Abstract
Background and Aims
Declaring the drought-resistance of plant communities in grassland and the underlying mechanisms is crucial under climatic change scenario. Here we aimed to quantify aboveground & belowground net primary productivity (ANPP & BNPP) and their allocation in response to extreme droughts, and to uncover any underlying factors of drought resistance.
Methods
We experimentally reduced growing season rainfall by 66% for four years in two grassland sites (Sandy and Loamy site respectively), and measured ANPP and BNPP yearly and evaluated the relatedness of drought-induced changes for them to plant traits (Plant height, δ13C, SLA and LNC) and community composition.
Results
Experimental drought decreased species richness, ANPP of forbs and the total at both sites, but decreased ANPP of grasses only at the Sandy site. Experimental drought has non-significant effect on total BNPP at both sites, but increased 10–20 cm BNPP and decreased 0–10 cm BNPP. BNPP ratio was kept unchanged at the Loamy site but increased at the Sandy site. Moreover, the communities at the Loamy site showed lower δ13C, SLA and LNC, but greater height.
Conclusions
Our study demonstrates that extreme drought can impact community productivity and structure, and highlights the importance of plant traits, community composition and soil properties in modulating the responses and resistance of steppe communities to extreme drought.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an ecosystem attribute, resistance is usually defined as the capacity of an ecosystem to remain relatively stable levels of structure and function in face of disturbance or extreme climatic fluctuations (Grime et al. 2000; Grman et al. 2010; Pfisterer and Schmid 2002). Mechanisms of ecosystem resistance have been a hot topic in ecology and have been intensively studied, but understanding is still limited due to the complexity of natural ecosystems (Donohue et al. 2013). A large number of studies have assessed community resistance to changes in precipitation, temperature and climate extremes by evaluating the changes of productivity, diversity, species composition and functional traits (Byrne et al. 2017; Griffin-Nolan et al. 2019; Knapp et al. 2015; Ruppert et al. 2015). However, the ecological mechanisms underlying community resistance are far from clear, probably because of previous preponderance of exploration of aboveground rather than belowground responses (Li et al. 2019).
Extreme drought events occur more and more frequently and produce profound influences on grassland ecosystem structure and functions through changing soil water content (Barbeta et al. 2015; Dong et al. 2011; Evans et al. 2011; Vicente-Serrano et al. 2013). Up to now, aboveground net primary productivity (ANPP) is the most common indicator or function for evaluating the community resistance to extreme drought (Hoover et al. 2014; Knapp et al. 2015; Ruppert et al. 2015), despite that belowground net primary productivity (BNPP) accounts for > 60% of total productivity especially in arid and semi-arid grasslands (Fan et al. 2009; Gao et al. 2011; Milchunas and Lauenroth 2001). Recent studies suggest that BNPP responses to reduced precipitation are different in magnitude or even opposite ways from those of ANPP (Byrne et al. 2013; Wilcox et al. 2017). Such difference in resistance between ANPP and BNPP is often explained by the optimal partitioning theory (OPT) (Shipley and Meziane 2002). According to OPT, plants usually allocate more biomass into roots for water uptake in response to decreased precipitation, thus leading to increased fraction of BNPP to NPP (referred to as fBNPP hereafter) and enhanced BNPP resistance (Mokany et al. 2006; Xu et al. 2015). However, OPT was not universally supported by empirical studies, and the plasticity for biomass allocation varied among communities with different soil water contents (Bernacchi et al. 2000; Frank 2007; Meier and Leuschner 2008). Hence, it is necessary to simultaneously consider both ANPP and BNPP when evaluating community resistance to extreme drought, because there is possibility of introducing bias when only ANPP is taken into account.
Since ecosystem attributes and environmental contexts varied among ecosystems or even among communities of the same grassland type, it is likely that extreme drought do not have consistent effects across grassland communities. Actually, it has been proved that soil water is an important limiting factor impacting communities’ responses to environmental changes, and that the arid and semiarid plant communities had weak responses to precipitation reduction (Gilgen and Buchmann 2009; Tielbörger et al. 2014; Vicente-Serrano et al. 2013), i.e., they had relatively high drought resistance. It was also reported that drought resistance in terms of ANPP and BNPP may be affected by community functional composition, such as community-weighted trait mean (CWM) and functional dispersion (FDis) (Griffin-Nolan et al. 2019). For instance, the resistance of community in dry site may be facilitated via selecting those species with lower leaf nitrogen content (LNC) and/or specific leaf area (SLA), the traits enabling plants to tolerate water deficits (Lavorel and Garnier 2002; Luo et al. 2018). Meanwhile, communities with higher drought-resistance may have a lower FDis value due to greater similarity of the coexisting species in related plant traits. Thus, comparing community responses to extreme drought among sites will help to uncover the potential mechanisms underlying differential resistance.
Hulunbeir grassland located in the east of Eurasian Steppes, providing a major forage food supply for domestic livestock in northeast China (Dong et al. 2011; Wang and Ba 2008). Although extreme droughts are predicted to increase dramatically in this area (Dai 2013; Li et al. 2017), yet their ecological consequences for this meadow steppe are scarcely explored, hampering the identification of the mechanisms underlying the resistance to climatic extremes. Therefore, we conducted a standardized extreme drought manipulative experiment by reducing 66% of the growing season precipitation over four consecutive years at two sites within Hulunbeir grassland. The two sites have same dominant species, species richness and average annual precipitation but differ in soil physical properties such as soil texture. Here, we attempt to address the following questions: (1) how does extreme drought affect the aboveground and belowground net primary productivity (ANPP and BNPP respectively), their allocation and the vertical distributions of BNPP in the soil? (2) how does the community-level drought resistance (in terms of drought-responses of ANPP and BNPP) differ between the two grassland sites? (3) how is the drought resistance of ANPP and/or BNPP at the community level related to functional traits and soil properties?
Materials and methods
Study area
In 2015, the experiment was carried out simultaneously at two grassland sites in Hulunbeir, east Inner Mongolia, China. One site was located in the field plot of the Erguna Forest-Steppe Ecotone Research Station, Institute of Applied Ecology, CAS (Sandy: 50.16°N, 119.39°E), and the other site was located nearby the National Hulunbeir Grassland Ecosystem Observation and Research Station, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences (Loamy: 49.35°N, 120.01°E). These grassland sites are quite well representative of the vegetation of the region, and have been enclosed to exclude grazing for at least three years when our experiments started. Both sites are classified as temperate steppe, with 80% of the annual precipitation falling from May to August. The mean annual precipitation is 362 mm and 349 mm, and the mean annual temperature is -2.4 °C and − 1.1 °C at the Sandy and Loamy sites (1957–2016), respectively.
Both communities are dominated by C3 perennial rhizomatous grass, Leymus chinensis. Common sub-dominant species include the perennial grass Stipa baicalensis and forbs Carex duriuscula and Pulsatilla turczaninovi at the Sandy site, and include the forbs P. turczaninovi, Artemisia tanacetifolia and Iris ventricosa at the Loamy site. The two grassland sites differ noticeably in soil properties, which, at least in part, lead to distinct community productivity and species abundance ranking within their communities, and may result in distinct drought resistance. Soil at the Loamy site not only has higher saturated moisture content, but also hold greater available P, total C and N than at the Sandy site. Meanwhile, soil at the Loamy site has lower bulk density, percentage of sands and available N, and is covered with thicker litter layer than at the Sandy site (Table 1).
Experimental design
At both sites, the experiment used a randomized block design with two treatments, including control and the drought treatment by intercepting 66% of growing season (May-August) precipitation from 2015 to 2018. The drought treatment can reduce the annual precipitation by approximately 50%, and actually result in an extreme drought, which is defined as annual precipitation below the 5th percentile of the historical probability distribution (Fig. S1) (Hoover et al. 2014; Luo et al. 2018; Zhang et al. 2019). Each treatment had six replicates. Each experimental plot was 36 m2 (6 m × 6 m) in size, including a central zone (4 m × 4 m) and a 1 m wide buffer belt at the periphery of the central zone to minimize edge effects. The buffer belt in each plot always received exactly the same treatment as the central zone. In each drought manipulated plot, the interception of 66% growing season rainfall was fulfilled by installing a rainout shelter and evenly arraying polypropylene strips to shield 2/3 vertical projected area of shelter roof, while minimally affecting microclimate (Fig. S1 and S2) (Hoover et al. 2014; Luo et al. 2018). To prevent the external subsurface water permeation into the experimental plots, watertight aluminum plates were buried around the plots by trenching to a depth of 1 m. Adjacent plots were located 2 m apart.
Measuring and monitoring
Before the start of the experiment, a square-shaped quarter (2 m × 2 m in size) of the central zone within each plot was selected and further divided into four quadrats (1 m × 1 m), which would be used in the four consecutive years from 2015 to 2018 (each quadrate for a year). Within each quadrate, two diagonal sub-quadrats (50 cm × 50 cm) were used to survey community composition and ANPP, while the other two sub-quadrates were used for functional trait investigation. During the peak of plant growth in each growing season, all aboveground live plant material was harvested in the ANPP survey sub-quadrates, and the plant samples were pooled together, sorted into species, then oven-dried and weighed. The aboveground biomass of the main quadrate was calculated as the sum of aboveground dry weight of all the species present in plant samples from the quadrats. We classified the species into two functional groups, i.e., forbs and grasses, to evaluate the difference of their responses to drought. ANPP was calculated as the aboveground biomass per square meter. We also calculated species richness (number of species present in each main plot), Shannon diversity index and evenness index for experimental plots (based on species relative biomass).
To examine the drought response of water-use efficiency at species level, we selected several dominant species in both control and drought-treated plots in 2016 and measured the foliar carbon isotope composition (δ13C). δ13C was determined using Finnigan continuous flow isotope ratio mass spectrometer (Delta C) via a ConFlo II interface (Thermo Finnigan, Bremen, Germany).
In 2017 and 2018, plant height, specific leaf area (SLA), leaf dry matter content (LDMC) and leaf nitrogen content (LNC) were measured for those species whose cumulative relative abundance reached at least 80% at each community. Five individuals (defined as clonal ramets and clusters for rhizomatous and bunch grass respectively) of each species per plot were selected and their traits were measured following standard protocols (Pérez-Harguindeguy et al. 2013).
BNPP was measured using root ingrowth core method (Persson 1980), from 2016 to 2018. In early May of the three years, two holes 5 cm in diameter were drilled to 20 cm deep into the ground at each plot, and re-filled with original soil surrounded by nylon net mesh (2 mm) from which original roots were already removed. At the end of August, soil cores were extracted, and each soil core was divided into 0 ~ 10 cm and 10 ~ 20 cm segments. Roots therein were picked out by hand washing, oven-dried and weighed to achieve dry weight. The dry root weight per square meter in the 0 ~ 10 cm and 10 ~ 20 cm segment were calculated as the belowground net primary productivity for the two layers (hereafter referred to as BNPP0 − 10 and BNPP10 − 20 respectively). So in the present study, the total BNPP was equaled to the sum of the BNPP0 − 10 and BNPP10 − 20 as defined above. The fBNPP was calculated as dividing BNPP by total NPP (BNPP/ [ANPP + BNPP]).
Four soil cores (0 ~ 10 cm) were collected from the two biomass survey sub-quadrats in each plot in August 2017 using a soil auger (3.5-cm diameter), and the soil samples were mixed by hand, and then passed through 2 mm sieve. Each sieved soil sample was divided into two sub-samples, with one stored at 4 °C as fresh soil for later measurement of the gravimetric moisture content, NH4+ and NO3−, with the other one dried and stored at room temperature for later measurement of total N, total C, pH, and available P. Fresh soil (10 g) was dried at 105 °C for 48 h to determine the gravimetric moisture content, and additional fresh soil (8 g) was mixed into 40 ml of 2 mol/L KCL, and the extract was used to measure NH4+ and NO3− using discontinuous analyzer (Cleverchem 200+, DeChem-Tech. GmbH, Germany). Total C and N were determined by Elementar, Vario EL III, and pH values were measured by PHS-25 pH meter. Available P was analyzed by the molybdenum blue-ascorbic acid method. Soil saturated moisture content was measured using cutting ring method only for the soil from control plots. Particle size analysis was conducted using Hydrometer method, with particles larger than 0.05 mm in size classified as sands and those smaller than 0.05 mm in size classified as clay and silt (Table S2).
Soil moisture sensors (PG-110; Jingchuang Electronic Technology Co., Handan, China) were installed in the center of each plot in the early spring of 2015, used to continuously measure 0 ~ 10 cm soil moisture and temperature in situ. Data from sensors were collected using Campbell Scientific CR1000. Daily precipitation data during 2016 to 2017 were obtained from the meteorological station near experimental area to evaluate the amount and proportion of reduced natural rainfall due to drought manipulation.
Statistical analyses
Community trait means for height, SLA, LDMC and LNC were calculated by the biomass-weighted average of all measured species in each plot according to Lepš et al. (2011).
where pi is the relative biomass of species i, xi is the trait value of species i, and S is the plant species number in the plot.
Functional dispersion (FDis), defined as the dissimilarity of functional trait among co-existing species (Laliberté and Legendre 2010), was quantified for height, SLA, LDMC and LNC, separately, using the dbFD function in the R package FD. FDis is calculated as:
where aj is the relative biomass of species j and zj is the distance of species j to the weighted centroid c. The centroid c is calculated as:
where xij is the value of species j for trait i.
For each site, the effects of extreme drought on all the response variables (including NPP components, species richness, diversity and evenness, as well as functional traits) were analyzed using repeated-measures mixed-effect models with drought as fixed factor, year as repeated factor, and block as a random factor. When interactive effects of drought treatment and year were significant, a post-hoc test of the binary treatments for each year was performed. To explain how community changes impact drought resistance for various productivity metrics, we performed repeated-measures mixed-effect models based on the later two years of sampling (2017 and 2018), with drought and richness as fixed factors, year as repeated factor, and block as a random factor. We also assessed the correlation of community productivity components with functional traits and species composition using mixed effect models with block as a random factor and year as a repeated factor.
Since δ13C value was measured only in 2016 for the selected species, the effect of extreme drought on δ13C was analyzed using mixed-effect model with drought as fixed factor, species as random factor nested within block.
To quantify the magnitude and direction of each individual species’ contribution to total ANPP response to extreme drought, we calculated a ratio of biomass change for a given species to summed absolute value of biomass changes for all species in the drought-manipulated plots compared with the control plots in each year, separately. To quantify the community structural difference between treatments at each site for each year, the analysis of similarities (ANOSIM) was conducted using species relative biomass and presence/absence data.
The community resistance was calculated for ANPP and BNPP as follows, referring to Isbell et al. (2015):
where \({\overline{Control_{Y}}}\) is the mean productivity value of control for Y year, and \({\overline{Drought_{Y}}}\) is mean productivity value of drought treatment for Y year. The low resistance value is characteristic of large proportional changes of community productivity, indicating higher drought sensitivity, and vice versa. The resistance has no upper boundary. All statistical analyses were performed in R 3.4.2 (R Core Team 2017).
Results
Validity of the experimental treatment
During the 4-yr experiment, both the annual mean precipitation and growing season precipitation were at similar level between the Sandy and the Loamy site (Table S1). Experimental drought reduced growing season precipitation to the level below the 5th percentiles of historic precipitation probability distribution functions based on nearly fifty year records for both sites, which is defined as an extreme drought (Fig. S1). Average soil moistures were 23% and 35% in control plots, and were reduced to 13% and 21% in extreme drought treatment plots at the Sandy and Loamy site respectively (Fig. S3), suggestive of the validity of the experimental treatment.
Productivity response to extreme drought
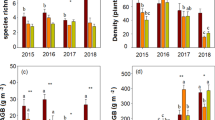
Under ambient conditions, the community at the Loamy site had greater ANPP and BNPP than at the Sandy site (Fig. 1). In response to experimental drought, the communities at the two sites behaved very differently: ANPP was significantly reduced by 45% and BNPP was kept unchanged at the Sandy site, while at the Loamy site ANPP and BNPP were significantly or marginally reduced by 11% and 15%, respectively (Fig. 1; Table 2). As a result, fBNPP was significantly increased at the Sandy site but was kept unchanged at the Loamy site (Fig. 3a). Apparently, BNPP showed greater drought resistance than ANPP at the Sandy site (t=-4.8, P = 0.035), but there was non-significant difference in drought resistance between ANPP and BNPP at the Loamy site (t = 0.12, P = 0.906). In the later two years of the experiment (2017 and 2018), both richness and drought showed significant effect on ANPP at the Sandy site, but only drought did at the Loamy site (Table S6).
Responses of above- (ANPP) and belowground net primary productivity (BNPP) to experimentally imposed growing season drought at the Sandy (a) and Loamy site (b). The open bars indicate the control, and the closed bars indicate the drought treatment. Statistical significance is depicted as: ^, 0.05 < p < 0.1; *, 0.01 < p < 0.05; **, 0.05 < p < 0.01; ***, p < 0.001
Experimental drought significantly reduced the ANPP of forbs by 63% and 23%, at the Sandy and Loamy sites, respectively, but reduced that of grasses (31%) only at the Sandy site (Fig. 2; Table 2). At the Sandy site, experimental drought significantly decreased the growth of P. turczaninovii, S. baicalensis and C. duriuscula, but not that of L. chinensis (Tables 3 and 4 and S3). At the Loamy site, experimental drought had no significant effect on growth of all the four species (including L. chinensis, A. tanacetifolia, I. ventricosa and P. turczaninovii), the main contributors to the changes of community biomass (Tables 3 and 4 and S3).
Responses of grass and forb productivity to experimentally imposed growing season drought at the Sandy (a) and Loamy site (b). The open circles indicate the control, and the closed circles indicate the drought treatment. Statistical significance is depicted as: ^, 0.05 < p < 0.1; *, 0.01 < p < 0.05; **, 0.05 < p < 0.01; ***, p < 0.001
Experimental drought significantly decreased BNPP0 − 10 by 33% and 37%, and significantly increased BNPP10 − 20 by 44% and 42%, at the Sandy and Loamy site, respectively (Fig. 3b, c). At the Sandy site, BNPP0 − 10 show significantly positive relationship with ANPP of both grass and forb, and BNPP10 − 20 show significantly negative relationship only with ANPP of forb; At the Loamy site, BNPP0 − 10 and BNPP10 − 20 show significantly positive and negative relationship with ANPP of forb and grass, respectively (Table S9). In the later two years of the experiment (2017 and 2018), BNPP0 − 10 and BNPP10 − 20 were affected by richness at the Sandy site, however, they were both affected by richness and drought at the Loamy site (Table S6).
Responses of fBNPP, BNPP0 − 10 and BNPP10 − 20 to experimentally imposed growing season drought at the Sandy site and the Loamy site. The open bars indicate the control, and the closed bars indicate the drought treatment. Statistical significance is depicted as: ^, 0.05 < p < 0.1; *, 0.01 < p < 0.05; **, 0.05 < p < 0.01; ***, p < 0.001
Responses of species diversity and functional traits to extreme drought
On average, 15 species per square meter were observed in the control plots of both sites, and species richness and diversity were significantly reduced by experimental drought (Table 2 and Fig. S4). Relative to the Loamy site, community structure in the Sandy site was significantly altered by experimental drought in 2017 and 2018, and a significant negative drought effect on evenness was observed (Table 2 and Table S4).
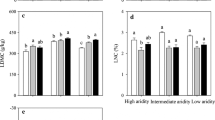
Under experimental drought, community-weighted mean and functional dispersion of SLA and LDMC remains unchanged at both sites. Community-weighted plant height decreased at both sites, while functional dispersion of plant height decreased at the Sandy site but remain unchanged at the Loamy site (Fig. 4 and Table S5). Conversely, community-weighted LNC increased at the Sandy site but remained unchanged at the Loamy site, while the functional dispersion of LNC decreased at both sites (Fig. 4 and Table S5). Species at the Loamy site showed lower δ13C than at the Sandy site (F = 196, P < 0.0001), and experimental drought did not exert any significant effect on δ13C at either site (Fig. 5). Communities at the Loamy site showed smaller SLA (F = 28.2, P < 0.0001) and LNC (F = 28.2, P < 0.0001), but greater height (F = 19.1, P = 0.001) than at the Sandy site (Fig. 4).
Response of community-weighted trait means and functional dispersion to experimentally imposed growing season drought at the Sandy and Loamy site in 2017 and 2018. The open bars indicate the control, and the closed bars indicate the drought treatment.. Statistical significance is depicted as: ^, 0.05 < p < 0.1; *, 0.01 < p < 0.05; **, 0.05 < p < 0.01; ***, p < 0.001
Discussion
Briefly, plant communities were affected by experimental drought, and their resistance differed between the two study sites. First, experimental drought biased the NPP allocation towards belowground by almost halving the ANPP at the Sandy site, while kept it unchanged at the Loamy site by reducing ANPP and BNPP simultaneously but to a lesser extent. Second, experimental drought affected the community structure reflected as the effects on functional groups (forb and grass) and species, but much more severely at the Sandy site than at the Loamy site. Moreover, experimental drought deepened the BNPP distribution and also reduced species richness and diversity at both sites. Communities and most species at the Sandy site showed greater δ13C, SLA and LNC, but smaller plant height than at the Loamy site. Under experimental drought, plant height decreased at both sites, but converged only at the Sandy site, whereas LNC converged at both sites, but its community-level mean increased only at the Sandy site. BNPP in both sites appeared less sensitive to extreme drought. Instead, total ANPP was reduced in the year starting extreme drought treatment at the Sandy site, while such reduction was not observed until the fourth year at the Loamy site, combining with the fact that community composition was altered significantly at the Sandy site but remained relatively stable at the Loamy site, suggesting that the communities at the Sandy site had higher resistance to drought. The responses and the resistance of the communities to extreme drought may be modulated by functional traits, community structure and soil water conditions.
From perspective of plant adaptation, the different resistance between the two sites might be attributed to distinct plant strategy of resource utilization reflected by functional leaf traits. Indeed, we found a great difference between the two sites in such functional traits of communities or species as plant height, SLA, leaf δ13C and LNC (Table 4 and S7), which are closely related to resource use strategies (Wright et al. 2004). Smaller SLA and LNC at the Loamy site suggested that communities were dominated by resource-conservative species, which may be less sensitive to resource changes like extreme drought (Reich 2014). The community-weighted mean and functional dispersion of height and LNC at the Sandy site were changed by experimental drought and linked to the changes of ANPP (Table S8), implying that experimental drought might have acted as an environmental filter to limit interspecific trait expression and thus affected the community aboveground productivity. Furthermore, we observed that all the studied species at the Sandy site had larger water use efficiency (lower leaf δ13C) than those at the Loamy site, even for co-dominant species L. chinensis and P. turczaninovii (Table 4 and S7). Since there’s little C4 plant species present at either site, we cannot refer to the C3 vs. C4 difference to explain this δ13C value discrepancy. We suspected that the higher δ13C value at the Sandy site is the evolutionary outcome under the long-term pressure of soil water deficit or that the plasticity in their morpho-physiology may play an important role for these species to behave more water efficient. Comparatively, at the Loamy site, the soil water deficit was not so severe due to the higher soil water content even under the similar ambient precipitation.
In our study, reduction in total ANPP was mainly driven by forbs at both sites. This suggested that forbs were more vulnerable to extreme drought than grasses, which coincides with previous work (Chelli et al. 2016; Hoover et al. 2014). Moreover, at the Sandy site the dominant species L. chinensis exhibited greater drought resistance than sub-dominants species, usually forbs, resulting in a significant shift in species composition. Many forbs have simpler taproots, while lack a large, extensive fibrous root system as grasses, which likely allows better tolerance to drought. Greater drought resistance for L. chinensis at the Sandy site may also benefit from its higher LNC, as plants usually increase their LNC with increasing natural aridity or under experimental drought (Liu et al. 2010; Luo et al. 2018). Increasing LNC would enhance photosynthesis and/or nutrient-rich soluble compound accumulation (Luo et al. 2015), helping plants better adapt to drought. Instead, we found no significant drought effect on individual species at the Loamy site, and this further supported that soil water deficit was not so severe at the Loamy site. In addition, we observed great difference in functional traits of L. chinensis between the two sites, as reflected by height, leaf δ13C, SLA, LDMC and LNC. Such variation in functional traits through phenotypic plasticity or genetic variation was speculated to help L. chinensis adapt to different soil water conditions. Although the communities at both sites were dominated by L. chinensis, they still exhibited different drought resistance in the present study. This implied that same dominant species does not necessarily render the communities similarly resistant to drought, because the drought-resistance of the communities could also be influenced by other factors, such as functional structure of communities and soil properties. However, L. chinensis was still important at both sites, and perhaps more important in the Sandy site.
Unlike ANPP, total BNPP at both sites was not significantly affected by experimental drought, suggesting that BNPP apparently had greater drought resistance than ANPP. Generally, root fraction was sensitive to changes of soil water content, because root allocation would always be adjusted to attain an optimal match between the above- and belowground resource acquisition, particularly in time of water shortage (Li et al. 2011; Pang et al. 2011; Xu et al. 2015). Although the drought-induced increase of community-level fBNPP at the Sandy site is obviously resulted by the unchanged BNPP and largely reduced ANPP, we still believe that the increase of fBNPP was a combination of coordinated plastic responses of every plant individual within the community, and the species turnover towards a more drought-resistant composition (Table S6 and S9). This suggested that communities at this place have great plasticity in root vs. shoot allocation and are able to allocate proportionally more biomass into roots, the structures responsible for water acquisition. Instead, the unchanged fBNPP in response to experimental drought at the Loamy site suggested that the communities had not experienced such high water limitation that could stimulate the adjustment of root vs. shoot allocation.
Different responses of biomass allocation between the two sites implied that it was not necessary for the communities to alter biomass allocation, unless extreme drought occurred. Instead, the communities tend to preferentially alter their vertical root distribution rather than adjust their root vs. shoot allocation in response to the experimental drought, as found by Zhang et al. (2019). In the present study, we did observe deepened root vertical distribution in response to experimental drought at both sites; however, changing of vertical root distribution mainly resulted from simultaneous increase of BNPP in 10–20 cm and decrease of BNPP in 0–10 cm. We acknowledged that roots deeper than 20 cm are not quantified, root sampling depth is insufficient to declare the panorama of BNPP, and root growth at deeper layer may be most important in regulating plant drought resistance. Therefore, deepened root vertical distribution and increased fBNPP are not to be taken as absolute estimates. Despite that, BNPP in 0–20 cm soil depth accounted for nearly 70 percent of belowground biomass according to the survey by Ma et al. (2008). After all, even based on this upper layer roots, we did observe the deepening of the root production in response to extreme drought, which is an important result that we expected. Adjusting root vertical distribution in response to experimental drought indicated strong root plasticity and flexible biomass allocation for plants to maximize water uptake. Under increased drought, the roots would be deepened because deeper roots are more accessible to water and thus facilitate their drought-resistance (Comas et al. 2013; Uga et al. 2013). Furthermore, we inferred that alteration of root vertical distribution at community level under drought condition was also driven by species composition changes and/or plastic adaptation (Table S6 and S9). For example, many forbs were identified as the shallow-rooted species, and decreasing proportion of forbs under experimental drought might underlie the lower BNPP in the shallow soil (Table S9). Instead, many grasses had a larger and deeper, more extensive fibrous root system and stronger capacity to shift the root vertical distribution (Skinner and Comas 2010). Therefore, the increased BNPP in 10–20 cm soil layer might be driven mainly by grasses.
Difference in drought resistance in terms of ANPP between the two sites could be attributed to the discrepancy of soil water content, as they were sharing dominant species, bearing same species richness and experiencing similar precipitation, which were usually identified as main drivers of variation in resistance for aboveground community attributes (Byrne et al. 2017; Isbell et al. 2015; Knapp et al. 2015; Sasaki and Lauenroth 2011). In our study, it may be such soil physical properties as smaller bulk density and finer texture that render better soil water condition at the Loamy site rather than at the Sandy site, leading to higher ANPP. As a result, greater litter coverage due to higher ANPP would further improve soil physical properties by increasing soil organic matter content, so a benign cycling was formed. In addition, the thicker litter layer, higher soil organic carbon and smaller specific leaf area would be more effective in preventing soil water losing through evaporation and plant transpiration. Both greater soil water retention and lower evapotranspiration resulted in higher soil water content at the Loamy than at the Sandy site. Indeed, soil moisture at the Loamy site under experimental drought was still about 20%, i.e., at the similar level as the control plots of the Sandy site. Thus, plant growth at the Sandy site was strongly limited by soil water and thus appeared more sensitive to experimental drought. Higher drought-resistance in terms of ANPP at the Loamy site suggested that soil water was not so scarce as to limit plant growth so much as at the Sandy site. Although the productivity of the temperate grasslands was usually co-limited by nitrogen and water (Lü et al. 2018), there is no evidence showing that nitrogen limitation levels differ between the two sites.
Conclusions
The responses and resistance of the steppe communities in terms of aboveground & belowground net primary productivity and their allocation are subject to physiological, morphological and community-level adjustments. Our study supports the theory of hierarchical reaction of plant communities, i.e., in response to drought, physiological reaction (modifying LNC and water use efficiency) is always the first, probably due to its reversibility and thus least costly and risky, then followed by morphological adjustment (changing productivity allocation, plant height and SLA), and lastly by community-level species turnover (shifting proportion of forbs and grass). Despite of the limitation of the experiment, i.e., lack of rigorous test due to only two study sites incorporated, our study highlighted the importance of plant traits, community composition and soil properties in modulating drought-resistance of plant communities in grasslands, and at least clued us on the underlying mechanisms.
References
Barbeta A, Mejia-Chang M, Ogaya R, Voltas J, Dawson TE, Penuelas J (2015) The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob Chang Biol 21:1213–1225
Bernacchi C, Coleman J, Bazzaz F, McConnaughay K (2000) Biomass allocation in old-field annual species grown in elevated CO2 environments: no evidence for optimal partitioning. Glob Change Biol 6(7):855–863
Byrne KM, Lauenroth WK, Adler PB (2013) Contrasting effects of precipitation manipulations on production in two sites within the central grassland region USA. Ecosystems 16(6):1039–1051
Byrne KM, Adler PB, Lauenroth WK (2017) Contrasting effects of precipitation manipulations in two Great Plains plant communities. J Veg Sci 28(2):238–249
Chelli S, Canullo R, Campetella G, Schmitt AO, Bartha S, Cervellini M, Wellstein C (2016) The response of sub-Mediterranean grasslands to rainfall variation is influenced by early season precipitation. Appl Veg Sci 19(4):611–619
Comas L, Becker S, Cruz VM, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4(442):442
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3(1):52–58
Dong G, Guo J, Chen J, Sun G, Gao S, Hu L, Wang Y (2011) Effects of spring drought on carbon sequestration, evapotranspiration and water use efficiency in the Songnen meadow steppe in northeast China. Ecohydrology 4(2):211–224
Donohue I, Petchey OL, Montoya JM, Jackson AL, McNally L, Viana M, Healy K, Lurgi M, O’Connor NE, Emmerson MC (2013) On the dimensionality of ecological stability. Ecol Lett 16(4):421–429
Evans SE, Byrne KM, Lauenroth WK, Burke IC (2011) Defining the limit to resistance in a drought-tolerant grassland: long‐term severe drought significantly reduces the dominant species and increases ruderals. J Ecol 99:1500–1507
Fan JW, Wang K, Harris W, Zhong HP, Hu ZM, Han B, Zhang WY, Wang JB (2009) Allocation of vegetation biomass across a climate-related gradient in the grasslands of Inner Mongolia. J Arid Environ 73(4):521–528
Frank DA (2007) Drought effects on above-and belowground production of a grazed temperate grassland ecosystem. Oecologia 152(1):131–139
Gao YZ, Chen Q, Lin S, Giese M, Brueck H (2011) Resource manipulation effects on net primary production, biomass allocation and rain-use efficiency of two semiarid grassland sites in Inner Mongolia, China. Oecologia 165(4):855–864
Gilgen AK, Buchmann N (2009) Response of temperate grasslands at different altitudes to simulated summer drought differed but scaled with annual precipitation. Biogeosciences 6(11):2525–2539
Griffin-Nolan RJ, Blumenthal DM, Collins SL, Farkas TE, Hoffman AM, Mueller KE, Ocheltree TW, Smith MD, Whitney KD, Knapp AK, Jones H (2019) Shifts in plant functional composition following long‐term drought in grasslands. J Ecol 107(5):2133–2148
Grime JP, Brown VK, Thompson K, Masters GJ, Hillier SH, Clarke IP, Askew AP, Corker D, Kielty JP (2000) The response of two contrasting limestone grasslands to simulated climate change. Science 289(5480):762–765
Grman E, Lau JA, Schoolmaster DR, Gross KL (2010) Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett 13(11):1400–1410
Hoover DL, Knapp AK, Smith MD (2014) Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95(9):2646–2656
Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, Bezemer TM, Bonin C, Bruelheide H, de Luca E, Ebeling A, Griffin JN, Guo Q, Hautier Y, Hector A, Jentsch A, Kreyling J, Lanta V, Manning P, Meyer ST, Mori AS, Naeem S, Niklaus PA, Polley HW, Reich PB, Roscher C, Seabloom EW, Smith MD, Thakur MP, Tilman D, Tracy BF, van der Putten WH, van Ruijven J, Weigelt A, Weisser WW, Wilsey B, Eisenhauer N (2015) Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526(7574):574–577
Knapp AK, Carroll CJW, Denton EM, La Pierre KJ, Collins SL, Smith MD (2015) Differential sensitivity to regional-scale drought in six central US grasslands. Oecologia 177(4):949–957
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91(1):299–305
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16(5):545–556
Lepš J, de Bello F, Šmilauer P, Doležal J (2011) Community trait response to environment: disentangling species turnover vs intraspecific trait variability effects. Ecography 34(5):856–863
Li JZ, Lin S, Taube F, Pan Q, Dittert K (2011) Above and belowground net primary productivity of grassland influenced by supplemental water and nitrogen in Inner Mongolia. Plant Soil 340(1):253–264
Li DH, Zou LW, Zhou TJ (2017) Changes of extreme indices over China in response to 1.5℃ global warming projected by a regional climate model. Adv Earth Sci 32(4):446–457
Li LF, Zheng ZZ, Biederman JA, Xu C, Xu ZH, Che RX, Wang YF, Cui XY, Hao YB (2019) Ecological responses to heavy rainfall depend on seasonal timing and multi-year recurrence. New Phytol 223(2):647–660
Liu GF, Freschet GT, Pan X, Cornelissen JHC, Li Y, Dong M (2010) Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol 188(2):543–553
Lü XT, Liu ZY, Hu YY, Zhang HY (2018) Testing nitrogen and water co-limitation of primary productivity in a temperate steppe. Plant Soil 432(1):119–127
Luo WT, Elser JJ, Lü XT, Wang ZW, Bai E, Yan CF, Wang C, Li MH, Zimmermann NE, Han XG, Xu ZW, Li H, Wu Y, Jiang Y (2015) Plant nutrients do not covary with soil nutrients under changing climatic conditions. Glob Biogeochem Cycles 29(8):1298–1308
Luo WT, Zuo XA, Ma W, Xu C, Li A, Yu Q, Knapp AK, Tognetti R, Dijkstra FA, Li MH, Han GD, Wang ZW, Han XG (2018) Differential responses of canopy nutrients to experimental drought along a natural aridity gradient. Ecology 99(10):2230–2239
Ma WH, Yang YH, He JS, Zeng H, Fang JY (2008) Above- and belowground biomass in relation to environmental factors in temperate grasslands, Inner Mongolia. Sci China Ser C Life Sci 51(3):263–270
Meier IC, Leuschner C (2008) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14(9):2081–2095
Milchunas DG, Lauenroth WK (2001) Belowground primary production by carbon isotope decay and long-term root biomass dynamics. Ecosystems 4(2):139–150
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Change Biol 12(1):84–96
Pang JY, Yang JY, Ward P, Siddique KHM, Lambers H, Tibbett M, Ryan M (2011) Contrasting responses to drought stress in herbaceous perennial legumes. Plant Soil 348(1):299
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61(3):167
Persson H (1980) Fine-root dynamics in a Scots pine stand with and without near-optimum nutrient and water regimes. Acta Phytogeographica Suecica 68:101–110
Pfisterer AB, Schmid BJN (2002) Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416(6876):84–86
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102(2):275–301
Ruppert JC, Harmoney K, Henkin Z, Snyman HA, Sternberg M, Willms W, Linstadter A (2015) Quantifying drylands’ drought resistance and recovery: the importance of drought intensity, dominant life history and grazing regime. Glob Chang Biol 21(3):1258–1270
Sasaki T, Lauenroth WK (2011) Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 166(3):761–768
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16(3):326–331
Skinner RH, Comas LH (2010) Root distribution of temperate forage species subjected to water and nitrogen stress. Crop Sci 50(5):2178–2185
Team RC (2017) R: a language and environment for Statitical computing. R Fundation for Statistical Computing, Vienna. Retrieved from http://www.R-project.org/
Tielbörger K, Bilton MC, Metz J, Kigel J, Holzapfel C, Lebrija-Trejos E, Konsens I, Parag HA, Sternberg M (2014) Middle-Eastern plant communities tolerate 9 years of drought in a multi-site climate manipulation experiment. Nat Commun 5:5102
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45(9):1097–1102
Vicente-Serrano SM, Gouveia C, Camarero JJ, Beguería S, Trigo R, López-Moreno JI, Azorín-Molina C, Pasho E, Lorenzo-Lacruz J, Revuelto J, Morán-Tejeda E, Sanchez-Lorenzo A (2013) Response of vegetation to drought time-scales across global land biomes. Proc Natl Acad Sci 110(1):52–57
Wang D, Ba L (2008) Ecology of meadow steppe in northeast China. Rangel J 30(2):247–254
Wilcox KR, Shi Z, Gherardi LA, Lemoine NP, Koerner SE, Hoover DL, Bork E, Byrne KM, Cahill J Jr, Collins SL, Evans S, Katarina Gilgen A, Holub P, Jiang L, Knapp AK, LeCain D, Liang J, Garcia-Palacios P, Penuelas J, Pockman WT, Smith MD, Sun S, White SR, Yahdjian L, Zhu K, Luo Y (2017) Asymmetric responses of primary productivity to precipitation extremes: a synthesis of grassland precipitation manipulation experiments. Glob Change Biol 23(10):4376–4385
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428(6985):821–827
Xu W, Cui KH, Xu AH, Nie LX, Huang JL, Peng SB (2015) Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant 37(2):9
Zhang BW, Cadotte MW, Chen SP, Tan XR, You CH, Ren TT, Chen ML, Wang SS, Li WJ, Chu CJ, Jiang L, Bai YF, Huang JH, Han XG (2019) Plants alter their vertical root distribution rather than biomass allocation in response to changing precipitation. Ecology 10(11):e02828
Acknowledgements
We are grateful to Professor Xiaoan Zuo and several students for additional help in collecting the data. We thank all who worked in the Extreme Drought in Grasslands Experiment (EDGE) project for assistance in planning and conducting the field experiment. This work is funded by the National Key Research and Development Program of China (2016YFC0500602) and the National Natural Science Foundation of China (31870422, 31570530).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Janusz J. Zwiazek.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2.54 MB)
Rights and permissions
About this article
Cite this article
Ma, W., Liang, X., Wang, Z. et al. Resistance of steppe communities to extreme drought in northeast China. Plant Soil 473, 181–194 (2022). https://doi.org/10.1007/s11104-020-04767-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04767-y