacterial diversity at abandoned uranium mining and milling sites in Bulgaria as revealed by 16S rRNA genetic diversity study. Water Air Soil Pollut 224:1748. https://doi.org/10.1007/s11270-013-1748-1
Raza S, Miao N, Wang P et al (2020) Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob Change Biol 26:3738–3751. https://doi.org/10.1111/gcb.15101
Sami A, Elimairi I, Patangia D et al (2021) The ultra-structural, metabolomic and metagenomic characterisation of the sudanese smokeless tobacco ‘Toombak.’ Toxicol Rep 8:1498–1512. https://doi.org/10.1016/j.toxrep.2021.07.008
Santos EF, Kondo Santini JM, Paixão AP et al (2017) Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol Biochem 113:6–19. https://doi.org/10.1016/j.plaphy.2017.01.022
Santoyo G, Urtis-Flores CA, Loeza-Lara PD et al (2021) Rhizosphere colonization determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology-Basel 10:475. https://doi.org/10.3390/biology10060475
Sarker A, Masud MAA, Deepo DM et al (2023) Biological and green remediation of heavy metal contaminated water and soils: a state-of-the-art review. Chemosphere 332:138861. https://doi.org/10.1016/j.chemosphere.2023.138861
Schmidt SB, Husted S (2019) The biochemical properties of manganese in plants. Plants-Basel 8:381. https://doi.org/10.3390/plants8100381
Shao Y (2017) Analysis of energy savings potential of China’s nonferrous metals industry. Resour Conserv Recycl 117:25–33. https://doi.org/10.1016/j.resconrec.2015.09.015
Shen X, Dai M, Yang J et al (2022) A critical review on the phytoremediation of heavy metals from environment: performance and challenges. Chemosphere 291:132979. https://doi.org/10.1016/j.chemosphere.2021.132979
Sheoran V, Sheoran AS, Poonia P (2016) Factors affecting phytoextraction: a review. Pedosphere 26:148–166. https://doi.org/10.1016/S1002-0160(15)60032-7
Song P, Xu D, Yue J et al (2022) Recent advances in soil remediation technology for heavy metal contaminated sites: a critical review. Sci Total Environ 838:156417. https://doi.org/10.1016/j.scitotenv.2022.156417
Steingräber LF, Ludolphy C, Metz J et al (2022) Uptake of lead and zinc from soil by blackberry plants (Rubus fruticosus L. agg.) and translocation from roots to leaves. Environ Adv 9:100313. https://doi.org/10.1016/j.envadv.2022.100313
Tang J, Zhang J, Ren L et al (2019) Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Environ Manag 242:121–130. https://doi.org/10.1016/j.jenvman.2019.04.061
Taylor AA, Tsuji JS, Garry MR et al (2020) Critical review of exposure and effects: implications for setting regulatory health criteria for ingested copper. Environ Manag 65:131–159. https://doi.org/10.1007/s00267-019-01234-y
Torbati S, Kangarloei BA, Khataee A (2021) Bioconcentration of heavy metals by three plant species growing in Golmarz wetland, in northwestern Iran: the plants antioxidant responses to metal pollutions. Environ Technol Innov 24:101804. https://doi.org/10.1016/j.eti.2021.101804
Upadhyay SK, Srivastava AK, Rajput VD et al (2022) Root exudates: mechanistic insight of plant growth promoting Rhizobacteria for sustainable crop production. Front Microbiol 13:916488. https://doi.org/10.3389/fmicb.2022.916488
Vaculík M, Konlechner C, Langer I et al (2012) Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ Pollut 163:117–126. https://doi.org/10.1016/j.envpol.2011.12.031
Vargas LK, Da Costa PB, Beneduzi A et al (2023) Soil fertility level is the main modulator of prokaryotic communities in a meta-analysis of 197 soil samples from the Americas and Europe. Appl Soil Ecol 186:104811. https://doi.org/10.1016/j.apsoil.2023.104811
Veerasamy V, Jagannathan UM, Arakkala SD et al (2023) Exploring the bacterial genetic diversity and community structure of crude oil contaminated soils using microbiomics. Environ Res 236:116779. https://doi.org/10.1016/j.envres.2023.116779
Wang C, Guo L, Shen RF (2023a) Rare microbial communities drive ecosystem multifunctionality in acidic soils of southern China. Appl Soil Ecol 189:104895. https://doi.org/10.1016/j.apsoil.2023.104895
Wang C, Wang Y, Yan S et al (2023b) Biochar-amended composting of lincomycin fermentation dregs promoted microbial metabolism and reduced antibiotic resistance genes. Biores Technol 367:128253. https://doi.org/10.1016/j.biortech.2022.128253
Wang J, Shi L, Zhai L et al (2021) Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: a review. Ecotoxicol Environ Saf 207:111261. https://doi.org/10.1016/j.ecoenv.2020.111261
Wang L, Ji B, Hu Y et al (2017) A review on in situ phytoremediation of mine tailings. Chemosphere 184:594–600. https://doi.org/10.1016/j.chemosphere.2017.06.025
Worlanyo AS, Jiangfeng L (2021) Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: a review. J Environ Manage 279:111623. https://doi.org/10.1016/j.jenvman.2020.111623
Wu B, Li L, Guo S, Li Y (2023) Source apportionment of heavy metals in the soil at the regional scale based on soil-forming processes. J Hazard Mater 448:130910. https://doi.org/10.1016/j.jhazmat.2023.130910
Wu Y, Santos SS, Vestergard M et al (2022) A field study reveals links between hyperaccumulating Sedum plants-associated bacterial communities and Cd/Zn uptake and translocation. Sci Total Environ 805:150400. https://doi.org/10.1016/j.scitotenv.2021.150400
Yang F, Huang M, Li C et al (2022) Vegetation restoration increases the diversity of bacterial communities in deep soils. Appl Soil Ecol 180:104631. https://doi.org/10.1016/j.apsoil.2022.104631
Yang M, Zhou D, Hang H, et al (2024) Effects of balancing exchangeable cations Ca, Mg, and K on the growth of tomato seedlings (Solanum lycopersicum L.) based on increased soil cation exchange capacity. Agronomy-Basel 14:629. https://doi.org/10.3390/agronomy14030629
Yu G, Ullah H, Wang X et al (2023) Integrated transcriptome and metabolome analysis reveals the mechanism of tolerance to manganese and cadmium toxicity in the Mn/Cd hyperaccumulator Celosia argentea Linn. J Hazard Mater 443:130206. https://doi.org/10.1016/j.jhazmat.2022.130206
Zakaria Z, Zulkafflee NS, Mohd Redzuan NA et al (2021) Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants-Basel 10:1070. https://doi.org/10.3390/plants10061070
Zeng F, Ali S, Zhang H et al (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Zhang C, Song Z, Zhuang D et al (2019) Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol Fertil Soils 55:229–242. https://doi.org/10.1007/s00374-019-01344-z
Zhang L, Zheng J, Leng H et al (2023) Effects of Al3+ on the microbial community, metabolic pathways, and morphological analysis of activated sludge. J Environ Chem Eng 11:110627. https://doi.org/10.1016/j.jece.2023.110627
Zhao X, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotox Environ Safe 170:218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Zhong X, Chen Z, Li Y et al (2020) Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in eastern China. J Hazard Mater 400:123289. https://doi.org/10.1016/j.jhazmat.2020.123289
Zuo Y, Li Y, Chen H et al (2023) Effects of multi-heavy metal composite pollution on microorganisms around a lead-zinc mine in typical karst areas, southwest China. Ecotoxicol Environ Saf 262:115190. https://doi.org/10.1016/j.ecoenv.2023.115190
Acknowledgements
This research was funded by the Key R&D Program of Guangxi, China (Grant No. AB22035038). We owe thank the Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, for their help with experimental test.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation were performed by Hua Deng, Dong Zhao and Shunyun Ye. Data collection and analysis were performed by Hua Deng and Dong Zhao. The first draft of the manuscript was written by Dong Zhao and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Juan Barcelo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, D., Deng, H., Hu, L. et al. Analysis of heavy metal content and microbial characteristics in the pioneer plant soil system of typical manganese tailing ponds in Guangxi. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06870-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06870-w