Abstract
Aims
The aims of the current study were to understand the variation in the abundance, diversity and structure of the diazotrophic communities in the rhizosphere soil of these three dominant plant species around Siding Pb–Zn mine.
Methods
Three dominant plant species (Pteris vittata, Miscanthus floridulus and Phragmites australis) were randomly selected, and rhizosphere soils were sampled from the rhizosphere of the plants.
Results
The nifH gene abundance in the rhizosphere soil of Pteris vittata was the highest among the three plant species. Variations in rhizosphere soil diazotrophic communities were mainly due to the changes in soil nutrient contents through plant‒soil system interactions. Diversity and structure of soil diazotrophic communities, including Alphaproteobacteria, Deltaproteobacteria and Cyanobacteria, were strongly influenced by soil heavy metals, ammonium nitrogen, soil moisture and available phosphorus contents. In addition, soil enzymes, especially urease, protease and alkaline phosphatase activities, also contributed to the structure of the diazotrophic communities. Alphaproteobacteria and Cyanobacteria play vital roles in the soil biological nitrogen fixation process. Heavy metal enrichment in mines provides electron donors for diazotrophs to support their activities in harsh environments. Diazotrophs can provide N to support plant growth in mines to help restore heavy metal-containing soil by dominant plants.
Conclusions
Our results showed the variations in diazotrophic community compositions in rhizosphere soil of three dominant plants and their impact on heavy metal accumulation. This study will help to determine the role and importance of soil properties and plant species in the soil biological nitrogen fixation process in highly contaminated mine areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is an essential element for all living organisms on Earth and is mainly required for the synthesis of key cellular components, such as nucleotides, amino acids and proteins (Kuypers et al. 2018). Over decades, human activities such as agricultural practices (fertilizer use and legume cultivation) and fossil fuel burning have had a profound effect on nitrogen inputs to terrestrial environments (Canfield et al. 2010; Wang et al. 2019b). Soil microorganisms play an important role in biogeochemical nitrogen cycling, which is generally controlled by the balance between microbiologically driven processes, including nitrogen fixation, nitrification and anammox/denitrification (Wise et al. 2020). Among nitrogen fixation methods, biological nitrogen fixation (BNF) is a process that converts atmospheric nitrogen into nitrogenous compounds that can be taken up and utilized by plants (Liu et al. 2012). The nifH gene is widely used as a key gene encoding the subunit protein of nitrogenase (Wang et al. 2019c), and diazotrophs containing the nifH gene have been discovered mainly in microbial phyla, including Alphaproteobacteria, Firmicutes and green sulfur bacteria (Yang et al. 2021, 2022). However, the low nitrogen content is often a limiting factor to the development of ecosystems in abandoned mines, while diazotrophs often act as early and frequent colonizers in this N-limited habitat (Li et al. 2019b). A number of soil properties are considered key factors influencing diazotrophic communities and nitrogen-fixing capacity. For example, Wang et al. (2020b) indicated that NH4+-N, as the main product of the NF process, influences the activity of soil enzymes by affecting diazotrophic community diversity. Additionally, Wang et al. (2022b) showed that the soil available P (AP) content is closely related to nifH gene abundance. Previous study results also confirmed the decisive influence of soil carbon (C) and N contents on diazotrophic communities (Coelho et al. 2008, 2009). Since the diversity of nifH genes is critically related to environmental factors in soil ecosystems, a better understanding of the NF process dominated by nifH can improve the bioavailability of nitrogen in mines and facilitate the process of mine rehabilitation.
Guangxi Province is known as the main area of nonferrous metals; there are more than 6800 mines of all kinds in Guangxi Province, the highest number of mines of any province in China (Xue et al. 2018). The Siding lead–zinc (Pb‒Zn) mine area is located in Liuzhou, Guangxi Province, China, with a total area of 13.64 km2 (Yin et al. 2008). Previously, field surveys conducted by Yin et al. (2008), Lu et al. (2010) and Cui et al. (2010) showed that the soil in the Siding mine area was severely polluted by heavy metals, such as Pb, Zn and Cd, and these authors also investigated the dominant plants that adapted to heavy metal (HM)-contaminated environments, such as Phragmites australis (Cav.) Trin. ex Steud (P. australis), Pteris vittata L. (P. vittata), Taraxacum mongolicum Hand.-Mazz. (T. mongolicum), Imperata cylindrica (L.) P. Beauv. (I. cylindrica), Miscanthus floridulus (Lab.) Warb. ex Schum. et Laut. (M. floridulus), Equisetum ramosissimum Desf. (E. ramosissimum), Buddleja officinalis Maxim. (B. officinalis) and Ficus tikoua Bur. (F. tikoua). Among these dominant plants, several plants exhibited good HM accumulation abilities, such as P. vittata, P. australis, I. cylindrica, and T. mongolicum. Dennis et al. (2010) and Hou et al. (2018) noted that terrestrial plants release a series of substrates through rhizodeposition, and these substrates may lead to interactions between plants and various soil microorganisms in the rhizosphere. During the BNF process, molecular nitrogen is reduced to NH4+ or other nitrogen-containing compounds by the action of corresponding biological enzymes and is thus taken up by plants (Hao et al. 2022). Several diazotrophs, including Mesorhizobium metallidurans and Paenibacillus graminis, have been isolated from plant rhizospheres during phytoremediation in mines (Sun et al. 2020). Therefore, nitrogen fixation is considered beneficial for the growth of higher-order plants, including hyperaccumulators, and can contribute to the phytoremediation of mine soils.
The different rhizosphere environments might lead to different effects on the diazotrophic community. In particular, nutrient-deficient mine soils with low organic matter content and limited bioavailable inorganic nutrients and diazotrophs that live symbiotically with pioneer plants should have adaptive mechanisms to cope with harsh mine conditions (Zhan and Sun 2012). Additionally, Kim et al. (2022) showed that plant species play vital roles in the variations in the diversity and structure of diazotrophic communities in mines. For instance, Li et al. (2015) showed that diazotrophic communities in the rhizosphere of Miscanthus giganteus contributed to plant growth and nitrogen use efficiency. In summary, there are differences in the soil NF process in different plant root environments due to regulation by environmental factors. Therefore, understanding nitrogen fixation processes in the rhizosphere soils of pioneer plants in mines will contribute to the sustainable development and remediation of mine ecosystems.
Accordingly, three dominant plant species (P. vittata, M. floridulus and P. australis) were selected from three different areas around the Siding Pb‒Zn mine to further understand the variation in the diazotrophic community in the rhizosphere soil of these three dominant plant species. The aims of this study were to (a) investigate the abundance, diversity, and community composition of the diazotrophs in the rhizosphere soils of the three plant species; (b) determine the distribution of the diazotrophic community under different rhizosphere conditions and elucidate the main properties that drive these diazotrophic communities; and (c) explore the relationship between nifH gene abundance and HM accumulation in dominant plant species. Thus, this study will help to determine the role and importance of rhizosphere soil properties and plant species in the BNF process around mine areas and to understand the impact of diazotrophs on the phytoremediation of heavy metals in contaminated soils.
Materials and methods
Study site description and sampling
This study was conducted in the Siding lead–zinc mining area, which is located in Liuzhou, Guangxi Province, China (109°13’-109°47’E, 24°46’-25°34’N). This area has a typical subtropical monsoon climate with an annual mean precipitation and annual mean temperature of 1985 mm and 17.8 °C, respectively. The mining area is approximately 13.64 km2 and ceased operations in 2006. There is a river flowing near the smelter, and the smelting sewage is discharged into the river through the sewage outlet (Li et al. 2022). Soil samples were collected on September 15th, 2021.
The soil type in the mine tailing region (MT region) was mainly a mixture of silt and ore waste screened out after mining, which was a sandy black soil with plant species such as M. floridulus, P. australis and Equisetum ramosissimum. Additionally, a highly contaminated region (4 km upstream of the mine tailing region, LC region) had clay and loess soil, and the plant species were mainly P. vittata, M. floridulus and P. australis. Moreover, another highly contaminated region (2 km downstream of the mine tailing region, HC region) had a sandy brown soil with P. vittata L., and M. floridulus as the main plant species. Four sites were selected in each region for the collection of plants and rhizosphere soils; these sites were L-1 to L-4 in the highly contaminated region in the upstream area (LC region), sites M-1 to M-4 in the MT region and sites H-1 to H-4 in the highly contaminated region in the downstream area (HC region). Site information is presented in Table S1. At each site, three samples of each plant species (P. vittata, M. floridulus and P. australis) were selected randomly. To prevent edge effects, we chose plants with adjacent plants on all four sides. Rhizosphere soils were taken around three types of plant species. Rhizosphere soils were sampled from the rhizosphere of the plants and adhered to the root crowns. After collection, each soil sample was divided into two parts. One portion of the fresh subsample was stored at 4 °C to determine the soil physicochemical properties. The other fresh subsamples were stored at -80 °C for DNA extraction. Plant samples were stored at -80 °C for further determination.
Soil and plant determination
Soil nitrate–N (NO3−) and ammonium-N (NH4+) were extracted 8.0 g fresh soil with 40 mL potassium chloride (KCl) (2 mol L−1) (soil mass: solution = 1:5) and determined by a continuous flow analyzer (Autoanalyzer AA3, Seal, Berlin, Germany) (Che et al. 2018). Fresh soil sample were air-dried at room temperature for 15 d and passed through a 2-mm sieve for further determine of the soil physicochemical properties. Soil total phosphorus (TP) was measured using a continuous flow analyzer after extraction with a mixture of sulfuric acid (H2SO4) and perchloric acid (HClO4) (Zhang et al. 2021), and available phosphorus (AP) was determined using the Olsen method (Olsen 1954). Soil total nitrogen (TN) and total carbon (TC) were analyzed in air-dried soils using an elemental analyzer (Vario MICRO cube, Elementar Instruments, Inc., Hanau, Germany). The soil moisture (MC) was determined after the fresh soil sample dried at 105 °C for 24 h until a constant weight was reached (Chen and Peng 2020). Soil pH was determined using a pH meter (A211 Thermo Scientific, USA) in H2O using a 1:2.5 (w/v) soil:solution ratio (Becerra-Castro et al. 2012). Soil metal (Pb, Zn, Cd, K, Ca, Na and Mg) concentrations were measured using the method described by Schlatter et al. (2019), in which the soil was digested and extracted with a mixture of 3:1:1 (v/v/v) concentrated hydrochloric acid (HCl), concentrated nitric acid (HNO3) and concentrated perchloric acid (HClO4) and measured using an atomic absorption spectrophotometer (AAnalyst 800, Perkin Elmer, MA, USA). Soil protease, urease, invertase, alkaline phosphatase and catalase activities were analyzed using the ninhydrin colorimetric method, hypochlorite-alkaline phenol method, 3,5-dinitrosalicylic acid method, phenyl disodium phosphate colorimetric method and potassium permanganate titration method, respectively (Ge et al. 2018; Watanabe and Hayano 1995; Wu et al. 2015; Yang et al. 2007).
Pb, Zn and Cd concentrations in the tissues (roots, stems and leaves) of P. vittata, M. floridulus and P. australis were determined using the method described by Li et al. (2020). Plant tissues were oven-dried at 105 °C for 30 min and then at 70 °C for 48 h until a constant weight was reached. And then, 0.25 g tissue sample were digested by 12 mL of a mixture of HCl and HClO4 at a ratio of 5:1 (v/v), and then measured using an atomic absorption spectrophotometer. The translocation value (TF value) was estimated as the ratio of HM concentrations in stems and leaves of the plant to the HM concentrations in roots (Yu et al. 2020c). The bioconcentration factor (BCF) was estimated as the ratio of HM concentrations in plant tissues (roots, stems and leaves) to the HM concentrations in soil (Khan et al. 2017).
Soil DNA extraction, real-time quantitative PCR and Illumina MiSeq sequencing
Soil genomic DNA was extracted using a Fast® DNA SPIN Kit (MP Biomedical, Solon, OH, USA) from 0.5 g of fresh soil previously stored at -80 °C, following the manufacturer’s instructions. The quality of the DNA samples was detected by 1% agarose gel electrophoresis. The abundance of nifH was determined using a quantitative PCR system (Quant Studio, Thermo Fisher, USA). The nifH gene was amplified using the primers nifHF (5′-AAAGGYGGWATCGGYAARTCCAC CAC-3′) and nifHR (5′-TTGTTSGCSGCRTACATSGCCATCAT-3′) (Chen et al. 2019b). Each reaction mixture (20 μL) contained 0.4 μL of DNA template, 0.6 μL of each primer (20 μmol L−1), 10 μL of SYBR Premix Ex Taq™ (TaKaRa Bio, China), and 8.4 μL of sterile H2O. The PCR was started with an initial denaturation at 95 °C for 60 s, followed by 40 cycles (95 °C for 15 s and 60 °C for 60 s), and finally an extension at 50 °C for 10 min. The PCR mixed product was recovered using an EZNA Gel Extraction Kit (Omega, USA). After that, the Illumina MiSeq PE300 platform (Illumina Inc., San Diego, CA, USA) was used to perform the microbial community analysis at Major Bio Biopharma Technology Co., Ltd. in Shanghai, China. The obtained raw data for nifH were analyzed using QIIME software (version 1.9.1). The sequences that failed to translate into nifH genes were removed, and the remaining sequencing reads were clustered into operational taxonomic units (OTUs) at 97% nucleotide identity using UPARSE software (version 7.0.1). The representative OTU sequences to reference nifH were compared to sequences in the NCBI database using BLAST to ensure sequence specificity for further analysis. All representative nifH sequences of OTUs with 97% similarity cut off were submitted to the NCBI archive under BioProject PRJNA869511, PRJNA869518 and PRJNA869522.
Statistical analyses
One-way ANOVA based on Tukey's HSD test was performed to determine the significant differences at p < 0.05 between the nifH gene abundance, α-diversity indices and HM concentration in plant tissues using SPSS 19.0 software. The α-diversity indices (including the ACE index, Shannon index and Simpson index) and β-diversity index (nonmetric multidimensional scaling score (NMDS1 score)) were calculated using QIIME software (version 1.9.1). Among the α-diversity indices, ACE indicates the species richness (i.e., the number of OTUs), whereas Simpson's and Shannon's indices indicate the diversity (which considers the number of OTUs and the frequency of each one). Two-way ANOVA was selected to examine the interactive effects of soil types and plant species on nifH gene abundance using SPSS 19.0 software. Multiple linear regression analysis was used to determine the relationships between diversity indices (including Shannon, Simpson and ACE index and NMDS1 score) and soil properties. Significant differences in α-diversity indices and nifH gene abundance of diazotroph communities were examined using Student’s t test. Principal component analysis (PCA) was used to reduce the dimension of factors in all samples, and the main factors were extracted affecting the characteristics of diazotrophic community. Pearson’s rank correlation was used to analyze the relationships between soil properties and the nifH gene abundance and relative abundance of diazotrophic community taxa at the class level. Pearson’s rank correlation was used to analyze the relationships between nifH gene abundance and HM concentration in plant tissues.
Results
Soil properties and soil enzyme activities
The soil properties and soil enzyme activities of the sample sites in different sample regions are presented in Tables S2-S4. A total of twelve contaminated sites in the upstream area, mine tailing area and downstream area with high concentrations of Pb, Zn and Cd around the Siding mine area were selected. At each site, rhizosphere soil samples were collected from three types of pioneer plant species, P. vittata, M. floridulus and P. australis. The Pb (average 6377.8 ± 378.7 mg kg−1), Zn (average 18,315.8 ± 2025.7 mg kg−1) and Cd (average 159.1 ± 12.5 mg kg−1) levels in the HC soil were significantly higher than those in the LC and MT soils (Fig. 1 (a)-(c)), and the Cd (average 61.7 ± 8.3 mg kg−1) level in the LC soil was significantly lower than those in the MT and HC soils. The TN, NH4+, TC, TP and AP levels in the LC soil were significantly higher than those in the MT and HC soils (Fig. 1 (d)-(i)), and TN, NH4+ and TC in the MT soil were significantly lower than those in the LC and HC soils. Furthermore, significant differences in NO3− levels were detected, with average values of 20.1 ± 5.6 mg kg−1 in the LC soil, 35.4 ± 3.2 mg kg−1 in the MT soil and 52.3 ± 7.7 mg kg−1 in the HC soil (Fig. 1 (e)). The variation in the enzyme activities in the rhizosphere soil is presented in Fig. 1 (l)-(p). Invertase (average 0.72 ± 0.22 mg glucose g−1 d−1), urease (average 22.7 ± 12.9 mg NH3-N g−1 d−1), protease (average 0.33 ± 0.10 mg NH3-N g−1 d−1) and alkaline phosphatase (average 124.4 ± 41.4 mg phenol g−1 d−1) activities in the LC soil were significantly higher than those in the MT and HC soils (Fig. 1 (l)-(o)). However, catalase activity in the HC soil was significantly higher than that in the LC and MT soils, with an average of 9.16 ± 3.07 mg KMnO4 g−1 d−1 (Fig. 1 (p)).
Variations of Pb (a), Zn (b), Cd (c), total nitrogen (d), NO3−-N (e), NH4+-N (f), total carbon (g), total phosphorus (h), available phosphorus (i), pH value (j), soil moisture (k), invertase activity (a), urease activity (b), protease activity (c), alkaline phosphatase (d) and catalase activity (e) in rhizosphere soil of Pteris vittata, Miscanthus floridulus and Phragmites australis in highly-contaminated region in upstream area (LC soil), mine tailing soil (MT soil) and highly-contaminated region in downstream area (HC soil) in Siding mine area. The different lowercase letters above the bars in the graph denote significant differences at p < 0.05 between the soil properties among different sample regions based on Tukey's HSD test
Relationship between nifH gene abundance and soil properties in the plant rhizosphere
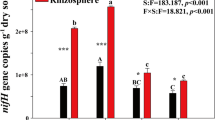
Variations in nifH gene abundance in the rhizosphere soil of P. vittata, M. floridulus and P. australis in the LC, MT and HC soils are presented in Fig. 2 and Figure S1. Two-way ANOVA results indicated that soil type, plant species and the interaction between them had a significant effect on the variations in nifH gene abundance (p < 0.001). In addition, the F value of soil type (F = 4699.8) was higher than that of plant species (F = 339.67), indicating that soil type had a greater impact on rhizosphere soil nifH gene abundance. Except for the nifH gene abundance in the rhizosphere soil of P. vittata in the LC soil, there were no significant changes in nifH gene abundance in the rhizosphere soil of the same plant in the same sample region (Figure S1). At the same time, significant differences in nifH gene abundance were detected among the sample regions (p < 0.001), with an average of (16.26 ± 2.55) × 107 gene copies g−1 dry soil in the LC soil, (1.79 ± 0.74) × 107 gene copies g−1 dry soil in the MT soil and (8.55 ± 2.28) × 107 gene copies g−1 dry soil in the HC soil (Fig. 2 (d)). However, significant differences in nifH gene abundance were detected among plant species in the LC, MT and HC soils (p < 0.001). The results indicated that the nifH gene abundance in the rhizosphere soil of P. vittata was significantly higher than that in the rhizosphere soil of M. floridulus and P. australis in the LC, MT and HC soils, with an average of (19.22 ± 0.79) × 107 gene copies g−1 dry soil in the LC soil, (2.65 ± 0.15) × 107 gene copies g−1 dry soil in the MT soil and (11.47 ± 0.85) × 107 gene copies g−1 dry soil in the HC soil (Fig. 2 (a)-(c)).
Abundance of nifH in rhizosphere soil of Pteris vittata, Miscanthus floridulus and Phragmites australis in a highly contaminated region in the upstream area (LC soil), mine tailing soil (MT soil) and a highly contaminated region in the downstream area (HC soil) in the Siding mine area. Student’s t test was used to examine the significance of differences among the nifH gene abundances in different plant species in LC soil (a), MT soil (b) and HC soil (c), and nifH gene abundance among different soil types (d). * indicates p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.001
The results of the PCA of the environmental factor data for the different ecological areas are shown in Table S5.The results of PCA revealed that the first four PCs had eigenvalues greater than unity and cumulatively explained 84.72%. The PCA loadings indicated that the PC I was highly correlated with nifH gene abundance, soil MC, NH4+-N, TC, Invertase, Protease, Alkaline phosphatase and Cyanobacteria, Deltaproteobacteria; PC II was highly correlated with soil NO3−-N, TN. C/N, Catalase, and Alphaproteobacteria; PC III was highly correlated with soil Urease and Betaproteobacteria; PC IV was highly correlated with soil pH and Proteobacteria. Two-way ANOVA showed that the effect of soil type on the rhizospheric diazotrophic community of the plants in this study was greater than that of plant type.
The correlations between nifH gene abundance and soil properties based on Pearson’s rank correlation analysis are presented in Fig. 3 and Figure S2. Strong and significant positive correlations were observed between nifH gene abundance and Pb, Zn, Ca, NH4+-N, TC, TP and AP levels and protease, urease, invertase and alkaline phosphatase activities (p < 0.001). In addition, strong and significantly negative correlations were observed between nifH gene abundance and Cd, Na, Mg and NO3−-N levels (p < 0.001). In particular, TP (R2 = 0.678, Pearson correlation = 0.823) and TC (R2 = 0.698, Pearson correlation = 0.836) and exhibited the most positive correlation with nifH gene abundance (Fig. 3 (c) and (e)).
Correlation between nifH gene abundance and soil properties (NH4+-N (a), NO3−-N (b), total phosphorus (c), available phosphorus (d), total carbon (e), Na (f), protease activity (g), invertase activity (h) and alkaline phosphatase activity (i)) in a highly contaminated region in the upstream area (LC soil), mine tailing soil (MT soil) and a highly contaminated region in the downstream area (HC soil) in the Siding mine area. Each point represents an individual sample. The solid lines indicate the linear regression, and the gray areas indicate the 95% confidence intervals. The Pearson correlation, statistical significance (p) and fitting coefficient (R2) are shown in the graphs
Variations in diazotrophic diversity
After quality filtering, we produced a nifH dataset of 294,964 high-quality sequences (ranging from 11,283 to 21,731 per sample) with 97% similarity. The α-diversity indices, including the ACE, Shannon and Simpson indices of the nifH genes, are presented in Table S7 and Fig. 4. Two-way ANOVA results indicated that soil type, plant species and the interaction between them had a significant effect on the variations in the α-diversity indices (p < 0.001) (Fig. 4). The ACE index for P. vittata displayed a higher value in the HC soil than in the other soils, the ACE index for M. floridulus displayed a higher value in the MT soil, and the ACE index for P. australis displayed a higher value in the LC soil (Fig. 4 (a)). Generally, there was a significant difference among the ACE index values among the different sample regions (p < 0.05 or p < 0.01) (Fig. 4 (b)). In addition, the Shannon index of P. australis displayed higher values (average 5.89 ± 0.40) in the LC soil than in the other soils (Fig. 4 (c)); in addition, there was no significant difference in the Shannon index between the MT and HC soils (Fig. 4 (d)). The Simpson index of P. vittata displayed higher values (average 0.238 ± 0.030) in the LC soil (Fig. 4 (e)), and there was no significant difference in the Simpson index between the LC soil and the other two soils (Fig. 4 (f)).
α-Diversity ((a)-(b) ACE index, (c)-(d) Shannon index and (e)-(f) Simpson index) of the diazotrophic communities in rhizosphere soil of Pteris vittata (P. vittata), Miscanthus floridulus (M. floridulus) and Phragmites australis (P. australis) in a highly contaminated region in the upstream area (LC soil), mine tailing soil (MT soil) and a highly contaminated region in the downstream area (HC soil) in the Siding mine area. The different lowercase letters above the bars in the graph denote significant differences at p < 0.05 between the α-diversity index in the same plant species among different soil types based on Tukey's HSD test. Student’s t test was used to examine the significant differences in α-diversity among different soil types. Two-way ANOVA results are presented in the graph. * indicates p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.001
The multiple linear regression between the α-diversity indices (including Shannon, Simpson and ACE indices) and β-diversity index (NMDS1 score) of diazotrophic communities and the tested soil properties are presented in Table 1. Soil moisture, TN, NH4+-N, TP and AP levels and invertase activity were highly correlated with α-diversity indices (p < 0.05, p < 0.01 or p < 0.001). In addition, Cd, K, Mg and NO3−-N levels and protease and alkaline phosphatase activities were highly correlated with the ACE and Shannon indices (p < 0.05, p < 0.01 or p < 0.001). For the β-diversity index, the soil moisture content; Pb, Zn, Ca, Na, NH4+-N, TP and AP levels; and invertase, protease and alkaline phosphatase activities were highly correlated with the NMDS1 score (p < 0.05, p < 0.01 or p < 0.001).
Variations in the diazotrophic community composition
Variations in the relative abundance of the diazotrophic community are presented in Fig. 5. Alphaproteobacteria and Deltaproteobacteria were the major classes of Protobacteria. Among them, Alphaproteobacteria existed at all sample sites, accounting for 18.4% to 87.6% in the LC soil, 40.8% to 52.9% in the MT soil and 42.5% to 68.8% in the HC soil. Deltaproteobacteria mainly existed in the rhizosphere soil of M. floridulus and P. australis in the LC and HC soils, accounting for 30.9% to 34.5% and 22.6% to 26.6% in LC soil, respectively. In addition, Betaproteobacteria mainly existed in the rhizosphere soil of M. floridulus, accounting for 3.73% to 8.39% in the LC soil. Specifically, the phylum Cyanobacteria existed at all sample sites in the MT soil, accounting for 10.6% to 29.2%; however, Cyanobacteria also existed in the rhizosphere soil of P. vittata and P. australis in the HC soil, accounting for 15.6% to 19.7% and 17.5% to 24.4%, respectively.
Relative abundances of diazotrophic communities in rhizosphere soil of Pteris vittata (P. vittata), Miscanthus floridulus (M. floridulus) and Phragmites australis (P. australis) in a highly contaminated region in the upstream area (LC soil, L-1 to L-4), mine tailing soil (MT soil, M-1 to M-4) and a highly contaminated region in the downstream area (HC soil, H-1 to H-4) in the Siding mine area at the class level. Classes with an average relative abundance higher than 0.1% are shown separately, and “Other” represents the sum of the proportions of the genera with abundances lower than 0.1%
Pearson’s rank correlation between soil physicochemical properties and soil enzyme activities and the relative abundance of diazotrophic community taxa at the class level are presented in Table 2. The soil Pb, Zn, Cd, Ca, Mg, and NO3−-N contents and catalase activity presented a positive correlation with Alphaproteobacteria (p < 0.05 or p < 0.01). However, the K, TN, NH4+-N and AP levels presented a negative correlation with Alphaproteobacteria (p < 0.05 or p < 0.01). In addition, the soil moisture content; Pb, Zn, TN, NH4+-N, TC, TP, and AP contents; and invertase, protease, urease and alkaline phosphatase activities were positively correlated with Deltaproteobacteria (p < 0.05 or p < 0.01) but negatively correlated with Cyanobacteria (p < 0.05 or p < 0.01). However, the Cd, Na, Mg and NO3−-N contents presented a negative correlation with Deltaproteobacteria (p < 0.05 or p < 0.01) but presented a positive correlation with Cyanobacteria (p < 0.05 or p < 0.01).
HM accumulation in plants and correlation with nifH gene abundance and soil properties
The Pb, Zn and Cd concentrations in the tissues of P. vittata, M. floridulus and P. australis in the LC, MT and HC soils are presented in Fig. 6. The Pb, Zn and Cd concentrations in the roots of the plants were higher than those in the stems and leaves. In addition, the Pb, Zn and Cd concentrations in the roots of the plants in the HC soil were higher than those in the LC and MT soils. The Pb, Zn and Cd concentrations in the roots of P. vittata, M. floridulus and P. australis in the HC soil were 666.5 ± 18.1, 987.2 ± 50.6 and 730.5 ± 17.9 mg kg−1 dry weight (DW) for Pb (Fig. 6 (a)); 2780.1 ± 182.4, 2722.0 ± 119.1 and 1114.4 ± 98.8 mg kg−1 DW for Zn (Fig. 6 (b)); and 33.9 ± 2.0, 44.6 ± 2.8 and 26.8 ± 2.9 mg kg−1 DW for Cd, respectively (Fig. 6 (c)). Generally, the TF and BCF values of Pb for P. vittata, M. floridulus and P. australis in the MT soil were significantly higher than those in the LC and HC soils (p < 0.05) (Table 3). For P. vittata, the TF values of Pb were higher than 1.0 in the three types of soil, while the TF values of Pb for both M. floridulus and P. australis in the LC and MT soils were higher than 1.0. Moreover, the BCF values of Cd for P. vittata, M. floridulus and P. australis in the LC soil were significantly higher than those in the MT and HC soils (p < 0.05) (Table 3).
Pb (a), Zn (b) and Cd (c) concentrations in roots, stems and leaves of Pteris vittata (P. vittata), Miscanthus floridulus (M. floridulus) and Phragmites australis (P. australis) in a highly contaminated region in the upstream area (LC soil), mine tailing soil (MT soil) and a highly contaminated region in the downstream area (HC soil) in the Siding mine area. The different lowercase letters above the bars in the graph denote significant differences at p < 0.05 between the HM concentration in the same tissues of different plant species in the same soil types based on Tukey's HSD test. Each bar represents all plants sampled in the same soil region (n = 12)
The correlations between nifH gene abundance and the Pb, Zn and Cd concentrations in the tissues of P. vittata, M. floridulus and P. australis in the LC, MT and HC soils are presented in Fig. 7. For P. vittata, strong and significant positive correlations were observed between nifH gene abundance and Pb, Zn, Cd concentrations in the leaves and Cd concentrations in the stems (p < 0.01 or p < 0.001) (Fig. 7 (b), (d) and (f)). In particular, the Zn (R2 = 0.897, Pearson correlation = 0.974) and Cd (R2 = 0.646, Pearson correlation = 0.804) concentrations in the leaves exhibited the most positive correlation with nifH gene abundance (Fig. 7 (d) and (f)). For M. floridulus, strong and significant positive correlations were observed between nifH gene abundance and the Pb, Zn, Cd concentrations in the stems and the Zn concentrations in the leaves (p < 0.01 or p < 0.001) (Fig. 7 (h), (j) and (l)). In particular, the Cd (R2 = 0.518, Pearson correlation = 0.719) concentrations in the stems exhibited the most positive correlation with nifH gene abundance (Fig. 7 (l)). However, the Pb (R2 = 0.260, Pearson correlation = -0.510) concentrations in the stems exhibited a significant negative correlation with nifH gene abundance (p < 0.01) (Fig. 7 (h)). For P. australis, a strong and significant positive correlation was observed between nifH gene abundance and the Zn (R2 = 0.332, Pearson correlation = 0.576) concentrations in the roots and the Cd (R2 = 0.909, Pearson correlation = 0.953) concentrations in the stems (p < 0.001) (Fig. 7 (o) and (r)). However, the Pb concentrations in the stems (R2 = 0.665, Pearson correlation = -0.816) and leaves (R2 = 0.733, Pearson correlation = -0.856) and the Zn concentrations in the stems (R2 = 0.461, Pearson correlation = -0.679) exhibited a significant negative correlation with nifH gene abundance (p < 0.001) (Fig. 7 (n) and (p)).
Correlation between nifH gene abundance and Pb, Zn and Cd concentrations in the roots, stems and leaves of Pteris vittata ((a)-(f)), Miscanthus floridulus ((g)-(l)) and Phragmites australis ((m)-(r)) in the Siding mine area. Each point represents an individual sample. The solid lines indicate the linear regression, and the gray areas indicate the 95% confidence intervals. The Pearson correlation, statistical significance (p) and fitting coefficient (R2) are shown in the graphs
Discussion
Soil diazotrophic community structure and composition can be influenced by many interactions among physical, chemical, biological and other variables (Quintela-Sabaris et al. 2019; Shu et al. 2019). Soil diazotrophic communities are the most important microorganisms involved in the nitrogen fixation process in the soil nitrogen cycle and are an important source of nitrogen input for the natural ecological restoration of abandoned mines. (Zhan and Sun 2011). Our study provided insight into the interactive effects of soil properties and dominant plant species (P. vittata, M. floridulus and P. australis) on the rhizosphere diazotrophic structures and community compositions in the Siding lead–zinc mining area. The HM-accumulator P. vittata, the HM-tolerant M. floridulus and the climate-regulating and water-providing P. australis contributed significantly to restoring the plant stability of the mine area (Kohda et al. 2022; Weis and Weis 2004; Wu et al. 2022).
Microbial communities established in the plant rhizosphere are not random and are influenced by different factors, such as plant species, soil types and seasonal variations (Hassani et al. 2018). Hence, our results indicated that nifH gene abundance varied among the soil types and plant species. Zhao et al. (2019) indicated that HM contamination can have two different effects on soil microbes: a decrease in the diversity and abundance of microbes that do not adapt to high concentrations or toxicity of HMs and an increase in the diversity and abundance of microbes that better adapt to a highly contaminated environment. In the present study, the concentrations of Pb, Zn and Cd varied considerably across regions and were highest in HC soil and influenced nifH gene abundance to some extent (Figure S2 (a)-(c)). A previous study pointed out that in metal-rich mine soil, metal metabolism is also critical for the survival of diazotrophs in mineral soils, which might be due to metals serving as electron donors to support microbial activity in mineral soils (Ullah et al. 2015).
Soil properties respond to plant‒soil interactions, and this process can be influenced by soil HMs (Qian et al. 2022). As presented in Tables S2-S4, the soil around the Siding mine areas was nutrient deficient and slightly acidic. In particular, N, P and C are known to be important components affecting soil enzyme activity, plant biomass and the microbial community (Li et al. 2019a; Yu et al. 2020b). We found that soil AN, TP, AP, TC and MC are important key factors influencing nifH genes in three different ecological areas of the Siding mine. In mineral soils with pioneer plants, diazotrophs growing between the rhizosphere can provide essential nutrients for plant growth by solubilizing inorganic phosphorus from immobile minerals in the soil or fixing atmospheric nitrogen (Xiao et al. 2019). Our results are consistent with the conclusion that the phenomenon of the highest nifH gene abundance in rhizosphere soils is normal in LC soil with high nutrient content. Among the soil N contents, NH4+-N and NO3−-N are considered vital and typical indicators of soil nutrient conditions because they represent the effective N in soil (Navarro-Noya et al. 2012; Wang et al. 2017). In addition, soil P is considered the other essential nutrient for the BNF process and is involved in the cell physiology of soil microbes, including the capacity of storage, metabolism and cell divisions, which also affect the abundance and community of soil diazotrophs (Bent et al. 2016; Samaddar et al. 2019). Notably, soil P is divided into inorganic P and organic P, with AP as an important indicator of utilization by soil microbes (Wu et al. 2017). The soil AP content and nifH gene abundance in the rhizosphere of the three species were highest in the LC soil and were in close positive correlation with each other. High levels of soil AP can indirectly reduce HM stress by immobilizing HMs through precipitation, which is beneficial for the survival of soil microbes (Komárek et al. 2013). In addition, soil C also plays a vital role in regulating soil nutrient cycling and increasing soil diazotrophic activity (Haiming et al. 2020). In the present study, soil TC was highly correlated with nifH gene abundance (Fig. 3 (g)). Decomposition of plant debris in rhizosphere soil may provide an additional C source for soil microbes (Maarastawi et al. 2019), which might explain the highly positive correlation that existed between TC and nifH gene abundance in this study. Another possible reason is that there are differences in TC content in the rhizosphere soil of pioneer plants in three different ecological zones (Table S2-S4). Due to the different degree of soil restoration, the relationship between the abundance of diazotrophs genes abundance in rhizosphere soil and the content of TC in soil also changes. In addition, the soil MC presented a highly positive correlation with the diversity and abundance of the diazotrophic community (Fig. 1 (f) and Table 1). But the Pearson correlation coefficient between TC and nifH gene abundance was greater than between soil MC and nifH gene abundance, so the main factors affecting nifH gene abundance were soil NH4+-N, AP and TC. In particular, the soil MC highly altered the NMDS1 scores. Morugan-Coronado et al. (2019) noted that soil MC had a strong impact on the soil microbial community, especially soil diazotrophs and denitrifiers, which emphasized the importance of water availability in drought environments. Therefore, the effect of soil NH4+-N and MC on the soil diazotrophic community was mainly reflected in β-diversity, which suggested that soil NH4+ and MC were the main reasons for the high similarity among the diazotrophic communities in the rhizosphere soil in P. vittata, M. floridulus and P. australis in the Siding mine area.
Soil enzymes are indicators of microbial activities and ecological function in soil (Zhou et al. 2020). In the plant rhizosphere, enzymes produced by soil microbes and plant roots are higher than those in bulk soil (Ma et al. 2018). Our study concluded that the soil enzyme activities among the three soil types impacted the nifH gene diversity, diversity and structure of the soil diazotrophic community. These findings were consistent with those of Wang et al. (2020c), who pointed out that soil enzymes play vital roles in the foundation of soil microbial communities. As presented in Table 1, invertase, protease and alkaline phosphatase activities were highly positively correlated with the α-diversity and β-diversity of soil diazotrophs. In particular, soil enzyme activity provides information about the interactions between plants and microbes that can reflect the diversity, composition and function of soil microbes (Razavi et al. 2016). A previous study indicated that invertase can be hydrolyzed into glucose and fructose, and then participate in C, P and N cycling in the soil environment (Gu et al. 2009). Thus, soil diazotrophs can survive well under the harsh conditions in mine areas by using glucose from the breakdown of invertase. In rhizosphere microecosystems, phosphorus mineralization is catalyzed by phosphatases produced by plants and microbes; microbes produce both acidic and alkaline phosphatases, and plants produce only acidic phosphatases (Chen and Moorhead 2022). Among the enzymes associated with microbially mediated mineralization of soil organophosphorus, the activity of alkaline phosphatase plays a vital role (Wang et al. 2019a). In our study, alkaline phosphatase presented a highly positive effect on nifH gene abundance and the diazotrophic community and simultaneously influenced the NMDS1 score. These findings were similar to those of Wang et al. (2022a), who noted that soil alkaline phosphatase activity was one of the most important factors influencing the soil diazotrophic community. Moreover, urease and protease are also considered vital enzymes involved in the soil N cycle. Protease converts soil organic N into inorganic nitrogen by mineralization, followed by ammonification into NH4+-N in the presence of urease; hence, plants can use NH4+-N to synthesize amino acids and proteins for growth and development (Lee et al. 2021; Lin et al. 2021; Xu et al. 2019). This catalytic effect of urease and protease makes it easier for plants to extract nutrients from the soil (Saarathandra et al. 1984). Thus, in our research, enzyme activities in the rhizosphere soil of plants played an important role in the variation in the diazotrophic community in the Siding mine area.
The α-diversity of the diazotrophic community in the rhizosphere also presented the same phenomenon among the soil types and plant species. Multiple linear regression analysis, Tukey's HSD test and Student's t test indicated that soil Cd content altered the α-diversity of the diazotrophic community (Table 1), while the diversity and richness also presented different responses to the existence of different plant species (Fig. 4), which was similar to the results of Geng et al. (2022). This result might have occurred because the root secretions of the different plant species had various effects on the soil diazotrophic community (Song et al. 2020). However, the three sample regions around the Siding mine area contained higher HM contents, and the high levels of HMs increased the establishment of tolerant microbial communities, which might have led to the reduction in microbial diversity and variations in the microbial communities (Guo et al. 2017).
In our study, the most dominant diazotrophic community was Proteobacteria at the phylum level, which mainly consisted of Alphaproteobacteria, Betaproteobacteria and Deltaproteobacteria at the class level (Fig. 5). A previous study noted that Proteobacteria in diazotrophs play an important role in metabolism and nutrient cycling, which are widespread in various environments (Chen et al. 2019a). The presence of diazotrophs in the plant rhizosphere indicated the potential importance of the NF process for nitrogen input in the Siding mine area. In addition, during prolonged HM contamination, soil microbes develop tolerance to HMs and react positively to them (Chen et al. 2018). In our study, tolerant bacteria generally occurred at high abundances in HM-contaminated environments (Chen et al. 2019a), and Alphaproteobacteria acted as a sensitive bacterial class in soil that can survive in nitrogen-deficient mine areas, presenting an unusual morphology, such as providing an N source for plants and symbiotic N fixation with plants (Shi et al. 2021). Cyanobacteria were also found in the MT and HT soils in the Siding mine area. Previous studies have indicated that the extracellular polysaccharides produced by Cyanobacteria contribute to their survival and the BNF process in arid and semiarid soil (Mager and Thomas 2011; Navarro-Noya et al. 2012); thus, they might survive under unfavorable drying conditions and low soil moisture content environments and contribute to the BNF process in mine areas.
Pb, Zn and Cd concentrations in the roots, stems and leaves of P. vittata, M. floridulus and P. australis are presented in Fig. 6. Our results indicated that the HM concentrations in the roots of the three plant species were higher than those in the stems and leaves. Diazotrophs indirectly increase plant growth by inhibiting harmful microorganisms and pathogens, which is a positive aspect for HM removal (Ullah et al. 2015). Similarly, Chandra and Kumar (2017) demonstrated that HMs mainly accumulated in the roots of plants, which could be due to the complexation that HMs form with the sulfhydryl groups in the root, and this scenario could have resulted in the low HM concentration in leaves and stems as well as the lower TF values. Diazotrophs can live freely in the soil or be combined with nodules, which act as metal buffers and provide further protection against metal ion penetration. It is thus a multistage metal biosorption process, as the roots and nodules adsorb metal ions in the first stage, followed by metal uptake by the roots and nodules and finally by the shoots of plants (Hu et al. 2022; Yang et al. 2020). Among the soil properties, the Ca and Mg levels were two of the important factors that influenced HM uptake by P. vittata, M. floridulus and P. australis in the Siding mine areas (Figure S3). Soil Ca content can help to increase the root length of plants, thereby increasing the surface area for roots to absorb water and nutrients, as well as HM accumulation (Rathika et al. 2020). In addition, our previous study showed that Ca plays a vital role in promoting the growth of plants and alleviating HM stress (Yu et al. 2020a). Diazotrophs not only improve the uptake of Ca and Mg in the soil by plants but also alleviate heavy metal toxicity by reducing ethylene production (Rajkumar et al. 2012). In addition, studies have indicated that organic acids, sugars and amino acids secreted by plant roots contribute to the growth and metabolism of microorganisms, in addition to the presence of soil available N (e.g., NH4+-N), which could lead to the selective enrichment of beneficial microorganisms to sustain plants in harsh environments (Olanrewaju et al. 2019; Yuan et al. 2018).
HMs are taken up by plants and then translocated to root vesicles and deposited, but the efficiency of relocation is strictly regulated by membrane proteins; thus, HMs mainly accumulate in the roots (Shah and Daverey 2020). The TF values of Pb, Zn and Cd for P. vittata were higher than those for M. floridulus and P. australis; however, the BCF values of Pb, Zn and Cd did not present significant differences among the three plant species. These three types of plant species cover the entire mine area, which might play an important role in plant stability and contribute to the ecological restoration of the mine area to some extent (Březinová and Vymazal 2022; Zeng et al. 2019). In addition, our results also showed a high correlation between nifH gene abundance and HM concentrations in plant tissues (Fig. 7). Specifically, among the three plant species, the Pb, Zn and Cd concentrations in both the stems and leaves of P. vittata presented a highly positive correlation with nifH gene abundance (Fig. 7 (b), (d) and (f)). This result might imply that HM contamination can be selective in different plant species, which could selectively decrease or increase nifH gene abundance in the rhizosphere soil, and this effect might impact HM accumulation in the aerial parts of plants (Wang et al. 2020a). Our results were consistent with those of Sarria Carabalí et al. (2020), who showed that Cd accumulation in Echinocactus platyacanthus was directly proportional to the increase in nifH gene abundance in the rhizosphere. Research has shown that metal resistance genes in bacteria (including diazotrophs) are an important strategy for microorganisms to survive in extreme environments to thrive in the presence of metal concentrations in mine soils, generating energy and helping plants cope with heavy metal stress (Sun et al. 2020). Diazotrophs are very beneficial because they bring metals to a more bioavailable form by the processes of methylation, chelation, leaching, and redox reactions and the production of siderophores as well as promote plant growth by synthesizing various compounds or stimulating other metabolic pathways in the soil, such as uptake of N, P, S and Mg, thereby facilitating the uptake of heavy metals. Soil microbes assist different reactions as well as metabolic processes occurring in biogeochemical cycles of nutrients, maintenance of soil structure, and detoxification of pollutants (Khan et al. 2010).
Conclusion
The current study presented the distribution patterns of the abundance, diversity and community composition of diazotrophs in the Siding mine area. First, HM concentrations greatly influenced diazotrophic community diversity and structure. Two-way ANOVA indicated that nifH gene abundance, Shannon index, Simpson index and ACE index were significantly altered by soil type and plant species. At the same time, the Cd, Pb, Zn, Ca, Mg, TP, AP and NH4+-N levels in the rhizosphere soil were significantly different among the plant species. Multiple linear regression and Pearson correlation analysis indicated that among the soil properties, soil TC, AP and NH4+-N were the key factors that strongly influenced soil diazotrophic community. In addition, soil enzymes, especially urease, protease and alkaline phosphatase activities, also contributed to the structure of the diazotrophic community. In terms of diazotrophic community composition, Alphaproteobacteria and Cyanobacteria might play vital roles in the soil BNF process. The HM concentrations in the roots of P. vittata, M. floridulus and P. australis were higher than those in their leaves and stems. A new argument emerging from our study is that HM concentrations in plant tissues presented a high correlation with nifH gene abundance. Consequently, our results investigated the variations in diazotrophic community compositions in the rhizosphere soil of P. vittata, M. floridulus and P. australis and their impact on HM accumulation for the first time. This study will help to determine the role and importance of soil properties and plant species in the soil BNF process in highly contaminated mine areas.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Becerra-Castro C, Monterroso C, Prieto-Fernández A, Rodríguez-Lamas L, Loureiro-Viñas M, Acea MJ, Kidd PS (2012) Pseudometallophytes colonising Pb/Zn mine tailings: a description of the plant-microorganism-rhizosphere soil system and isolation of metal-tolerant bacteria. J Hazard Mater 217–218:350–359
Bent E, Németh D, Wagner-Riddle C, Dunfield K (2016) Residue management leading to higher field-scale N2O flux is associated with different soil bacterial nitrifier and denitrifier gene community structures. Appl Soil Ecol 108:288–299
Březinová TD, Vymazal J (2022) Distribution of heavy metals in Phragmites australis growing in constructed treatment wetlands and comparison with natural unpolluted sites. Ecol Eng 175
Canfield DE, Glazer AN, Falkowski PG (2010) The Evolution and Future of Earth’s Nitrogen Cycle. Science 330:192–196
Chandra R, Kumar V (2017) Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilised distillery sludge as a prospective tool for in situ phytoremediation of industrial waste. Environ Sci Pollut Res Int 24:2605–2619
Che RX, Deng YC, Wang F, Wang WJ, Xu ZH, Hao YB, Xue K, Zhang B, Tang L, Zhou HK, Cui XY (2018) Autotrophic and symbiotic diazotrophs dominate nitrogen-fixing communities in Tibetan grassland soils. Sci Total Environ 639:997–1006
Chen J, Moorhead DL (2022) Progressively decreased nitrogen-stimulation of soil phosphatase activity with long-term nitrogen addition. Appl Soil Ecol 169:104213
Chen WB, Peng SL (2020) Land-use legacy effects shape microbial contribution to N2O production in three tropical forests. Geoderma 358:113979
Chen Y, Jiang Y, Huang H, Mou L, Ru J, Zhao J, Xiao S (2018) Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ 637–638:1400–1412
Chen J, Shen W, Xu H, Li Y, Luo T (2019a) The composition of nitrogen-fixing microorganisms correlates with soil nitrogen content during reforestation: A comparison between legume and non-legume plantations. Front Microbiol 10:508
Chen J, Wang PF, Wang C, Wang X, Miao LZ, Liu S, Yuan QS (2019b) Dam construction alters function and community composition of diazotrophs in riparian soils across an environmental gradient. Soil Biol Biochem 132:14–23
Coelho MR, de Vos M, Carneiro NP, Marriel IE, Paiva E, Seldin L (2008) Diversity of nifH gene pools in the rhizosphere of two cultivars of sorghum (Sorghum bicolor) treated with contrasting levels of nitrogen fertilizer. FEMS Microbiol Lett 279:15–22
Coelho MRR, Marriel IE, Jenkins SN, Lanyon CV, Seldin L, O’Donnell AG (2009) Molecular detection and quantification of nifH gene sequences in the rhizosphere of sorghum (Sorghum bicolor) sown with two levels of nitrogen fertilizer. Appl Soil Ecol 42:48–53
Cui X, Guo W, Chen X (2010) Enrichment characteristics of heavy metals by dominant plants in Siding Lead-zine Abandoned Mine area. Metal Mine 406:180–182 (in Chinese)
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–327
Drewniak L, Sklodowska A (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Pollut Res 20:7728–7739
Ge YY, Wang QL, Wang L, Liu WX, Liu XY, Huang YJ, Christie P (2018) Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch Agron Soil Sci 64:708–717
Geng H, Wang F, Yan C, Ma S, Zhang Y, Qin Q, Tian Z, Liu R, Chen H, Zhou B, Yuan R (2022) Rhizosphere microbial community composition and survival strategies in oligotrophic and metal(loid) contaminated iron tailings areas. J Hazard Mater 436:129045
Gu Y, Wang P, Kong CH (2009) Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur J Soil Biol 45:436–441
Guo H, Nasir M, Lv J, Dai Y, Gao J (2017) Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotox Environ Saf 144:300–306
Haiming T, Xiaoping X, Chao L, Xiaochen P, Kaikai C, Weiyan L, Ke W (2020) Microbial carbon source utilization in rice rhizosphere and nonrhizosphere soils with short-term manure N input rate in paddy field. Sci Rep 10:6487
Hao J, Feng Y, Wang X, Yu Q, Zhang F, Yang G, Ren G, Han X, Wang X, Ren C (2022) Soil microbial nitrogen-cycling gene abundances in response to crop diversification: A meta-analysis. Sci Total Environ 838:156621
Hassani MA, Duran P, Hacquard S (2018) Microbial interactions within the plant holobiont. Microbiome 6:58
Hou S, Ai C, Zhou W, Liang G, He P (2018) Structure and assembly cues for rhizospheric nirK- and nirS-type denitrifier communities in long-term fertilized soils. Soil Biol Biochem 119:32–40
Hu T, Chen A, Jiang Y, Sun C, Luo S, Shao J (2022) Application of a newly recorded diazotrophic cyanobacterium in acidified and Cd contaminated paddy soil: Promotes rice yield and decreases Cd accumulation. Sci Total Environ 814:152630
Khan S, Hesham AE-L, Qiao M, Rehman S, He J-Z (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res 17:288–296
Khan AR, Ullah I, Waqas M, Park G-S, Khan AL, Hong S-J, Ullah R, Jung BK, Park CE, Ur-Rehman S, Lee I-J, Shin J-H (2017) Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotox Environ Saf 136:180–188
Kim H, Lee DK, Voigt TB, Tian G, Yannarell AC (2022) Agricultural practices of perennial energy crops affect nitrogen cycling microbial communities. Appl Soil Ecol 172:104366
Kohda YH, Endo G, Kitajima N, Sugawara K, Chien MF, Inoue C, Miyauchi K (2022) Arsenic uptake by Pteris vittata in a subarctic arsenic-contaminated agricultural field in Japan: An 8-year study. Sci Total Environ 831:154830
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides – A review. Environ Pollut 172:9–22
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263
Lee JK, Park HJ, Cha SJ, Kwon SJ, Park JH (2021) Effect of pyroligneous acid on soil urease, amidase, and nitrogen use efficiency by Chinese cabbage (Brassica campestris var. Pekinensis). Environ Pollut 291 118132
Li D, Voigt TB, Kent AD (2015) Plant and soil effects on bacterial communities associated with Miscanthus × giganteus rhizosphere and rhizomes. GCB Bioenergy 8:183–193
Li Y, Liu K, Zhu J, Jiang Y, Huang Y, Zhou Z, Chen C, Yu F (2019a) Manganese accumulation and plant physiology behavior of Camellia oleifera in response to different levels of nitrogen fertilization. Ecotox Environ Saf 184:109603
Li Y, Wu Z, Dong X, Jia Z, Sun Q (2019b) Dependency of biological nitrogen fixation on organic carbon in acidic mine tailings under light and dark conditions. Appl Soil Ecol 140:18–25
Li Y, Lin J, Huang Y, Yao Y, Wang X, Liu C, Liang Y, Liu K, Yu F (2020) Bioaugmentation-assisted phytoremediation of manganese and cadmium co-contaminated soil by Polygonaceae plants (Polygonum hydropiper L. and Polygonum lapathifolium L.) and Enterobacter sp. FM-1. Plant Soil 448:439–453
Li Y, Zhang HC, Liu Y, Wei JT, Wang C, Liang Y, Liu KH, Yu FM (2022) Characteristics on the community structure and abundance of diazotrophs from the soil profile in the Siding mine area. China Environ Sci 42:1819–1828 (in Chinese)
Lin H, Liu C, Li B, Dong Y (2021) Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J Hazard Mater 402 123829
Liu WQ, Song YS, Wang B, Li JT, Shu WS (2012) Nitrogen fixation in biotic crusts and vascular plant communities on a copper mine tailings. Eur J Soil Biol 50:15–20
Lu C, Wang Y, Yang J (2010) Soil heavy metal pollution and dominant plants selection in Pb-Zn Mining areas of Guangxi. Chinese J Soil Sci 41:1471–1475 (in Chinese)
Ma X, Zarebanadkouki M, Kuzyakov Y, Blagodatskaya E, Pausch J, Razavi BS (2018) Spatial patterns of enzyme activities in the rhizosphere: Effects of root hairs and root radius. Soil Biol Biochem 118:69–78
Maarastawi SA, Frindte K, Bodelier PLE, Knief C (2019) Rice straw serves as additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol Biochem 135:235–238
Mager DM, Thomas AD (2011) Extracellular polysaccharides from cyanobacterial soil crusts: A review of their role in dryland soil processes. J Arid Environ 75:91–97
Morugan-Coronado A, Garcia-Orenes F, McMillan M, Pereg L (2019) The effect of moisture on soil microbial properties and nitrogen cyclers in Mediterranean sweet orange orchards under organic and inorganic fertilization. Sci Total Environ 655:158–167
Navarro-Noya YE, Hernández-Mendoza E, Morales-Jiménez J, Jan-Roblero J, Martínez-Romero E, Hernández-Rodríguez C (2012) Isolation and characterization of nitrogen fixing heterotrophic bacteria from the rhizosphere of pioneer plants growing on mine tailings. Appl Soil Ecol 62:52–60
Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO (2019) Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biot 103:1155–1166
Olsen SR (1954) Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. United States Department Of Agriculture, Washington
Qian F, Huang X, Su X, Bao Y (2022) Responses of microbial communities and metabolic profiles to the rhizosphere of Tamarix ramosissima in soils contaminated by multiple heavy metals. J Hazard Mater 438:129469
Quintela-Sabaris C, Auber E, Sumail S, Masfaraud JF, Faucon MP, Watteau F, Saad RF, van der Ent A, Repin R, Sugau J, Nilus R, Echevarria G, Leguedois S (2019) Recovery of ultramafic soil functions and plant communities along an age-gradient of the actinorhizal tree Ceuthostoma terminale (Casuarinaceae) in Sabah (Malaysia). Plant Soil 440:201–218
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Rathika R, Khalifa AYZ, Srinivasan P, Praburaman L, Kamala-Kannan S, Selvankumar T, Kim W, Govarthanan M (2020) Effect of citric acid and vermi-wash on growth and metal accumulation of Sorghum bicolor cultivated in lead and nickel contaminated soil. Chemosphere 243:125327
Razavi BS, Zarebanadkouki M, Blagodatskaya E, Kuzyakov Y (2016) Rhizosphere shape of lentil and maize: Spatial distribution of enzyme activities. Soil Biol Biochem 96:229–237
Saarathandra SU, Perrott KW, Upsdell MP (1984) Microbiological and biochemical characteristics of a range New Zealand soils under estabilised pasture. Soil Biol Biochem 16:177–183
Samaddar S, Chatterjee P, Truu J, Anandham R, Kim S, Sa T (2019) Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl Soil Ecol 134:111–115
Sarria Carabalí MM, García-Oliva F, Cortés Páez LE, López-Lozano NE (2020) Effect of cadmium contamination on the rhizosphere bacterial diversity of Echinocactus platyacanthus. Rhizosphere 13
Schlatter DC, Reardon CL, Johnson-Maynard J, Brooks E, Kahl K, Norby J, Huggins D, Paulitz TC (2019) Mining the Drilosphere: Bacterial Communities and Denitrifier Abundance in a No-Till Wheat Cropping System. Front Microbiol 10:1339
Shah V, Daverey A (2020) Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ Technol Inno 18:100774
Shi L, Zhang P, He Y, Zeng F, Xu J, He L (2021) Enantioselective effects of cyflumetofen on microbial community and related nitrogen cycle gene function in acid-soil. Sci Total Environ 771:144831
Shu X, Zhang K, Zhang Q, Wang W (2019) Ecophysiological responses of Jatropha curcas L. seedlings to simulated acid rain under different soil types. Ecotox Environ Saf 185: 109705
Song Y, Li X, Yao S, Yang X, Jiang X (2020) Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci Total Environ 728:138439
Sun X, Kong T, Haggblom MM, Kolton M, Li F, Dong Y, Huang Y, Li B, Sun W (2020) Chemolithoautotropic Diazotrophy Dominates the Nitrogen Fixation Process in Mine Tailings. Environ Sci Technol 54:6082–6093
Ullah A, Mushtaq H, Ali H, Munis MFH, Javed MT, Chaudhary HJ (2015) Diazotrophs-assisted phytoremediation of heavy metals: a novel approach. Environ Sci Pollut Res 22:2505–2514
Wang C, Zheng M, Song W, Wen S, Wang B, Zhu C, Shen R (2017) Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol Biochem 113:240–249
Wang F, Kertesz MA, Feng G (2019a) Phosphorus forms affect the hyphosphere bacterial community involved in soil organic phosphorus turnover. Mycorrhiza 29:351–362
Wang S, Liu W, Zhao S, Wang C, Zhuang L, Liu L, Wang W, Lu Y, Li F, Zhu G (2019b) Denitrification is the main microbial N loss pathway on the Qinghai-Tibet Plateau above an elevation of 5000 m. Sci Total Environ 696:133852
Wang X, Liu B, Ma J, Zhang Y, Hu T, Zhang H, Feng Y, Pan H, Xu Z, Liu G, Lin X, Zhu J, Bei Q, Xie Z (2019c) Soil aluminum oxides determine biological nitrogen fixation and diazotrophic communities across major types of paddy soils in China. Soil Biol Biochem 131:81–89
Wang J, Li Q, Shen C, Yang F, Wang J, Ge Y (2020b) Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes in a long-term paddy field fertilization experiment. Land Degrad Dev 32:420–429
Wang C, Wei M, Wang S, Wu B, Du D (2020a) Cadmium influences the litter decomposition of Solidago canadensis L. and soil N-fixing bacterial communities. Chemosphere 246: 125717
Wang L, Pang X, Li N, Qi K, Huang J, Yin C (2020c) Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 194
Wang G, Jin Z, Wang X, George TS, Feng G, Zhang L (2022a) Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl Soil Ecol 170
Wang L, Zhang H, Wang J, Wang J, Zhang Y (2022b) Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl Soil Ecol 170
Watanabe K, Hayano K (1995) Seasonal variation of soil protease activities and their relation to proteolytic bacteria and Bacillus spp in paddy field soil. Soil Biol Biochem 27:197–203
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700
Wise BR, Roane TM, Mosier AC (2020) Community Composition of Nitrite Reductase Gene Sequences in an Acid Mine Drainage Environment. Microbial Ecol 79:562–575
Wu LS, Feng S, Nie YY, Zhou JH, Yang ZR, Zhang J (2015) Soil cellulase activity and fungal community responses to wetland degradation in the Zoige Plateau, China. J Mount Sci 12:471–482
Wu W, Dong C, Wu J, Liu X, Wu Y, Chen X, Yu S (2017) Ecological effects of soil properties and metal concentrations on the composition and diversity of microbial communities associated with land use patterns in an electronic waste recycling region. Sci Total Environ 601:57–65
Wu B, Luo S, Luo H, Huang H, Xu F, Feng S, Xu H (2022) Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic. Sci Total Environ 808:151995
Xiao E, Ning A, Xiao T, Sun W, Qiu Y, Zhang Y, Chen J, Gou Z, Chen Y (2019) Variation in rhizosphere microbiota correlates with edaphic factor in an abandoned antimony tailing dump. Environ Pollut 253:141–151
Xu L, Han Y, Yi M, Yi H, Guo E, Zhang A (2019) Shift of millet rhizosphere bacterial community during the maturation of parent soil revealed by 16S rDNA high-throughput sequencing. Appl Soil Ecol 135:157–165
Xue S, Wang J, Wu C, Li S, Hartley W, Wu H, Zhu F, Cui M (2018) Physiological response of Polygonum perfoliatum L. following exposure to elevated manganese concentrations. Environ Sci Pollut Res 25:132–140
Yang CL, Sun TH, He WX, Zhou QX, Chen S (2007) Single and joint effects of pesticides and mercury on soil urease. J Environ Sci 19:210–216
Yang Y, Ding J, Chi Y, Yuan J (2020) Characterization of bacterial communities associated with the exotic and heavy metal tolerant wetland plant Spartina alterniflora. Sci Rep 10:17985
Yang L, Cao XY, Chen XY, Deng QH, Wan LL, Li XW, Zhou YY, Song CL (2021) Community composition and functional genes explain different ecological roles of heterotrophic bacteria attached to two bloom-forming cyanobacterial genera. Sci Total Environ 758:143850
Yang Z, Chen X, Hou J, Liu H, Tan W (2022) Soil texture and pH exhibit important effects on biological nitrogen fixation in paddy soil. Appl Soil Ecol 178
Yin R, Luo Y, Li J, Zhu Y (2008) Evaluation of the Potential Ecological Risk of Heavy Metal Pollution in Soil and Bioaccumulation Characteristics of Dominant Plants in Siding Pb-Zn Mine. J Agro-Environ Sci 27:2158–2165 (in Chinese)
Yu F, Li C, Dai C, Liu K, Li Y (2020a) Phosphate: Coupling the functions of fertilization and passivation in phytoremediation of manganese-contaminated soil by Polygonum pubescens blume. Chemosphere 260:127651
Yu F, Lin J, Xie D, Yao Y, Wang X, Huang Y, Xin M, Yang F, Liu K, Li Y (2020b) Soil properties and heavy metal concentrations affect the composition and diversity of the diazotrophs communities associated with different land use types in a mining area. Appl Soil Ecol 155
Yu G, Jiang P, Fu X, Liu J, Sunahara GI, Chen Z, Xiao H, Lin F, Wang X (2020c) Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: A long-term field study. Environ Pollut 266: 115408
Yuan J, Zhao J, Wen T, Zhao M, Li R, Goossens P, Huang Q, Bai Y, Vivanco JM, Kowalchuk GA, Berendsen RL, Shen Q (2018) Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 6:156
Zeng P, Guo Z, Xiao X, Peng C, Feng W, Xin L, Xu Z (2019) Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci Total Environ 650:594–603
Zhan J, Sun Q (2011) Diversity of free-living nitrogen-fixing microorganisms in wastelands of copper mine tailings during the process of natural ecological restoration. J Environ Sci 23:476–487
Zhan J, Sun Q (2012) Diversity of free-living nitrogen-fixing microorganisms in the rhizosphere and non-rhizosphere of pioneer plants growing on wastelands of copper mine tailings. Microbiol Res 167:157–165
Zhang Q, Jia X, Li T, Shao M, Yu Q, Wei X (2021) Decreased soil total phosphorus following artificial plantation in the Loess Plateau of China. Geoderma 385:114882
Zhao X, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotox Environ Saf 170:218–226
Zhou T, Wang C, Zhou Z (2020) Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol Biochem 149:107915
Acknowledgements
This paper is sponsored by the National Natural Science Foundation of China (grant number 41967019, 41907096), Guangxi Key Research and Development Program (AB21220057), Research Funds of The Guangxi Key Laboratory of Landscape Resources Conservation and Sustainable Utilization in Lijiang River Basin, Guangxi Normal University (grant number LRCSU21Z0210, LRCSU21Z0211) and Natural Science Foundation of Guangxi Province (grant number 2021GXNSFAA220024).
Author information
Authors and Affiliations
Contributions
YL: Writing-original draft, Conceptualization, Supervision, Funding acquisition. XC: Investigation, Data curation. CT: Software, Validation, Data curation. MZ: Software, Investigation. SL: Data curation, Formal analysis. QL: Software, Data curation. KL: Writing- review & editing. JM: Funding acquisition. ST: Writing-original draft, Investigation, Data curation. FY: Supervision, Writing-review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this work. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Juan Barcelo.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Chen, X., Tang, C. et al. Variations on the diazotrophic community in the rhizosphere soil of three dominant plant species in a lead–zinc mine area. Plant Soil 489, 155–175 (2023). https://doi.org/10.1007/s11104-023-06003-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06003-9