Abstract
Background and aims
Medicago sativa L. is widely grown in southern Australia, but is poorly adapted to dry, hot summers. This study aimed to identify perennial herbaceous legumes with greater resistance to drought stress and explore their adaptive strategies.
Methods
Ten herbaceous perennial legume species/accessions were grown in deep pots in a sandy, low-phosphorus field soil in a glasshouse. Drought stress was imposed by ceasing to water. A companion M. sativa plant in each pot minimised differences in leaf area and water consumption among species. Plants were harvested when stomatal conductance of stressed plants decreased to around 10% of well watered plants.
Results
A range of responses to drought stress were identified, including: reduced shoot growth; leaf curling; thicker pubescence on leaves and stems; an increased root:shoot ratio; an increase, decrease or no change in root distribution with depth; reductions in specific leaf area or leaf water potential; and osmotic adjustment. The suite of changes differed substantially among species and, less so, among accessions.
Conclusions
The inter- and intra-specific variability of responses to drought-stress in the plants examined suggests a wide range of strategies are available in perennial legumes to cope with drying conditions, and these could be harnessed in breeding/selection programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
South-western Australia has a typical Mediterranean climate, characterised by hot, dry summers that restrict plant growth. In such regions, the replacement of deep-rooted native perennial species by annual crop and pasture species has led to rising water tables and dryland salinity (Dear and Ewing 2008; Lambers 2003) and, thus, to a call for greater inclusion of perennials into farming systems (Cocks 2001; Ward et al. 2006). In recent decades, the perennial pasture legume lucerne (Medicago sativa L.) has been more widely sown in south-western Australia (Cocks 2001). However, it is poorly adapted to the dry, hot summers in areas with <350 mm average annual rainfall (Loo et al. 2006) and to acidic sandy soils (Humphries and Auricht 2001). Furthermore, reliance on a small diversity of species within agricultural systems is considered inadequate to respond to the multitude of environmental conditions present (Dear et al. 2008). So, there is an urgent need to expand the range of perennial legumes that grow well in areas with <350 mm average annual rainfall and can be included in grazing systems where lucerne is either poorly adapted or has reached the limits of adaptation. The provision of out-of-season (summer/autumn) green feed from these novel perennial legumes in a permanent grazing situation or in rotation with crops would provide considerable impetus for adoption.
A large research effort has recently evaluated novel, including undomesticated, native and exotic perennial legumes as alternatives to lucerne in south-western Australia and across Australia (Bell et al. 2006; Li et al. 2008; Snowball et al. 2010). Traits viewed as favourable in novel germplasm include better drought resistance than lucerne and tolerance of low-nutrient, especially low-phosphorus, soils. Novel species, especially Australian natives in the genera Cullen and the exotic Bituminaria bituminosa var. albo-marginata have performed favourably (Bennett et al. 2011; Pang et al. 2010a; Pang et al. 2010b; Suriyagoda et al. 2010). There are few detailed studies on the response of herbaceous perennial legumes to drought stress. The present glasshouse study examined the plant growth response to an imposed drought stress under low-P conditions of a range of perennial legumes with potential for use in Mediterranean pasture systems: B. bituminosa var. albomarginata (3 accessions), C. australasicum (2 accessions), C. pallidum (1 accession), C. cinereum (1 accession), Macroptilium atropurpureum (1 cultivar), Kennedia prostrata (1 accession) and M. sativa (lucerne, 1 cultivar). The specific aims were to (1) identify species with greater tolerance of drought stress than M. sativa, (2) establish whether variation in resistance to drought stress is present among accessions of B. bituminosa var. albomarginata and C. australasicum, and (3) identify the range of strategies employed by the plants to cope with drought stress.
Material and methods
Plant material and growth conditions
Plants (See Table 1 for species details) were grown in 19.1-L free-draining PVC pots (1 m deep and 16 cm diameter). Each pot was cut lengthwise on both sides and securely taped. Pots were filled with yellow loamy sand soil which was air-dried and passed through a 2-mm sieve. Soil analyses on subsamples of soil were conducted by CSBP FutureFarm analytical laboratories (Bibra Lake, Australia). The loamy sand contained 1 μg g−1 of nitrate-N, 1 μg g−1 of ammonium-N, 1 μg g−1 of bicarbonate-extractable P, and had a pH (CaCl2) of 5.6 and a phosphorus-retention index of 4.6. Thus, the soil was extremely low in N and P and had a high P-retention index. In each pot, 400 g of coarse gravel was placed at the bottom and 15 kg of air-dried soil, without extra nutrient supply, was added above the gravel. Another 12 kg of soil mixed thoroughly with basic nutrients was then added on the top. Soil in the pots had a bulk density of 1.41 g cm−3. The basic nutrients provided were 126.6 mg kg−1 Ca(NO3)2.4H2O, 42.8 mg kg−1 NH4NO3, 43.9 mg kg−1 KH2PO4, 178 mg kg−1 K2SO4, 101 mg kg−1 MgSO4.7H2O, 11 mg kg−1 CaCl2.2H2O, 12 mg kg−1 MnSO4.H2O, 8.8 mg kg−1 ZnSO4.7H2O, 1.96 mg kg−1 CuSO4.5H2O, 0.68 mg kg−1 H3BO3, 1.01 mg kg−1 NaMoO4.2H2O and 32.9 mg kg−1 FeNaEDTA. Thus, only 10 mg P kg−1 dry soil was provided in order to mimic a low P agricultural soil, while providing adequate supply of other nutrients. The provision of N, as a mixture of NH4NO3 and Ca(NO3)2, was to ensure an initial adequate supply of N after germination, prior to nodulation by rhizobia.
Before planting, seeds were scarified and pre-germinated in Petri dishes at staggered times according to their pre-determined germination time. Three germinated seedlings were planted in each pot and thinned to one plant after 1 week. In each pot, three M. sativa seedlings were planted at the same time on the other side of the pot, and later thinned to one plant after 1 week. The purpose of planting lucerne in each pot was to minimise differences in leaf area between pots and thus, once watering ceased, to cause each species, irrespective of plant size, to experience a similar degree of drought stress. All seedlings were inoculated with an appropriate strain of rhizobium provided by the Rutherglen Centre, Department of Primary Industries, Victoria, Australia (Table 1). The experiment was carried out in a naturally lit temperature-controlled glasshouse at The University of Western Australia, Perth, Australia. The temperature in the glasshouse ranged from 17°C to 35°C in January 2008 and from 12°C to 28°C in June 2008, and relative humidity was maintained at ~70% during the experiment period.
For each species/accession, 12 pots were set up. Four months after planting, just before drought stress was imposed, four replicate pots of each species/accession were harvested. Of the eight remaining pots, four continued to be well watered while watering ceased for the other four pots. When originally filling pots with soil, in some pots (2 per species/accession per treatment, 40 pots in total), two frequency domain reflectometer (FDR) probes (Model CS615, Campbell Scientific Ltd, Logan, Utah, USA) were installed vertically between 0.05 and 0.35 m and 0.5–0.8 m from the top to obtain hourly changes in volumetric soil water content. The FDR sensor consists of two stainless steel rods (0.3 m long, 0.0032 m in diameter, 0.032 m spacing). For each species, all pots were watered to field capacity every other day as determined by the change in water content in the two pots containing the FDR probes. All pots were watered to field capacity on the afternoon of 11 May 2008; watering was then stopped for the drought-stressed plants. Well watered control pots continued to be watered to field capacity.
Physiological measurements
Predawn leaf water potential was measured (0300–0500 h) in a pressure chamber (Soil moisture Equipment Corp., Santa Barbara, CA, USA) on petioles of young fully expanded leaves. The same leaf samples were immediately placed in a water-tight vial, snap frozen in liquid N2 and stored in a freezer at −20°C. Samples were later thawed, sap expressed using a leaf press, and sap osmolality measured using a freezing point osmometer, which was calibrated against 50 and 850 mOsm kg−1 standard solutions (Fiske Associates, Norwood, MA, USA). Osmotic potential (Ψπ, MPa) of samples was then calculated from osmolality (Eq. 1).
Osmotic adjustment was calculated as the difference in Ψπ at full turgor (Ψπ 100; Eq. 2; i.e. 100% relative leaf water content) between drought-stressed and well watered plants according to the method of Ludlow et al. (1983). Relative leaf water content was determined as outlined by Turner (1981). Whole leaves were removed adjacent to those used for water potential measurements and fresh weight (Leaf FW) measured immediately. Turgid weight (Leaf TW) was measured after whole leaflets were floated on deionised water overnight (approximately 16 h) in a Petri dish placed in a laboratory with ambient air temperature of 25°C. Dry weight (Leaf DW) was measured after samples were dried in an oven at 70°C for 2 days.
Measurements of gas exchange on the youngest fully expanded leaves were carried out between 0900 and 1200 h using a LICOR–6400 with red/blue LED light source (LI-COR, Lincoln, NE, USA). Photosynthetic photon flux density at the leaf surface was set at 1,500 μmol m−2 s−1, leaf temperature at 25°C, flow rate at 500 μmol s−1 and ambient CO2 concentration of incoming gas stream at 380 μmol mol−1.
Plant analyses
After drought stress was imposed, all plants were harvested when stomatal conductance of the youngest fully expanded leaf in the drought-stressed plants was around 10% of that of well watered plants, as stomatal conductance was used as an integrative parameter reflecting the drought stress experienced by plants (Medrano et al. 2002). Hence plants of each species were harvested at different times, but when the drought-stressed plants were showing a similar level of stress reaction.
At harvest, the tape was removed and the pots split open which allowed root distribution to be examined with minimal soil disturbance. Roots in each pot were washed carefully in a water tub and separated carefully from the top of roots into the legume of interest and M. sativa. The root system was then laid down on a flat surface and divided into 0.1 m segments. Shoots were separated from roots at the crown and partitioned into leaves and stems (including petiole). The shoots and roots were dried at 70°C for 72 h and dry weight (DW) was measured. Green leaf area was determined using a WinRHIZO scanner and software (Regent Instruments Inc., Quebec City, QC, Canada). Specific leaf area was calculated as green leaf area per unit dry weight (m2 kg−1).
Statistical analysis
The experiment was a two-factorial (species/accession and water treatment) randomised complete block design. Data for growth and other parameters were analysed by general analysis of variance (ANOVA) in Genstat version 13.1 (Lawes Agricultural Trust, Rothamsted Experimental Station, UK, 2007).
Results
Soil water depletion and time for final harvest after drought stress
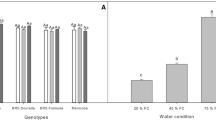
At the start of drought stress, soil water content was maintained at around 12% (v/v) in all pots (Fig. 1). The imposition of drought stress reduced soil water content continuously in all drought-stressed pots (Fig. 1). Fourteen days after drought stress was imposed, water content was reduced to 6–8% (v/v) in all species/accessions. After that, soil water content only decreased further in M. atropurpureum, resulting in the lowest soil water content (~4% (v/v)) at harvest. During this period, soil water content in all well watered pots was maintained at ~12% (v/v) (data not shown).
Volumetric soil water content in pots with drought-stressed plants after cessation of watering. Note, not all standard errors are presented to avoid crowding. Data for each species/accession are the average of soil moisture data from the top and bottom of pots installed with moisture probes. Species abbreviations are BB (B. bituminosa var. albomarginata), CA (C. australasicum), CC (C. cinereum), CP (C. pallidum), KP (K. prostrata), MS (M. sativa), MA (M. atropurpureum)
The time of final harvest after drought stress differed among species/accessions as it occurred when stomatal conductance of the drought-stressed plants was reduced to ~10% of that of the well watered plants. B. bituminosa var. albomarginata accession 6 and accession 10, C. australasicum SA42762, C. pallidum, C. cinereum, K. prostrata and M. sativa were harvested 16 days after drought stress was imposed, C. australasicum SA44239 and M. atropurpureum were harvested 21 days after drought stress was imposed and B. bituminosa accession 22 was harvested 35 days after drought stress was imposed.
Phenological difference of species/accessions
There were differences in phenology among species/accessions at the start of drought stress. Whilst experiencing the drought stress, some species/accessions were vegetative, e.g. all accessions of B. bituminosa var. albomarginata and K. prostrata, while the remaining species were flowering and/or setting seeds.
Shoot and root dry biomass
Initial shoot DW, prior to the imposition of drought stress, differed among species (Fig. 2a, Table 2). At this time, both accessions of C. australasicum, along with C. pallidum and M. atropurpureum had much more shoot DW than M. sativa, while only M. atropurpureum had more root DW than M. sativa (Fig. 2b). B. bituminosa var. albomarginata accession 22 had the lowest shoot DW among the species. There was a significant interaction between species/accessions and water treatment at final harvest (Fig. 2a, Table 2). After drought stress was imposed, B. bituminosa var. albomarginata accession 22 and M. atropurpureum gained little shoot DW in both well watered and drought-stressed plants (Fig. 2a). In these species, drought-stressed plants had similar shoot DWs to well watered plants at the final harvest. Little shoot DW was also gained in drought-stressed plants of B. bituminosa var. albomarginata accession 6 and M. sativa, while shoot DW increased in well watered plants until final harvest. Shoot DW increased in drought-stressed plants of B. bituminosa var. albomarginata accession10 and both C. australasicum accessions after watering ceased, but increased more in well watered plants. For C. cinereum, C. pallidum and K. prostrata, shoot DW of drought-stressed plants continued to increase after drought stress was imposed and these species had similar shoot DWs in drought-stressed plants and well watered plants at the final harvest.
Shoot dry weight (a), root dry weight (b) and root/shoot ratio (c) at the start of drought stress (white bars), in well watered plants (grey bars) and drought-stressed plants (black bars) at final harvest. Data are means ± s.e. (n = 4). Species abbreviations are BB (B. bituminosa var. albomarginata), CA (C. australasicum), CC (C. cinereum), CP (C. pallidum), KP (K. prostrata), MS (M. sativa), MA (M. atropurpureum)
Initial root DW varied greatly and there was a significant interaction between species/accessions and water treatment at final harvest (Fig. 2b, Table 2). After drought stress commenced, root DW changed little in B. bituminosa var. albomarginata accessions 6 and 22, C. cinereum, C. pallidum and K. prostrata. In contrast, root DW continued to increase after drought stress was imposed in B. bituminosa var. albomarginata accession 10, but it remained less than the well watered plants at the final harvest. Root DW of drought-stressed plants was close to that of well watered plants for both accessions of C. australasicum, along with M. sativa and M. atropurpureum (Fig. 2b).
Root:shoot ratio at final harvest
Root:shoot ratio and its response to drought stress varied greatly among species at the final harvest (Fig. 2c). There was a significant interaction between species/accessions and water treatment (Table 2, Fig. 2c). M. sativa and M. atropurpureum had the highest root:shoot ratios (> 1.75), while that of the other species varied between 1.35 and 0.25. Some species/accessions, e.g. B. bituminosa var. albomarginata accession 22, both accessions of C. australasicum and, most notably, M. sativa, increased their root:shoot ratio in response to drought stress. In contrast, root:shoot ratio decreased in B. bituminosa var. albomarginata accession 10 and C. cinereum in response to drought stress (Fig. 2c).
Root distribution down the profile at final harvest
Normalised root DW (percentage of total root dry biomass present in each depth increment of the pot) at the final harvest was affected by the interaction of species/accessions, root depth and water treatment (Fig. 3, Table 2). For well watered plants, B. bituminosa var. albomarginata accession 22, M. sativa and M. atropurpureum had 53–62% of roots in the top 30 cm and thus the proportion of root DW decreased with depth. In B. bituminosa var. albomarginata accessions 6 and 10, both accessions of C. australasicum and C. cinereum, roots were distributed relatively evenly with depth. For K. prostrata, roots in the top 15 cm contributed ~ 35% of total root DW, with roots distributed evenly across other depths. C. pallidum also had ~35% of roots in the top 15 cm, with the proportion of root DW then decreasing with depth before increasing at 90–105 cm (Fig. 3). In response to drought stress, the proportion of roots at greater depths increased for C. australasicum accession SA44239, C. pallidum and, most notably, for B. bituminosa var. albomarginata accession 22 where the proportion of root DW at 90–105 cm changed from 3 to 23% although the absolute root biomass of this accession remained very low. The proportion of roots at shallow depths increased for B. bituminosa var. albomarginata accession 10 and, in particular, for C. cinereum (28 to 45%) in response to drought stress; there was little change for the remaining species (Fig. 3).
Green leaf area, senesced leaves and leaf morphology at final harvest
For green leaf area and the percentage of senesced leaf to total leaf DW at final harvest, there was significant interaction between species/accessions and water treatment (Fig. 4, Table 2). For well watered plants, C. cinereum had the lowest green leaf area (~100 cm2), followed by B. bituminosa var. albomarginata accession 22 and M. sativa. The leaf area of the other species/accessions ranged from 540 to 1235 cm2. In response to drought stress, green leaf area decreased in all species except M. sativa and M. atropurpureum (Fig. 4a). All species under drought stress increased the percentage of senesced leaf to total leaves (Fig. 4b). The highest percentage of leaf senescence in drought-stressed plants occurred in K. prostrata and the lowest for M. sativa and M. atropurpureum. A relatively high percentage of leaf senescence was also observed in well watered B. bituminosa var. albomarginata accession 22 and C. cinereum.
Green leaf area (a), the percentage of senesced to total leaf dry weight (b), and specific leaf area (c) in well watered plants (grey bars) and drought-stressed plants (black bars) at final harvest. Data are means ± s.e. (n = 4). Species abbreviations are BB (B. bituminosa var. albomarginata), CA (C. australasicum), CC (C. cinereum), CP (C. pallidum), KP (K. prostrata), MS (M. sativa), MA (M. atropurpureum)
A number of leaf morphological responses to water deficit were observed and are summarised in Table 3. Specific leaf area (average of all green leaves on plants) varied among legume species in response to drought stress (Fig. 4c, Table 2). In well watered plants, K. prostrata and M. sativa had the highest specific leaf area, while C. cinereum and C. pallidum had the lowest (Fig. 4c). In response to drought stress, both accessions of C. australasicum and C. pallidum had ~30% less specific leaf area while M. sativa had 17% less; the other species did not show a response. Thick stands of leaf hair were observed on stems and leaves of M. atropurpureum and C. pallidum which were enhanced in the drought-stressed treatment. In all Cullen species, inward leaf curling was observed after drought stress. In M. sativa, leaflets folded to form a cup.
Physiological adaptations
The effect of drought treatment on pre-dawn leaf water potential at final harvest differed among species/accessions (Fig. 5a, Table 2). Leaf water potential was reduced most by drought stress, being less than −2.5 MPa, in both C. australasicum accessions, along with C. pallidum, K. prostrata and M. sativa, while it remained higher than −1.0 MPa in B. bituminosa var. albomarginata accession 6 and 10, and M. atropurpureum.
Predawn leaf water potential (a), osmotic potential (b), and calculated osmotic potential at full turgor (c) in leaves of well watered plants (grey bars) and drought-stressed plants (black bars) at final harvest. Data are means ± s.e. (n = 4). Species abbreviations are BB (B. bituminosa var. albomarginata), CA (C. australasicum), CC (C. cinereum), CP (C. pallidum), KP (K. prostrata), MS (M. sativa), MA (M. atropurpureum)
The effect of drought treatment on osmotic potential at final harvest also differed among species/accessions (Fig. 5b, Table 2). Osmotic potential was reduced greatly in response to drought stress in all species except C. cinereum and M. atropurpureum. In drought-stressed plants, osmotic potential was lower than −3.0 MPa in C. australasicum SA44239 and K. prostrata, maintained at around −1.0 MPa in C. cinereum and M. atropurpureum and ranged from −2.0 to −3.0 MPa in the remaining species. The leaf water potential of all species/accessions except B. bituminosa var. albomarginata accessions 6 and 10, and M. atropurpureum was close to, or lower than, the osmotic potential, which indicated that leaf turgor was close to zero or lost at the final harvest (Fig. 6a and b).
Photosynthetic rate before (−7 days) and after (1–32 days) the start of the drought-stress treatment. Data are means ± s.e. (n = 4). Species abbreviations are BB (B. bituminosa var. albomarginata), CA (C. australasicum), CC (C. cinereum), CP (C. pallidum), KP (K. prostrata), MS (M. sativa), MA (M. atropurpureum)
The effect of drought treatment on osmotic potential at full turgor at the final harvest differed among species/accessions (concentration effects on changes in leaf osmotic potential were removed by presenting osmotic potential at full turgor) (Fig. 5c, Table 2). Osmotic potential at full turgor was clearly lower in drought-stressed than well watered plants of B. bituminosa var. albomarginata accessions 6 and 10, both C. australasicum accessions and K. prostrata.
Photosynthetic responses
Prior to imposition of drought stress, photosynthetic rate varied among species (Fig. 6, Table 2) with the highest rates (~25 μmol m−2 s−1) recorded for all Cullen species/accessions (Fig. 6). At all subesquent dates, there was a significant interaction between species/accession and water treatment (Table 2). Upon imposition of drought stress, C. pallidum, M. sativa and K. prostrata quickly, i.e. within 7 days, reduced their photosynthetic rate (Fig. 6). In contrast, M. atropurpureum and B. bituminosa var. albomarginata accession 22 maintained their pre-stress photosynthetic rate for 14 and 18 days, respectively. The other species showed an intermediate response.
Discussion
The 10 perennial legume species/accessions in the present study showed large and contrasting differences in growth, and physiological and morphological traits, when exposed to drying soil under conditions of low available soil P. The implications of these diverse responses, and other major findings of this study, are discussed below.
Physiological adaptations to drought stress
Osmotic adjustment is a commonly reported response to drought stress. The accumulation of osmotically active compounds such as sucrose, glucose, fructose, potassium and chloride ions lowers the osmotic potential of cells which in turn allows the maintenance of full or partial turgor pressure at a low leaf water potential (Morgan 1984). In our study only some species showed osmotic adjustment, that is, B. bituminosa var. albomarginata accessions 6 and 10, both C. australasicum accessions and K. prostrata. Differences in osmotic adjustment among species of perennial legumes were also reported by Bell et al. (2007), who observed an accumulation of osmotic solutes in Dorycnium hirsutum and D. rectum in response to drought stress, but no accumulation in M. sativa. However, others did report that osmoprotectants accumulated in leaves and phloem of M. sativa exposed to drought stress (Girousse et al. 1996; Irigoyen et al. 1992). Bell et al. (2007) hypothesised that the absence of osmotic solutes in M. sativa was due to the rapid development of drought stress (see Turner and Jones 1980) or reflected genetic differences among M. sativa cultivars. In the present study, relatively rapid soil water depletion due to the sandy soil and large plant size when watering ceased might be the reason for limited osmotic adjustment in some legume species/accessions.
The ability to maintain leaf turgor which may be aided by osmotic adjustment, is considered important for maintaining stomatal conductance and photosynthesis under low soil water potential (Jones et al. 1980). For instance, leaf turgor was maintained until final harvest in M. atropurpureum which may have contributed to its relatively long period of continuing photosynthesis (18 days after the imposition of drought stress). Although B. bituminosa var. albomarginata accessions 6 and 10 also maintained leaf turgor until final harvest, stomatal conductance decreased dramatically and these accessions did not maintain a high photosynthetic rate (i.e. there was a rapid reduction of photosynthesis 9 days after the supply of water ceased). As the leaf water potential in these two accessions was maintained at a high level, we hypothesise that leaves of these two species tended towards being isohydric through a strong feedforward stomatal control, which allowed maintenance of a high leaf water potential at a low soil water content (Lambers et al. 2008). Abscisic acid (ABA) is a chemical signal to control stomatal behaviour, and differences between species/accessions in maintenance of photosynthesis under drought stress might be due to differential sensitivity of stomata to ABA (Lambers et al. 2008). C. australasicum accession SA44239 also continued to photosynthesise, albeit at a low rate, at very low leaf water potential. In this accession, osmotic adjustment would have assisted the maintenance of very low leaf water potentials. In contrast, Bell et al. (2007) found that osmotic adjustment had little effect on maintaining leaf photosynthesis or transpiration in both D. hirsutum and M. sativa, as it occurred when leaf turgor was lost and later than the reduction of stomatal conductance and photosynthesis.
Shoot and root morphological adaptations to drought stress
Morphological adaptations to drought stress were observed in some species/accessions. For instance, a thicker layer of white leaf hairs was observed on stems and leaves of M. atropurpureum and C. pallidum. Leaf hairs may help to reflect solar radiation, thus increasing resistance to transpirational water loss and moderating leaf temperature (Ehleringer and Björkman 1978; Grammatikopoulos et al. 1994). In addition, leaf curling (cupping) was observed in all Cullen species and M. sativa. Such a phenomenon has been recorded previously in perennial legumes in response to reduced leaf water potential (Bell et al. 2007; Travis and Reed 1983). Cupping also reduces light interception by leaves through reducing the angle at which leaves are exposed to direct solar radiation (Koller 1990). Leaf hairs and leaf curling also reduce transpiration rates by increasing the thickness of the laminar layer over the leaf.
Root systems can show great plasticity in response to environmental stress, and an increase in root mass ratio is a typical response to drought (Gregory et al. 1996). In the present study, M. sativa, B. bituminosa var. albomarginata accession 22 and both accessions of C. australasicum increased root:shoot ratio in response to drought stress. The increased root:shoot ratio in these species/accessions was due to root biomass in drought-stressed plants accumulating at the same rate as the well watered plants, while shoot growth was reduced. Skinner and Comas (2010) present similar results in temperate forage species under drought stress. M. sativa has also been shown in two soil types to have consistently lower root development under drought stress, associated with a higher root:shoot ratio, compared with favourable conditions when grown in large boxes with the dimension of 55 cm × 12 cm × 75 cm (Annicchiarico 2007). The high root:shoot ratio of M. sativa was mainly due to the presence of a large tap-root which could have a major function as a storage organ. An increased root:shoot ratio is presumably advantageous because it enhances soil resource acquisition, but it does so at the expense of photosynthetic carbon gain (Ho et al. 2005). As a result, a carbon cost for increased allocation to roots could ultimately reduce plant growth (Nielsen et al. 2001).
Deep root systems may allow plants to maintain water uptake as top soil dries (Ho et al. 2005). In this study, all perennial species/accessions reached the bottom of the pots, i.e. 1.05 m. However, there were large differences in the distribution of root systems down the profile among species/accessions, with roots of some species (e.g. B. bituminosa var. albomarginata accessions 6 and 10, both accessions of C. australasicum and C. cinereum) being evenly distributed and those of other species (e.g. B. bituminosa var. albomarginata accession 22, M. sativa and M. atropurpureum) having a high concentration in the topsoil. In response to drought stress, only three out of 10 species/accessions, C. australasicum accession SA44239, C. pallidum and most particularly, B. bituminosa var. albomarginata accession 22, increased the proportion of roots at depth. In the present study, pots dried out throughout the profile during drought stress. Suriyagoda et al. (2010) reported that C. australasicum accessions SA 42762 and SA4966 did not enhance the proportion of deep roots when pots dried throughout the profile, while the proportion of deep roots increased when only the top half of the pots dried while the bottom half was kept moist. These authors used washed river sand, which has a lower water-holding capacity than the field soil used in the present study, so pots dried out even more quickly. Skinner and Comas (2010) found that in 50-cm deep pots drought stress had no effect on the proportion of deep roots in legumes and forbs, but significantly increased it in grasses.
Two species (B. bituminosa var. albomarginata accessions 10 and, in particular, C. cinereum) increased the proportion of roots in the top soil in response to drought stress. In this study, since nutrients were only supplied in the top 40 cm of soil, to mimic a field situation, and P supply was low, an increased proportion of roots in the top soil may have helped plants acquire more nutrients under drought stress, especially relatively immobile nutrients such as P. Root foraging in top soil is considered an effective strategy to acquire P from low-P soils as P levels in top soil are generally higher due to decaying litter, higher organic matter and microbial activity (Ho et al. 2005; Lynch and Brown 2001). Top soil root foraging for P has been reported in a similar set of perennial legumes (Denton et al. 2006; Suriyagoda et al. 2010) and further study is warranted on the interactions between low-P and drought stress.
Root system responses probably also contributed to drought-stressed and well watered plants of C. pallidum and K. prostrata accumulating approximately the same amount of shoot DW by final harvest in spite of photosynthetic rate decreasing immediately upon drought stress being imposed. Both these species accumulated little additional root dry weight after drought stress was imposed, thus maybe were able to invest more C in shoot growth for both drought-stressed and well watered plants. In addition, a range of changes associated with the imposition of drought stress then allowed shoot growth to continue under water limitation until harvest. For C. pallidum an increased proportion of roots at depth may have enhanced access to soil water at depth, and a large reduction in leaf area probably reduced water loss. For K. prostrata, osmotic adjustment may have helped plants to extract water from the soil and adjust their water potential. In addition, K. prostrata had an initially high leaf area ratio (the ratio of leaf area and total plant weight) before the initiation of drought stress and a high specific leaf area, which might have contributed to its relatively high accumulation of shoot biomass during drought stress and high shoot DW of this species at final harvest. These results are consistent with those of Poorter and Remkes (1990) who found a very strong positive correlation between relative growth rate and leaf area ratio, and between relative growth rate and specific leaf area in 24 herbaceous C3 species.
Variation among accessions
A key finding from the present experiment was differences between accessions of both B. bituminosa var. albomarginata and C. australasicum. For example, B. bituminosa var. albomarginata accessions 6 and 10 in response to drought stress accumulated osmotic solutes and maintained a higher leaf water potential at final harvest than accession 22. Instead, accession 22 increased root:shoot ratio and the proportion of roots deep in the profile. Whilst differences between accessions within species were generally not as marked as differences between species, it may be possible to match them with field performance and hence they could provide useful information for future selection/breeding programs.
Extrapolating results to field conditions
Some caution is necessary when using the results of this glasshouse experiment to predict responses in a field setting. In the field, drought stress may be imposed more slowly and last for longer, and roots of well-established plants may have access to stores of water deep in the soil profile. In addition, in the field plants may be preconditioned by experiencing periods of milder drought prior to such a serious period of drought stress. Additionally, climatic conditions in the greenhouse may differ substantially from the field, particularly in terms of wind. Also, for a perennial pasture on a commercial farm, in addition to the important issue of ability to grow and provide livestock feed under dry conditions which was assessed in the current experiment, the ability to survive an extended dry period is also crucial. This was not assessed in this experiment.
Conclusions
The present glasshouse study has shown that novel perennial legumes use a range of quite contrasting morphological and physiological strategies, which often differ from those used by M. sativa, to cope with drought stress. The companion M. sativa plants in each pot in this study minimised the difference in soil water reduction. M. atropurpureum extracted more water from the soil and achieved the lowest soil water content at harvest of all species/accessions. Leaf adaptations upon drought stress were observed in all four Cullen species and in M. sativa (leaf curling), and in M. atropurpureum and C. pallidum (denser layers of long trichomes on stems and leaves). B. bituminosa accession 22, both accessions of C. australasicum and M. sativa increased their root:shoot ratio in response to drought stress. Leaf osmotic adjustment in response to drought stress was observed in B. bituminosa var. albomarginata accessions 6 and 10, both accessions of C. australasicum and K. prostrata. Leaf water potential was maintained at a high level in B. bituminosa var. albomarginata accessions 6 and 10 and M. atropurpureum. The inter- and intra-specific variability of responses to drought-stress in the plants examined here suggests a wide range of strategies in perennial herbaceous legumes to cope with drying conditions. These findings offer great encouragement for the development of new perennial legume forages for a wide range of drought-prone agricultural regions in breeding/selection programs.
References
Annicchiarico P (2007) Lucerne shoot and root traits associated with adaptation to favourable or drought-stress environments and to contrasting soil types. Field Crop Res 102:51–59
Bell LW, Ryan MH, Moore GA, Ewing MA (2006) Comparative water use by Dorycnium hirsutum-, lucerne-, and annual-based pastures in the Western Australian wheatbelt. Aust J Agric Res 57:857–865
Bell LW, Williams AH, Ryan MH, Ewing MA (2007) Water relations and adaptations to increasing water deficit in three perennial legumes, Medicago sativa, Dorycnium hirsutum and Dorycnium rectum. Plant Soil 290:231–243
Bennett RG, Ryan MH, Colmer TD, Real D (2011) Prioritisation of novel pasture species for use in water-limited agriculture: a case study of Cullen in the Western Australian wheatbelt. Genet Resour Crop Evol 58:83–100
Cocks PS (2001) Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and waterlogging in dryland cropping systems. Aust J Agric Res 52:137–151
Dear BS, Ewing MA (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Aust J Exp Agric 48:387–396
Dear BS, Reed KFM, Craig AD (2008) Outcomes of the search for new perennial and salt tolerant pasture plants for southern Australia. Aust J Exp Agric 48:578–588
Denton MD, Sasse C, Tibbett M, Ryan MH (2006) Root distributions of Australian herbaceous perennial legumes in response to phosphorus placement. Funct Plant Biol 33:1091–1102
Ehleringer JR, Björkman O (1978) Comparison of photosynthetic characteristics of Encelia species possessing glabrous and pubescent leaves. Plant Physiol 62:185–190
Girousse C, Bournoville R, Bonnemain JL (1996) Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol 111:109–113
Grammatikopoulos G, Karabourniotis G, Kyparissis A, Petropoulou Y, Manetas Y (1994) Leaf hairs of olive (Olea europaea) prevent stomatal closure by ultraviolent-B radiation. Aust J Plant Physiol 21:293–301
Gregory PJ, Palta JA, Batts GR (1996) Root systems and root:mass ratio—Carbon allocation under current and projected atmospheric conditions in arable crops. Plant Soil 187:221–228
Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32:737–748
Humphries AW, Auricht GC (2001) Breeding lucerne for Australia’s southern dryland cropping environments. Aust J Agric Res 52:153–169
Irigoyen JJ, Emerich DW, Sanchezdiaz M (1992) Water-stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Jones MM, Osmond CB, Turner NC (1980) Accumulation of solutes in leaves of sorghum and sunflower in response to water deficits. Aust J Plant Physiol 7:193–205
Koller D (1990) Light-driven leaf movements. Plant Cell Environ 13:615–632
Lambers H (2003) Dryland salinity: a key environmental issue in southern Australia. Plant Soil 257:v–vii
Lambers H, Pons TL, Chapin FSIII (2008) Plant physiological ecology, 2nd edn. Springer, New York
Li GD, Lodge GM, Moore GA, Craig AD, Dear BS, Boschma SP, Albertsen TO, Miller SM, Harden S, Hayes RC, Hughes SJ, Snowball R, Smith AB, Cullis BC (2008) Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Aust J Exp Agric 48:449–466
Loo C, Dolling PJ, Mokhtari S (2006) Lucerne. In: Moore G, Sanford P, Wiley T (eds) Perennial pastures for Western Australia, Bulletin 4690. Department of Agriculture and Food Western Australia, South Perth, pp 59–75
Ludlow MM, Chu ACP, Clements RJ, Kerslake RG (1983) Adaptation of species of Centrosema to water stress. Aust J Plant Physiol 10:119–130
Lynch JP, Brown KM (2001) Topsoil foraging - an architectural adaptation of plants to low phosphorus availability. Plant Soil 237:225–237
Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89:895–905
Morgan JM (1984) Osmoregulation and water stress in higher plants. Ann Rev Plant Physiol 35:299–319
Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52:329–339
Pang J, Ryan MH, Tibbett M, Cawthray GR, Siddique KHM, Bolland MDA, Denton MD, Lambers H (2010a) Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 331:241–255
Pang J, Tibbett M, Denton MD, Lambers H, Siddique KHM, Bolland MDA, Revell CK, Ryan MH (2010b) Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant Soil 328:133–143
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83:553–559
Skinner RH, Comas LH (2010) Root distribution of temperate forage species subjected to water and nitrogen stress. Crop Sci 50:2178–2185
Snowball R, D’Antuono MF, Cohen BJ, Gajda K, Bennett R (2010) The value of germplasm nurseries in selecting species for field evaluation. Crop Pasture Sci 61:957–969
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann Bot 105:755–767
Travis RL, Reed R (1983) The solar tracking pattern in a closed alfalfa canopy. Crop Sci 23:664–668
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366
Turner NC, Jones MM (1980) Turgor maintenance by osmotic adjustment. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 87–104
Ward PR, Micin SF, Dunin FX (2006) Using soil, climate, and agronomy to predict soil water use by lucerne compared with soil water use by annual crops or pastures. Aust J Agric Res 57:347–354
Acknowledgements
This work was funded by the Australian Research Council (ARC), Rural Industries Research and Development Corporation (RIRDC), the Department of Agriculture and Food Western Australia (DAFWA), Heritage Seeds, the Chemistry Centre of Western Australia, and the Facey Group and Mingenew Irwin Group. We thank Dr Daniel Real (DAFWA), Dr Matthew Denton (DPI, Victoria) and Mr Richard Bennett (UWA) who provided legume seeds and rhizobia, and Mr. Darryl McClements (DAFWA) who provided technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Caixian Tang.
Rights and permissions
About this article
Cite this article
Pang, J., Yang, J., Ward, P. et al. Contrasting responses to drought stress in herbaceous perennial legumes. Plant Soil 348, 299–314 (2011). https://doi.org/10.1007/s11104-011-0904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0904-x