Abstract
Background
Sorghum roots release two categories of biological nitrification inhibitors (BNIs) – hydrophilic-BNIs and hydrophobic-BNIs. Earlier research indicated that rhizosphere pH and plasma membrane (PM) H+ATPase are functionally linked with the release of hydrophilic BNIs, but the underlying mechanisms are not fully elucidated. This study is designed to reveal further insights into the regulatory mechanisms of BNIs release in root systems, using three sorghum genetic stocks.
Methods
Sorghum plants were grown in a hydroponic system with pH of nutrient solutions ranging from 3.0 ̴ 9.0. Pharmacological agents [(fusicoccin and vanadate) and anion-channel blockers (−niflumic acid (NIF) and anthracene-9-carboxylate (A9C)] were applied to root exudate collection solutions; BNI activity was determined with luminescent Nitrosomonas europaea bioassay. Sorgoleone levels in root exudates and H+ excretion from roots were determined. Two-phase partitioning system is used to isolate root plasma membrane (PM) and H+ ATPase activity was determined.
Results
A decrease in rhizosphere pH improved the release of hydrophilic-BNIs from roots of all the three sorghum genotypes, but had no effect on the release of hydrophobic-BNIs. Hydrophobic-BNI activity and sorgoleone levels in root-DCM wash are positively correlated. Fusicoccin promoted H+extrusion and stimulated the release of hydrophilic-BNIs. Vanadate, in contrast, suppressed H+ extrusion and lowered the release of hydrophilic-BNIs. Anion-channel blockers did not inhibit the release of hydrophilic BNIs, but enhanced H+-extrusion and hydrophilic-BNIs release.
Conclusion
Rhizosphere pH has a major influence on hydrophilic-BNIs release, but not on the release of hydrophobic-BNIs. The low rhizosphere pH stimulated PM-H+ ATPase activity; H+-extrusion is closely coupled with hydrophilic-BNIs release. Anion-channel blockers stimulated H+ extrusion and hydrophilic-BNIs release. Our results indicate that some unknown membrane transporters are operating the release of protonated BNIs, which may compensate for charge balance when transport of other anions is suppressed using anion-channel blockers. A new hypothesis is proposed for the release of hydrophilic-BNIs from sorghum roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern productive agriculture is highly concerned with the threats generated from loss of nitrogen (N) and the associated environmental pollution, leading to significant reductions in N use efficiency (NUE). These risks are linked with the fate of soil-N [i.e. from mineralization of SOM (soil organic matter)] and N-fertilizers applied to agricultural systems. Nitrate (NO3 −) is liable to leach out of the rhizosphere and into underground water bodies (Amberger 1989). Furthermore, denitrification of NO3 − produces various gaseous-N forms (N2O, NO, N2), which reduces agronomic-NUE, and contributes to greenhouse gas emissions and global warming (Parker 1972; Meinshausen et al. 2009). Nitrification and denitrification in agricultural soils are largely responsible for the ineffective N use in production systems worldwide (Raun and Johnson 1999; Subbarao et al. 2015, 2017). Keeping soil-N in NH4 + form for an extended period by inhibiting nitrification can improve N recovery and agronomic-NUE (Slangen and Kerkhoff 1984; Subbarao et al. 2006a, 2012, 2015, 2017).
Currently, application of synthetic nitrification inhibitors is the only practical method being used to control soil nitrification in agricultural systems (Slangen and Kerkhoff 1984; Amberger 1989; Zerulla et al. 2001). The newly emerging research areas suggest that nitrification can also be controlled by in situ production of nitrification inhibitors from plants root systems, termed as “biological nitrification inhibition” (BNI) (Subbarao et al. 2009, 2015, 2017), and microorganisms in the rhizosphere (Smart and Bloom 2001; Weiske et al. 2001). Among field crops, sorghum is reported to release a significant amount of BNIs from root systems (Alsaadawi et al. 1986; Hossain et al. 2008; Subbarao et al. 2007a, 2013; Tsehaye et al. 2014).
Sorghum roots release two different categories of nitrification inhibitors - one category of inhibitors is water soluble, hereafter referred as hydrophilic-BNIs. Methyl 3-(4-hydroxyphenyl) propionate (MHPP) is identified as one of the hydrophilic-BNIs released from sorghum roots (Hossain et al. 2008). The second category of inhibitors is soluble only in acidified-DCM (Dichloromethane), obtained by washing roots with DCM, and is highly hydrophobic in nature, hereafter referred as hydrophobic-BNIs (Subbarao et al. 2013). Sorgoleone is a major component in the root-DCM wash and accounts for 80% of the hydrophobic-BNI activity (Czarnota et al. 2003; Subbarao et al. 2013).
Hydrophilic- and hydrophobic- BNIs vary in their distribution in the rhizosphere; hydrophobic-BNIs are likely to remain close to the root and is strongly adsorbed to the soil particles upon release and their movement in soil is mostly via diffusion across the concentration gradient and likely tobe confined to the rhizosphere (Dayan et al. 2010; Subbarao et al. 2013). In contrast, the hydrophilic-BNIs may move further from the point of release due to their solubility in water, which improve their capacity to control nitrifier activity beyond the rhizosphere. It is likely that hydrophobic- and hydrophilic-BNIs will have complimentary role in the control of nitrifier activity (such as differential inhibitory impact on AOB (ammonia oxidizing bacteria) and AOA (ammonia oxidizing archea) (Subbarao et al. 2013).
Presence of NH4 + in the rhizosphere is known for its stimulatory effect on BNIs release in sorghum roots (Hossain et al. 2008; Subbarao et al. 2007b, c). NH4 + uptake in plant roots is coupled with H+ release and acidification of the rhizosphere (Lewis et al. 1982; Marschner 1995). Results from our previous research suggested that NH4 + uptake, rhizosphere acidification and plasma membrane (PM) H+ ATPase activity are functionally interconnected with hydrophilic-BNIs release from sorghum roots (Zhu et al. 2012; Zeng et al. 2016). The regulatory mechanism involved in hydrophobic-BNIs release in sorghum is largely unknown at present.
Plasma membrane H+ ATPase (PM H+ATPase) is a universal electrogenic H+ pump, which generates H+ electrochemical gradient to provide the driving force for active transport, i.e. influx and efflux of ions and metabolites across the cell PM (Palmgren 2001) and is hypothesized to be involved in the release of hydrophilic-BNIs from sorghum roots. As BNIs are suggested to be anionic substances (Yamashita et al. 1996; Zhu et al. 2005; Subbarao et al. 2007a), the question is whether they are released through anion channels as has been suggested earlier (Zhu et al. 2012), requires confirmation.
During the present study, rhizosphere pH, PM H+ ATPase, pharmacological agents that influence H+ATPases (either stimulate or suppress) and anion-channel blockers are investigated for their role in the release of hydrophilic- and hydrophobic- BNIs from root systems using three genetic stocks of sorghum. This is to develop further insights into the underlying mechanisms/pathways operating for the release of BNIs in sorghum root systems.
Materials and methods
Plant cultivation
To test the rhizosphere pH influence on the release of hydrophobic BNIs from sorghum roots, seedlings were raised from three genetic stocks of sorghum (Sorghum bicolor L. Moench) – ‘Hybridsorgo’, PVK 801 and 296B using seedling-growth-box system (Fig. 1a, b). The cultivation solution contained 0.5 mM (NH4)2SO4 and 200 μM CaSO4 with pH ranging from 3.0–9.0. Plant seedlings were grown in a growth chamber with a day: night temperature regime of 30:28 °C, a photosynthetic photon flux averaging at 300 μmol m−2 s−1 and a 14: 10 h light: dark photoperiod.
Sorghum seedlings are cultivated using the seedling-growth-box system under nutrient solution pH of 3.0, 5.0, 7.0 and 9.0 (a). Plants roots are grown between two layers of filter paper to get the solution in the growth box (b). During the collection of root exudates, the pH-stat system was used to maintain the treatment pH of root exudate collection solutions
Extraction of hydrophilic-BNIs from root exudation and analysis of BNI activity by bioassay
Intact plant roots were immersed in 1 L aerated collection solution for 1 h; the solution contained 1 mM NH4Cl and 1 mM CaCl2 and the treatment pH of the cultivation solution and root exudate collection solutions were maintained using pH-stat systems (Subbarao et al. 2013; Zhu et al. 2012).
For determination of hydrophilic-BNI activity, the root exudates (as referred to hydrophilic-BNIs) in collection solutions were evaporated to dryness using a rotary evaporator under vacuum at 40 °C, followed by extraction with 20 mL of methanol. The methanol extract of hydrophilic BNIs was further evaporated to dryness using a rotary evaporator at 40 °C and the residue was extracted with 200 μL of DMSO. 1 μL of this aliquot was used for the determination of hydrophilic-BNI activity using the bioassay and expressed in ATU (Subbarao et al. 2006b).
Extraction of hydrophobic BNIs from root exudation and analysis of sorgoleone
Hydrophobic BNI-activity was collected from sorghum roots as described earlier (Subbarao et al. 2013). In brief, roots were excised from seedlings and dipped into acidified-DCM (1% v/v of acetic/DCM) for 1 min. The root-DCM wash was then filtered and evaporated to dryness using a rotary evaporator at 35 °C (Buchi, V-850, Flawil, Switzerland); the residue was re-extracted with 10 mL methanol and evaporated to dryness again. The residue was transferred to Eppendorf tube after filtering through 0.20 μm syringe membrane filter; the filtrate was evaporated to dryness using a centrifugal evaporator at 35 °C. Residues were dissolved in 500 μL acetonitrile and filtered again through 0.20 μm syringe membrane filter to remove any insoluble compound. An aliquot (10 μL in acetonitrile) from the sample was injected into HPLC with isocratic flow (2 mL min−1) of the eluent (55% acetonitrile and 45% of 0.5% formic acid) and sorgoleone was detected at 280 nm.
Determination of hydrophobic-BNI activity in root exudates using recombinant luminescent bioassay
A sub-sample of the acetonitrile-soluble fraction prepared for sorgoleone analysis was taken and evaporated to dryness by the centrifugal evaporator (Buchi, V-850, Flawil, Switzerland) at 35 °C. The residue was then dissolved in dimethyl sulfoxide (DMSO) and 1 μL of this aliquot was used for the determination of hydrophobic BNI activity using the bioassay described earlier (Iizumi et al. 1998; Subbarao et al. 2006b). BNI activity is expressed in unit defined in terms of the action of a standard inhibitor, allylthiourea (AT), where the inhibitory effect of 0.22 μM AT in an assay containing 18.9 mM of NH4 + is defined as one AT unit (ATU) of activity (Subbarao et al. 2006b).
PM isolation and H+-ATPase activity analysis
After collection of root exudates, the plant roots were used for plasma membrane (PM) isolation according to Yan et al. (2002). Roots were cut and washed with deionized water and ground in ice-cold homogenization buffer. The homogenate was filtered through two layers of Miracloth (Calbiochem-Novabiochem, San Diego, USA) and centrifuged in a fixed rotor at 11,700 g (RP42A rotor, 94 mL × 6, HITACHI Koki; Himac CP100WX Hitachi Ultra Centrifuge) and 0 °C for 10 min. The supernatants were centrifuged at 106,000 g for 35 min. The microsomal pellets were resuspended and fractionated by two-phase partitioning in aqueous dextran T-500 (Sigma) and polyethylene glycol (Sigma) according to the method of Larsson (1985). The upper phases obtained after four times of separations were diluted and centrifuged at 188,000 g (RP42A rotor, 94 mL × 6) for 40 min. The pellets were re-suspended and immediately stored in liquid nitrogen.
PM H+ ATPase activity was determined by measuring the Pi amount after 30 min hydrolysis reaction (Baginski et al. 1967; Yan et al. 2002). ATPase activity was calculated as the phosphate liberated in excess of a boiled-membrane control.
Treatment of different pharmacological agents on plant roots during root exudate collection
The plants were cultivated in nutrient solution as described before (Zhu et al. 2012). Intact plant roots were immersed for 1 h in 1 L aerated collection solutions containing 1 mM NH4Cl and 1 mM CaCl2 and amended with various pharmacological agents.
To test the effect of PM H+ ATPase on the release of BNIs, two pharmacological agents were applied to the collection solutions to either stimulate or inhibit the activity of PM H+ ATPase in situ. Fusicoccin, a known stimulator of PM H+ ATPase phosphorylation and release of H+ (de Boer and de Vries-van Leeuwen 2012), was applied to root exudate collection solution at 1 μM. Vanadate (Na3VO4), a known inhibitor of PM H+ATPase (vanadate inhibits phosphorylation of PM H+ ATPase, which in turn blocks H+ excretion) (Palmgren 2001), was applied to root exudate solutions at 0.5 mM. Root exudates were collected, processed and BNI activity was determined as described earlier.
To understand anion-channels role in hydrophilic-BNIs release, two anion-channel blockers [Niflumic acid and anthracene-9-carboxylate, known for their inhibitory effect on trans-membrane transport of anions, such as Cl−, SO4 2−, citrate, malate etc. (Test 1990, Tyerman 1992, Schwartz et al. 1995)] were applied to root exudate collection solutions - niflumic acid (NIF) at 200 μM and 400 μM; whereas, anthracene-9-carboxylate (A9C) at 200 μM and 300 μM. Root exudates were collected using collection solutions (pH maintained at 6.0 by pH-stat systems) amended with anion-channel blockers mentioned above.
H+extrusion measurement
About one-tenth root exudates collection solution (100 ml) was titrated to pH 7.0 against 10 mM NaOH with a pH meter electrode inside the solution. NaOH amount was recorded to calculate the H+ extrusion rate (Zhu et al. 2005). Collection solution without root exudation was titrated as blank.
Statistical analysis
All experiments were repeated three times. Data from experiments were pooled for calculations of means and ± SE and analyzed by one-way ANOVA followed by the LSD test at P ≤ 0.05 to determine the statistical significance of the differences between individual treatments, indicated by letters above bars.
Results
Rhizosphere pH effects on the release of hydrophobic-BNI activity and sorgoleone from sorghum roots
Changes in rhizosphere pH (from 3.0 to 9.0) did not have much impact on the release of hydrophobic-BNIs and sorgoleone in root systems of ‘Hybridsorgo’ and 296B; in PVK 801, there is a marginal increase in sorgoleone release and hydrophobic-BNIs from root systems (Figs. 2a, b amd 3a, b). The hydrophobic-BNI activity and sorgoleone release in PVK 801 were significantly higher than 296B in all rhizosphere-pH treatments (Figs. 2b and 3b). A linear relationship was observed between sorgoleone levels in the root-DCM wash and hydrophobic-BNI activity (Fig. 4), reinforcing the functional relationship between hydrophobic-BNI activity and sorgoleone levels in sorghum (Fig. 4).
Rhizosphere pH influence on the release of hydrophilic-BNIs and PM H+ ATPase activity in sorghum root systems
Rhizosphere pH (ranging from 3.0 to 9.0) has a significant negative effect on the release of hydrophilic-BNIs from sorghum root systems (Fig. 5a, b); all the 3 sorghum genetic stocks used in this study showed a similar response, i.e. release of hydrophilic-BNIs was negatively impacted by an increase in rhizosphere pH. There were significant genotypic differences in hydrophilic-BNIs release; ‘Hybridsorgo’ consistently had higher BNI release (nearly 2-fold higher) across pH range (i.e. from 3.0 to 9.0) compared to PVK 801 and 296B (Fig. 5a, b). Thus, sensitivity of hydrophilic-BNIs release to higher rhizosphere pH depends on genotype; ‘Hybridsorgo’ appears to be less sensitive to higher rhizosphere pH (about 35% decline in hydrophilic-BNIs release at pH 9.0 compared to pH 3.0) compared to PVK 801 and 296B (about 65% decline in hydrophilic-BNIs release at pH 9.0 compared to pH 3.0) (Fig. 5a, b). Similarly, the PM H+-ATPase activity levels declined with increasing rhizosphere pH (from 3.0 to 6.0) (Fig. 6a, b). For ‘Hybridsorgo’, PM H+-ATPase activity levels declined to 75% at rhizosphere pH of 9.0 compared to 3.0; for PVK801 and 296B, there was only a 50% decline when rhizosphere pH increased from 3.0 to 9.0 (Fig. 6a, b). In general, PM H+-ATPase activity levels in ‘Hybridsorgo’ were significantly higher at all rhizosphere pH treatments compared to PVK 801 and 296B.
Statistical analysis indicates no relationship between PM H+ATPase and hydrophobic-BNI release in sorghum root systems (Fig. 7a). However, the release of hydrophilic-BNIs is positively linked with PM H+-ATPase activity levels in sorghum roots (Fig. 7b).
Influence of pharmacological agents on root exudation of H+ and hydrophilic-BNIs from sorghum roots
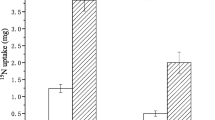
Fusicoccin, a H+ ATPase stimulator, enhanced H+extrusion (nearly 4-times higher) and BNIs activity release (about 30% increase) in ‘Hybridsorgo’ roots compared to control (Fig. 8a, b). Vanadate, which suppresses H+ ATPase activity, significantly inhibited the release of H+ and hydrophilic-BNIs from ‘Hybridsorgo’ roots (Fig. 8a, c). PVK 801 and 296B responded in a similar manner to the presence of fusicoccin and vanadate in root exudate collection solutions (3B,3D).
Influence of fusicoccin and vanadate in root exudate collection solutions on H+ exudation rate and hydrophilic-BNIs release in roots of ‘Hybridsorgo’ (a and c) and PVK 801 and 296 B (b and d). Bars represent means of 3 replicates ± SE. Letters above the bars indicate the significant difference between treatments (P ≤ 0.05)
Anion-channel blockers, NIF and A9C significantly stimulated H+ extrusion and release of hydrophilic-BNIs in all three sorghum genetic stocks (Fig. 9a, b, c, d). NIF 200 μM and 400 μM showed no difference in H+ extrusion rate and release of hydrophilic-BNIs in ‘Hybridsorgo’ (9A,9C). However, A9C resulted in a higher rate of H+ extrusion at 300 μM compared to 200 μM (Fig. 9a), but no difference in hydrophilic-BNIs release in ‘Hybridsorgo’ (Fig. 9c). For PVK 801 and 296B, anion-channel blockers have stimulated both H+-extrusion and hydrophilic-BNIs release, akin to ‘Hybridsorgo’ (Fig. 9b, d). H+-extrusion rate and hydrophilic-BNIs release were linearly correlated in all the 3 sorghum genotypes (Fig. 10).
Influence of anion-channel blockers (in root exudate collection solutions) on H+ exudation rate and hydrophilic-BNIs release in sorghum roots; ‘Hybridsorgo’ (a and c); PVK 801 and 296 B (b and d). Bars represent means of 3 replicates ± SE. Letters above the bars indicate the significant difference between treatments (P ≤ 0.05)
Discussion
Sorghum roots release two categories of nitrification inhibitors from roots, i.e. hydrophilic BNIs and hydrophobic BNIs (Subbarao et al. 2013). Previous research indicated that hydrophilic BNIs release from ‘Hybridsorgo’ is linked to PM-H+ ATPase caused by ammonium uptake (Zhu et al. 2012), and ammonium assimilation in root cells (Zeng et al. 2016). In the present study, we further establish that rhizosphere pH has a regulatory role in the release of hydrophilic-BNIs (pH ranges tested from 3.0 to 9.0), based on three sorghum genotypes tested (Fig. 5a, b). In addition, the functional link between PM H+ ATPase activity and hydrophilic-BNIs release has been demonstrated further, using root systems of three sorghum genotypes (Fig. 6a, b and 7a, b).
These results suggest that rhizosphere acidification caused by ammonium uptake could trigger PM H+ ATPase activity which in turn stimulated hydrophilic-BNIs release (Zhu et al. 2012). Apart from ammonium uptake, its assimilation in the root cells is also critical to trigger H+ pumps running to sustain the accelerated release of hydrophilic-BNIs (i.e., ammonium assimilation produces H+ in the cytoplasm of root cells; to keep the pH constant in cytoplasm, PM H+ ATPase activity is activated to pump H+ outside root cells, which in turn facilitates hydrophilic-BNIs release) (Zeng et al. 2016).
Presence of ammonium in rhizosphere thus stimulates BNIs release, thereby protecting ammonium from nitrifiers, which turn facilitates ammonium uptake and assimilation; the interplay among various processes associated with ammonium uptake, assimilation, operation of H+-pumps (in PM) determines the release of hydrophilic-BNIs from roots and acts as a feedback mechanism between ammonium uptake and BNI release. The high-BNI release capacity in plant root systems can thus lead to higher acidification of rhizosphere, lowers nitrifier activity, and trigger BNIs release that lead to suppression of nitrifier activity, which further improves ammonium availability in the rhizosphere, thus, facilitates its uptake and assimilation – acting as a feedback loop and is part of ecological adaptation to low-N production environments and evolution of BNI function as an adaptive trait.
The present study further confirms the functional link between PM H+ ATPase and release of hydrophilic BNIs using pharmacological agents. Fusicoccin that serves as PM-H+ ATPase stimulator simultaneously enhanced H+-extrusion and BNIs activity released from roots of ‘Hybridsorgo’, PVK801 and 296B; Vanadate, which is a PM-H+ ATPase inhibitor, significantly inhibited H+ extrusion and hydrophilic-BNIs release in ‘Hybridsorgo’, PVK801 and 296B (Fig. 8), conforming earlier reports (Zhu et al. 2012).
Unlike hydrophilic-BNIs release, hydrophobic-BNIs release appears to be largely unaffected by rhizosphere pH in all the 3 sorghum genetic stocks (Fig. 3). These findings are in support of our earlier observations that suggest hydrophobic BNIs being stable under a range of pH (pH from 3 to 9) and continue their inhibiting activity (Subbarao et al. 2013). Also, a nonlinear relationship between the PM-H+ ATPase activity and hydrophobic-BNI activity was detected for ‘Hybridsorgo’, PVK 801 and 296B (Fig. 7a), suggesting that the release of hydrophobic-BNIs is independent of PM H+ ATPase activity. These results suggested that the mechanisms operating for hydrophobic-BNIs release are likely to be different from hydrophilic-BNIs release in sorghum roots and require further research.
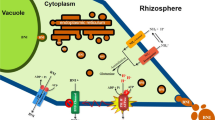
We have earlier proposed hydrophilic BNIs to be anionic substances, and are likely to be released through anion-channels (Subbarao et al. 2006b; Zhu et al. 2012). However, the two commonly used anion-channel blockers did not inhibit the release of hydrophilic-BNIs from roots of all the three sorghum genotypes (Fig. 9). The observed linear relationship between H+ extrusion and hydrophilic-BNIs release provided a direct evidence for the existence of a strong connection between H+ and BNIs release in sorghum roots (Fig. 10). Since the anion-channel blockers did not inhibit plasma membrane H+ ATPase (data not shown), which is also reported by other researchers (Zhu et al. 2005), we hypothesize that a part of these H+ might be released with hydrophilic-BNIs (i.e. protonated BNIs?) together through an unknown BNI channels. Hydrophilic-BNIs seem to carry more negative charges, which can be protonated in cytoplasm. When the release of anions (i.e., Cl−, SO4 2−) were inhibited by anion-channel blockers, the charge balance across the plasma membranes can be disturbed by a decrease in anion release, thus, triggering the release of protonated BNIs to keep the charge balance, and to release H+ into the rhizosphere. Hence, a new hypothesis is proposed for the transport of hydrophilic-BNIs from root systems of sorghum (Fig. 11).
Proposed new hypothesis for the release of hydrophilic-BNIs in sorghum roots. Schematic description of the BNIs release process; the uptake of ammonium/ammonia causes release of H+ in rhizosphere, and this triggers PM H+ ATPase activity, which in turn stimulates release of anionic BNIs through an unknown BNI-channel. In addition, assimilation of ammonium/ammonia in root cells produce H+, which needs to be pumped out of the root cells to prevent cytosolic acidification; PM H+ ATPase can also be activated by cytosolic H+, which can regulate hydrophilic-BNIs release. It is interesting to note that BNIs may carry more negative charges, which can be protonated in cytoplasm before release. When the release of anions (eg. Cl−, SO4 2−) were inhibited by anion-channel blockers, the charge balance across the plasma membranes can be disrupted from the decrease in anion release, and this can trigger release of protonated BNIs to maintain charge balance in the cytoplasm of root cells; this can also bring H+ from outside into root cells together
Conclusion and potential implications
We conclude that PM H+ATPase plays a major role in the regulation of hydrophilic BNIs release from sorghum roots. However, the release of hydrophobic-BNIs from sorghum roots is not influenced by the rhizosphere pH, suggesting that hydrophobic-BNIs release may follow other transport processes. Hydrophilic-BNIs are transported through some unknown anion-channels, which are voltage dependent on the activity of PM H+ ATPase, and might be also transporting protonated BNIs. Further research is needed to establish this hypothesis (Fig. 11).
Sorgoleone release from root systems contributes to about 50% of the BNI potential in sorghum roots (Subbarao et al. 2013). The pH insensitivity for sorgoleone release will have implications for BNI-trait expression in soil types with low buffering capacity. Genetic improvements in sorgoleone release can thus result in future sorghum varieties with high-BNI capacity in root systems where the trait is expressed in a range of soil-types with varying pH, thus can have wider adaptation to low-N production environments with improvements in agronomic-NUE.
References
Alsaadawi IS, Al-Uquili JK, Alrubeaa AJ, Al-Hadithy SM (1986) Allelopathic suppression of weed and nitrification by selected cultivars of Sorghum Bicolor (L.) Moench. J Chem Ecol 12:209–219
Amberger A (1989) Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plant Anal 20:1933–1955
Baginski ES, Foa PP, Zak B (1967) Determination of phosphate: study of labile organic phosphate interference. Clin Chim Acta 15:155–158
de Boer AH, de Vries-van Leeuwen IJ (2012) Fusicoccanes: diterpenes with surprising biological functions. Trends Plant Sci 17:360–368
Czarnota MA, Rimando AM, Weston LA (2003) Evaluation of seven sorghum (Sorghum sp) accessions. J Chem Ecol 29:2073–2083
Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO (2010) Sorgoleone. Phytochemistry 71:1032–1039
Hossain AKMZ, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum Bicolor). New Phytol 180:442–451
Iizumi T, Mizumoto M, Nakamura KA (1998) Bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl Environ Microbiol 64:3656–3662
Larsson C (1985) Plasma membrane. In: Modern methods of plant analysis (eds H.F. Linskens & J.F. Jackson). Springer-Verlag, Berlin, pp 85–104
Lewis OAM, James DM, Hewitt EJ (1982) Nitrogen assimilation in barley (Hordeum vulgare L. cv. Mazurka) in response to nitrate and ammonium nutrition. Ann Bot 49:39–49
Marschner H (1995) Mineral nutrition Plant of Higher Plants. Academic, San Diego
Meinshausen M, Meinshausen N, Hare W, Raper SCB, Frieler K, Knutti R, Frame DJ, Allen MR (2009) Greenhouse-gas emission targets for limiting global warming to 2°C. Nature 458:1158–1162
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Parker JH (1972) How fertilizer moves and reacts in soil. Crops Soils 72:7–11
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363
Schwartz A, Ilan N, Schwarz M, Scheaffer J, Assmann SM, Schroeder JI (1995) Anion-channel blockers inhibit S-type anion channels and abscisic acid response in guard cells. Plant Physiol 109:651–658
Slangen J, Kerkhoff P (1984) Nitrification inhibitors in agriculture and horticulture: a literature review. Fertil Res 5:1–76
Smart DR, Bloom AJ (2001) Wheat leaves emit nitrous oxide during nitrate assimilation. Proc Nat Acad Sci (USA) 98:7875–7787
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006a) Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Crit Rev Plant Sci 25:303–335
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006b) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007a) Biological nitrification inhibition (BNI) – is it a widespread phenomenon? Plant Soil 294:5–18
Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL (2007b) NH4 + triggers the synthesis and release of biological nitrification inhibition compounds in Brachiara humidicola roots. Plant Soil 290:245–257
Subbarao GV, Ban T, Kishi M, Ito O, Samejima H, Wang HY, Pearse SJ, Gopalakrishnan S, Nakahara K, Hossain AKMZ, Tsujimoto H, Berry WL (2007c) Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 299:55–64
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Nat Acad Sci (PNAS) (USA) 106:17302–17307
Subbarao GV, Sahrawat KL, Nakahara K, Ishikawa T, Kishii M, Rao IM, Hash CT, George TS, Srinivasa Rao P, Nardi P, Bonnett D, Berry W, Suenaga K, Lata JC (2012) Biological nitrification inhibition (BNI) – a novel strategy to regulate nitrification in agricultural systems. Adv Agron 114:249–302
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Zhu Y, Zakir HAKM, Deshpande SP, Hash CT, Sahrawat KL (2013) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata JC, Kishii M, Braun H-J (2015) Suppression of soil nitrification by plants. Plant Sci 233:155–164
Subbarao GV, Arango J, Masahiro K, Hooper AM, Yoshihashi T, Ando Y, Nakahara K, Deshpande S, Ortiz-Monasterio I, Ishitani M, Peters M, Chirinda N, Wollenberg L, Lata JC, Gerard B, Tobita S, Rao IM, Braun HJ, Kommerell V, Tohme J, Iwanaga M (2017) Genetic mitigation strategies to tackle agricultural GHG emissions: the case for biological nitrification inhibition technology. Plant Sci 262:165–168
Test M (1990) Tansley review no. 1 plant ion channels: whole-cell and single-channel studies. New Phytol 114:305–340
Tsehaye T, Yoshinaga H, Deshpande SP, Srinivasa Rao P, Sahrawat KL, Ando Y, Nakahara K, Hash CT, Subbarao GV (2014) Biological nitrification inhibition in sorghum: the role of sorgoleone production. Plant Soil 379:325–335
Tyerman SD (1992) Anion channels in plants. Annu Rev Plant Physiol Plant Mol Biol 43:351–357
Weiske A, Benckiser G, Ottow JCG (2001) Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr Cycl Agroecosys 60:57–64
Yamashita K, Yamamoto Y, Matsumoto K (1996) Characterization of an anion transporter in the plasma membrane of barley roots. Plant Cell Physiol 37(7):949–956
Yan F, Zhu Y, Muller C, Zorb C, Schubert S (2002) Adaptation of H+-pumping and PMH+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Zeng H, Di T, Zhu Y, Subbarao GV (2016) Transcriptional response of plasma membrane H+-ATPase genes to ammonium nutrition and its functional link to the release of biological nitrification inhibitors from sorghum roots. Plant Soil 398:301–312
Zerulla W, Barth T, Dressel J, Erhardt K, Von-Locquenghien KH, Pasda G, Radle M, Wissemeier H (2001) 3, 4- Dimethylpyrazole phosphate (DMPP)-a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84
Zhu Y, Yan F, Zörb C, Schubert S (2005) A link between citrate and proton release by Proteoid roots of WhiteLupin (Lupinus albus L.) grown under phosphorus-deficient conditions? Plant Cell Physiol. 46(6):892–901
Zhu YY, Zeng HQ, Shen QR, Ishikawa T, Subbarao GV (2012) Interplay among NH4 + uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant Soil 358:131–141
Acknowledgments
The research presented here is funded by grant-in-Aid for scientific research from Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) to JIRCAS under BNI project. Funding support also came from Natural Science Foundation of China (NSFC 31172035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap.
Rights and permissions
About this article
Cite this article
Di, T., Afzal, M.R., Yoshihashi, T. et al. Further insights into underlying mechanisms for the release of biological nitrification inhibitors from sorghum roots. Plant Soil 423, 99–110 (2018). https://doi.org/10.1007/s11104-017-3505-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3505-5