Abstract
Background and aims
Increasing atmospheric nitrogen (N) deposition and biological invasion have become major concerns with global environmental change. This study aimed to determine the effects of an exotic species on a native one under increasing N deposition.
Methods
We conducted a greenhouse experiment in which the exotic species Robinia pseudoacacia and the native species Quercus acutissima were grown in mixture and monocultures under four levels of simulated N deposition (0, 3, 6, 12 g m−2 year−1). After 12 weeks of treatment, plant growth, leaf physiological traits and soil chemical properties were determined.
Results
With its strong capability for nutrient absorption and carbon assimilation, R. pseudoacacia dominated in competition. R. pseudoacacia reduced the growth of Q. acutissima, but the relative competition index decreased with increasing N deposition. At the end of the experiment, the soil available phosphorus (P) in mixture was significantly lower than that in the monoculture of Q. acutissima, while the soil available N in the two cultivations did not show obvious differences.
Conclusions
Increased N deposition alleviated the competitive effects of R. pseudoacacia on Q. acutissima. In the future, besides N, increased P availability should also be considered in the interaction between the two species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of its huge ecological impacts, increasing atmospheric nitrogen (N) deposition has aroused widespread concern (Reay et al. 2008; Stevens et al. 2011; Liu et al. 2013b). Owing to frequent anthropogenic activities, such as fossil fuel burning and fertilizer use, atmospheric N deposition has risen sharply since the Industrial Revolution (Reay et al. 2008; Maskell et al. 2010). Elevated N deposition can enhance the content of soil available N and has been proven to promote plant production (Bai et al. 2010; Zhang et al. 2013). On the other hand, excess N may cause a series of problems such as N and base cation leaching and soil acidification (Fang et al. 2009; Lu et al. 2009), and thus limit plant growth, reduce biological diversity and affect ecosystem functioning (Reich 2009; Bobbink et al. 2010; Zhang et al. 2013). In addition, the level of N deposition is also considered to relate to the invasibility of ecosystems (Davis et al. 2000). Positive correlations have been found between N deposition and the abundance of invasive species at different scales (Scherer-Lorenzen et al. 2007).
As an important component of human-caused global climate change, biological invasion is considered one of the most severe environmental problems (Sala et al. 2000; Jiang et al. 2009). Generally, invasive plants grow fast and are capable of allocating more resources to photosynthesis and using resources more efficiently. With these strategies, they may out-compete the co-occurring native species (Jiang et al. 2009). Additionally, invasive species may alter the soil nutrient conditions, modify the soil biota and release allelochemicals (Callaway and Ridenour 2004; Jordan et al. 2008; Perkins and Nowak 2013), all of which may decrease the fitness of native species. At a larger scale, biological invasion may threaten the species richness and diversity of native communities, change ecosystem carbon and nitrogen cycles and affect ecosystem structure and functioning (Ehrenfeld 2010; Vilà et al. 2011; Pyšek et al. 2012).
In the context of increasing atmospheric N deposition, the magnitude and pattern of the influence of invasive species on natives may be changed (Dukes and Mooney 1999; Bradford et al. 2007). As pointed out by Dukes and Mooney (1999), increased N deposition may benefit fast-growing invasive species and therefore threaten slow-growing native species adapted to low resource levels. Several studies have demonstrated that elevated N availability increases the competitive ability of invasive species over natives (Vasquez et al. 2008; He et al. 2012). However, other research has not confirmed such a conclusion (e.g. Thomsen et al. 2006; Bradford et al. 2007). If the growth of exotic and native species is restricted by different resources, elevated N availability may not exacerbate the competitive effects of exotics (Bradford et al. 2007). On the whole, experimental evidence is still insufficient and the impact of increasing atmospheric N deposition on the competitive effects of invasive species remains to be confirmed.
To examine how the impacts of invasive species on natives respond to increasing N deposition, a greenhouse experiment was conducted with an exotic tree species (Robinia pseudoacacia) and a native tree species (Quercus acutissima). R. pseudoacacia is a deciduous member of the Fabaceae family. Native to North America, it is now widespread across Europe and Asia (Von Holle et al. 2006; Cierjacks et al. 2013) and is accounted one of the most invasive species in the world (Boring and Swank 1984). Kawaletz et al. (2013a, b) reported that strong competitiveness enabled R. pseudoacacia to suppress the growth of native species. However, because of its N2-fixation ability, R. pseudoacacia is also considered to facilitate the growth of neighboring plants (Von Holle et al. 2006; Ding et al. 2012). Therefore, the relationships between R. pseudoacacia and native species still remain an open question. Q. acutissima, a deciduous member of the Fagaceae family, is one of the most important constructive species (dominant species in the tree layer of a community) of forests in North China (Wang and Zhou 2000). Both species are widely used in reforestation, therefore mixed forests of R. pseudoacacia and Q. acutissima are very common (Wang and Zhou 2000). It was reported that a strong capacity for root lateral extension and clonal growth enabled R. pseudoacacia to intrude into nearby Q. acutissima forest and suppress the growth of young oaks (Wang and Wang 1996; Zhang et al. 2008). A similar phenomenon was also found in South Korea (Lee et al. 2004).
In our greenhouse experiment, seedlings of R. pseudoacacia and Q. acutissima were grown both in monocultures and mixture. Four levels of N were applied to simulate increasing atmospheric N deposition. The following questions were addressed:
-
1.
Is there a competition between R. pseudoacacia and Q. acutissima in mixture? If so, what plant traits contribute to the competitiveness of R. pseudoacacia?
-
2.
Is the competitive pressure of R. pseudoacacia on Q. acutissima exacerbated with increasing N levels?
Materials and methods
Study site
The experiment was carried out at the Fanggan Research Station of Shandong University, Shandong Province, China (36°26′N, 117°27′E). The station is located in the Central Mountainous Region of Shandong Province. This area has a typical temperate monsoon climate with a mean annual temperature of 13 ± 1 °C and a mean annual precipitation of 700 ± 100 mm. The soil type is a yellow cinnamon soil with limestone as the parent material (Wang and Zhou 2000). The whole experiment was carried out in a greenhouse at the station to ensure a controlled and homogenous environment. Made up of steel pipes, the framework of the greenhouse was covered by plastic film. By rolling up the plastic film at the sides, the greenhouse was kept well ventilated. Using a portable temperature and humidity data logger (MicroLog EC650, Fourtec, USA), the microclimate in the greenhouse was monitored. During the growth period of the plants, the average temperature in the greenhouse ranged from 20.3 to 36.2 °C during the day and 10.0 to 26.7 °C during the night. The average day length relative humidity varied from 30.9 to 88.0 %.
Plant materials
Seeds of R. pseudoacacia and acorns of Q. acutissima were collected from hills near the research station in October, 2012 and stored at 0–4 °C during the winter. In mid-April 2013, they were soaked in distilled water for 24 h and stimulated to germinate in a growth chamber. When the radicles were about 2 cm long, healthy and uniform seedlings were selected and transplanted into plastic pots (25 cm × 24 cm, height × diameter). Each pot was filled with 6.3 kg loam and 2.1 kg sand, which were carefully sieved to remove debris and stones. The substrates were mixed thoroughly and their chemical properties were determined as pH 6.51, available N 50.20 mg kg−1 and available P 31.14 mg kg−1. During the experiment, all of the pots received enough water. Weeds and insects were controlled manually.
Experimental design
The seedlings were arranged into three cultivation types: monocultures with two seedlings of the same species (R. pseudoacacia or Q. acutissima) and a mixture with one seedling of each species. Each cultivation type received four N treatments: 0, 3, 6 and 12 g m−2 year−1 (designated N0, N1, N2 and N3). N0 was set as the control; N1 and N2 corresponded to levels of N deposition already recorded in some areas of northern and southern China (Lü and Tian 2007; Zhang et al. 2011); N3 represented a high deposition level that may be reached in the future. There were 12 treatment combinations in total and each combination contained six pots as replicates. All of the pots were arranged randomly and rearranged regularly during the experiment.
Beginning on June 16, the N treatments were applied biweekly seven times in total, ending on September 8. According to the report that the ratio of NH4-N and NO3-N in atmospheric N deposition in China was about two in recent years (Liu et al. 2013b), we simulated the N deposition by adding mixed solutions of (NH4)2SO4 and KNO3 (1:1, M/M). K2SO4 and KCl solutions of different concentrations were also added to different N treatments to ensure that all treatments received the same amount of K as well as S. Since about 70 % of the annual precipitation falls during June to August in North China (Wang and Zhou 2000), solutions corresponding to 10 % of the annual N deposition were added each time. The compositions and concentrations of the solutions applied to the four N treatments during the experiment are shown in Table 1.
Measurements
Seedling height (H) was measured at the beginning and end of the N treatment. The relative growth rate of height (RGRH) during the N treatment was calculated with the formula: RGRH = (lnH2 − lnH1)/t, in which H2 and H1 stood for the seedling height at the end and the beginning of the N treatment, and t stood for the time duration (88 days).
After 64 days of N treatment, the maximum net photosynthetic rate (A max ) was measured between 8:30 and 12:00 from 19 to 21 August, 2013. All 3 days were sunny. Using a portable leaf gas exchange system (GFS-3000, Walz GmbH, Effeltrich, Germany), a PPFD of 1,000 μmol m−2 s−1 was provided by a red-blue light-emitting diode to ensure that all seedlings were light-saturated (derived from a pre-experiment, data not shown). Three to four fully expanded leaves from the upper shoots were selected in each treatment (one leaf per pot for monocultures and one leaf per species per pot for mixture) and the measurements were conducted alternately among different treatments. The mean air temperature, relative humidity and CO2 concentration values in the leaf cuvette were 27.51 °C, 51.0 % and 399.5 μmol mol−1.
Leaf morphology was measured at the beginning of September. Five to six fully expanded leaves from the upper shoots in each treatment (one leaf per pot for monocultures and one leaf per species per pot for mixture) were scanned and images were obtained. Then, the leaf area was calculated with the WinFOLIA Pro 2009a software (Regent Instruments, Inc., Quebec, Canada). After scanning, these leaves were oven-dried at 80 °C for 48 h and weighed. Specific leaf area (SLA) was calculated as leaf area/leaf dry mass.
At the end of the experiment, all seedlings were harvested and the roots were washed carefully with tap water. Each whole seedling was divided into four parts: leaf, stem, main root and lateral root. The main root was determined to be the one that grew directly from the seed, while the lateral roots were those that branched laterally from the main root (Guo et al. 2013). All seedling parts were oven-dried at 80 °C for 48 h and weighed. Total biomass and biomass allocation were calculated as follows:
The total biomass in monocultures was averaged from the two individuals in the same pot.
The effect of interaction between the two species was assessed by the relative competition index (RCI). The RCI was calculated with the following equation (Werner et al. 2010):
where TBii stands for the average total biomass of one species in monoculture, and TBij for the total biomass of the same species in mixture.
After the measurement of biomass, 4 to 6 leaf samples in each treatment were kept for the determination of leaf nitrogen (LN) and leaf phosphorus (LP) concentrations with the Kjeldahl method and the Molybdenum antimony-D-isoascorbic-acid colorimetry method (Yuan et al. 2013). Then the photosynthetic nitrogen use efficiency (PNUE) and photosynthetic phosphorus use efficiency (PPUE) (Hidaka and Kitayama 2009) were calculated as follows:
where LMA means leaf mass per area, the reciprocal of SLA; 14 and 31 are the relative atomic masses of N and P.
Soil samples were collected from all pots after the experiment for chemical analyses. Soil available N was analyzed with the alkaline hydrolysis diffusion method (Zhang et al. 2013) and soil available P was analyzed colorimetrically after extraction by sodium bicarbonate (Olsen et al. 1954). The chemical analyses of soil were performed at Shandong Agricultural University.
Statistical analysis
Two-way analysis of variance (ANOVA) was performed to test the effects of cultivation and N treatment and their interactions. One-way ANOVA and Duncan’s multiple range tests (DMRT) at p ≤ 0.05 were conducted to find the differences among treatments. Before ANOVA, the data were checked for normality and homogeneity of variance. When necessary, log transformation or square root transformation was applied. All statistical analyses were performed with the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). All figures were drawn with the Origin 8.0 software (Originlab Co., Northampton, MA, USA).
Results
Plant growth
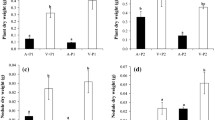
The seedling height (H), relative growth rate of height (RGRH) and total biomass (TB) of R. pseudoacacia were much higher than those of Q. acutissima (Fig. 1). For R. pseudoacacia, mixed cultivation significantly increased H (Table 2, Fig. 1). In mixture, H of R. pseudoacacia tended to be higher compared with monoculture, but the differences were mostly not statistically significant (Fig. 1a). No significant interaction was confirmed between cultivation and N addition (Table 2). For Q. acutissima, H, RGRH and TB were all significantly decreased by mixed cultivation, but N addition did not show significant effects (Table 2, Fig. 1). H was 40.1, 18.0 and 36.7 % lower in mixture than in monoculture under the N0, N1 and N2 treatments and significant differences were observed for the N0 and N2 treatments (Fig. 1b). In mixture, RGRH was lower than that in monoculture, and the differences were statistically significant in all N treatments except for N1 (Fig. 1d). TB was 54.5, 29.1 and 39.1 % lower in mixture than in monoculture under the N0, N1 and N2 treatments, and significant differences were observed for the N0 and N2 treatments (Fig. 1f). Specifically, we found a significant interaction between cultivation and N addition on the TB of Q. acutissima (Table 2). In monoculture, TB did not exhibit significant changes among N treatments; in mixture, TB showed an obvious rising trend with the addition of N and was significantly higher in N3 than in the N0 treatment (Fig. 1f).
Seedling height (H), relative growth rate of height (RGRH) during the nitrogen treatment and total biomass (TB) of R. pseudoacacia (a, c, e) and Q. acutissima (b, d, f) under different cultivations and nitrogen additions. The values are shown as mean ± SE (n = 4–6). Different letters denote significant differences (p ≤ 0.05) with Duncan’s test
Biomass allocation
The root to shoot ratio (RSR) and main root to lateral root ratio (MLR) of R. pseudoacacia were both distinctly lower than those of Q. acutissima in all N treatments (Fig. 2). For Q. acutissima, RSR was significantly increased by mixed cultivation (Table 2, Fig. 2). In mixture, it was significantly higher than in monoculture except for the N1 treatment (Fig. 2b).
Leaf traits
Nitrogen addition did not significantly affect the leaf traits of the two species, and cultivation only significantly influenced the PPUE and SLA of Q. acutissima (Table 2). The A max , PNUE, PPUE and SLA of R. pseudoacacia were all much higher than those of Q. acutissima (Fig. 3).
Maximum net photosynthetic rate (A max ) (n = 3–4), photosynthetic nitrogen use efficiency (PNUE) (n = 3–4), photosynthetic phosphorus use efficiency (PPUE) (n = 3–4) and specific leaf area (SLA) (n = 5–6) of R. pseudoacacia (a, c, e, g) and Q. acutissima (b, d, f, h) under different cultivations and nitrogen additions. The values are shown as mean ± SE. Different letters denote significant differences (p ≤ 0.05) with Duncan’s test
In addition, the leaf N:P ratios of the two species in mixture were significantly affected by N addition. The leaf N:P ratio of R. pseudoacacia under the N3 treatment was significantly lower than that under N1 (Fig. 4a), while for Q. acutissima, it was significantly lower with N addition than in the control group (Fig. 4b).
Competitive effects of R. pseudoacacia on Q. acutissima
The relative competition index (RCI, derived from the total biomass of Q. acutissima) was significantly affected by N addition. With increasing N deposition, it showed an obvious declining trend and a significant difference was found between the N0 and N3 treatments. With the highest N addition level, the RCI was negative, while in the other three N treatments it was positive (Fig. 5).
Soil available N and P
After the experiment, the soil available N was not significantly different among the three cultivation types. However, the soil available P in the monoculture of Q. acutissima was significantly higher than that in mixture and the monoculture of R. pseudoacacia among most N treatments (Table 3).
Discussion
Plant growth
In our experiment, the seedling height (H), relative growth rate of height (RGRH) and total biomass (TB) of Q. acutissima all tended to be lower in mixture than in monoculture under the same N treatment. Significant differences were observed for H and TB under the 0 and 6 g m−2 year−1 N levels and RGRH under all but the 3 g m−2 year−1 N level. These results suggested that the growth of Q. acutissima was inhibited by R. pseudoacacia. For R. pseudoacacia, H, RGRH and TB were mostly not significantly different under the same N treatment between the two cultivation types, indicating that it was largely unaffected by Q. acutissima. Therefore, we can conclude that there was an obvious competition between the two species and that R. pseudoacacia was the superior competitor. R. pseudoacacia was also reported to be strongly competitive with some other native species (Kawaletz et al. 2013a, b). Although it has been confirmed that some exotic species from the Fabaceae family can facilitate the growth of co-occurring native species by sharing the products of N2-fixation (Yang et al. 2009; Hellmann et al. 2011), they may compete with native plants if other limiting resources exist, such as water or soil phosphorus (Kawaletz et al. 2013b).
Functional traits under competition
Invasive species are often characterized by a series of functional traits that act synergistically to confer competitive success (Morris et al. 2011). These traits usually include high growth rate, biomass production, photosynthetic rate, and SLA, and efficient use of available resources (Jiang et al. 2009; Lamarque et al. 2011). In our experiment, R. pseudoacacia showed a much higher relative growth rate of height (RGRH) than Q. acutissima. From the perspective of community succession, R. pseudoacacia is an early successional species that is usually typified by fast growth, especially in the early stage (Boring and Swank 1984; Closset-Kopp et al. 2007; Callaway et al. 2011). This high relative growth rate may lead R. pseudoacacia to acquire more resources from the soil, resulting in resource depletion and hindering the growth of neighboring plants (Kawaletz et al. 2013b). Above the ground, the fast-growing R. pseudoacacia may overtop Q. acutissima rapidly and out-compete it for light since height is the key factor in determining the success of light competition between individuals (Gorchov and Trisel 2003).
According to Hoffmann and Poorter (2002), A max and SLA are the most important factors influencing relative growth rate. Generally, SLA is highly negatively correlated with cell wall mass (Onoda et al. 2004), which is linked to leaf toughness, a basic defensive trait (Feng et al. 2009). To grow faster, plants have to distribute more resources to the photosynthetic apparatus, and thus less to structural defense (Feng et al. 2009). Therefore, the much higher SLA of R. pseudoacacia may indicate that compared with Q. acutissima, it allocated less resources to structural defense and more to photosynthesis, leading to a high A max and growth rate. In addition, the greater SLA may help R. pseudoacacia increase light capture and reduce CO2 diffusion resistance, and thus to maintain a high A max and biomass production (Hanba et al. 1999; Hidaka and Kitayama 2009).
The root structure and strategy of biomass allocation might also confer R. pseudoacacia a strong competitive ability. The nutrient acquisition of plants is largely affected by root structure, which includes root spatial distribution, surface area and biomass (Lambers et al. 2008). R. pseudoacacia is able to both produce deep roots and spread roots horizontally (Stone and Kalisz 1991; Cierjacks et al. 2013), forming an extensive root system. In our experiment, R. pseudoacacia produced a much lower main root to lateral root ratio (MLR) than Q. acutissima. Therefore, R. pseudoacacia occupied most of the rooting space of the pot in mixture with Q. acutissima. By intruding into the shared rooting space, R. pseudoacacia may obtain additional resources from the soil and hamper the growth of Q. acutissima by decreasing its resource supply. Additionally, the large lateral root biomass produced by R. pseudoacacia conferred it a large root surface area, which also helps plants absorb nutrients effectively (Yuan et al. 2013). Compared with Q. acutissima, R. pseudoacacia showed a distinctly lower root to shoot ratio. However, because of its strong photosynthetic capacity and high growth rate, R. pseudoacacia in mixture produced 3.4 to 9.8 times more total biomass than Q. acutissima under different N levels. Therefore, even with a much lower root to shoot ratio, it was still able to maintain 1.0 to 2.8 times greater root biomass than Q. acutissima and acquire a large amount of nutrients. Moreover, by allocating more biomass aboveground, R. pseudoacacia was able to achieve a tall shoot and a large canopy, which enabled it to capture light effectively and suppress the growth of Q. acutissima by shading it.
In addition to active nutrient acquisition, R. pseudoacacia also showed higher photosynthetic nitrogen and phosphorus use efficiency than Q. acutissima in all treatments. Invasive species usually use resources more efficiently than native species (Jiang et al. 2009; Godoy et al. 2011) and this has been predicted to be a crucial feature determining the invasiveness of exotic species (Funk and Vitousek 2007). This strong ability to use resources effectively may also confer R. pseudoacacia an advantage to outperform native plants.
Under the strong competition of R. pseudoacacia, Q. acutissima also changed its strategy for growth and biomass allocation. Compared with monoculture, Q. acutissima grown in mixture significantly increased its biomass allocation to roots. This was consistent with the balanced growth hypothesis, which suggests that plants may increase the biomass allocated to organs involved in nutrient acquisition when faced with deficient resource supply (Shipley and Meziane 2002). Because of the high growth rate and strong ability to take up nutrients of R. pseudoacacia, Q. acutissima may well face a resource deficiency. Therefore, the altered biomass allocation was a response to limited soil resource availability. Although the light available to Q. acutissima also decreased because of the shading by R. pseudoacacia, alleviating the belowground resource deficiency by increasing biomass partitioning to roots was more important for Q. acutissima. Similar results were also reported by Jose et al. (2003). By contrast, there were no significant changes in the biomass allocation of R. pseudoacacia between above- and belowground organs, suggesting its strong competitiveness or increased resource acquisition efficiency (Kawaletz et al. 2013a). Although Q. acutissima adopted this strategy, it still could not offset the strong competition from R. pseudoacacia. Its relative growth rate was significantly lower in mixture than in monoculture and its biomass production was also lower in most N treatments.
Competitive effects of R. pseudoacacia on Q. acutissima under increasing N deposition
According to Dukes and Mooney (1999), increased N deposition may create beneficial conditions for fast-growing invasive species, thus disadvantaging slow-growing native species. Many studies have also confirmed that under increased N availability, the rapid response to N enrichment favored invasive species and the competition pressure on less N-responsive native species was intensified (Vasquez et al. 2008; He et al. 2012). However, in our experiment, N addition alleviated the competitive effects of R. pseudoacacia on Q. acutissima, as indicated by the RCI, which showed a declining trend with increasing N level. At the highest N addition level, RCI was negative and significantly different from that in the control group. Nonetheless, we could not conclude that the growth of Q. acutissima was facilitated by R. pseudoacacia under the highest N level, since there was no significant increase in the growth of Q. acutissima compared with monoculture.
The negative impacts of R. pseudoacacia on Q. acutissima were mitigated because in mixture the total biomass of Q. acutissima gradually increased while that of R. pseudoacacia remained unchanged with increasing N deposition. In monoculture, the total biomass of Q. acutissima was not significantly affected by N addition and the soil available N after the experiment was not significantly different from that in mixture, suggesting that the increasing total biomass of Q. acutissima in mixture was not promoted by N itself. Combined with the fact that Q. acutissima is well adapted to nutrient-poor habitats (Wang and Zhou 2000), we consider that N was not a major limiting factor for the growth of Q. acutissima. By contrast, in mixture, the soil available P after the experiment was significantly lower than that in the monoculture of Q. acutissima and showed an obvious decline relative to the soil before the experiment, which indicates that R. pseudoacacia may possess a strong competitive capacity for soil P. Other researchers have also concluded that N2-fixing species may absorb disproportionately larger amounts of P since the N2-fixation process requires a high P level (Killingbeck 1993; Uliassi and Ruess 2002). Additionally, in view of the report that most soils in China are P-limited for plants (Han et al. 2005) and the limited amount of soil used in the pot experiment, Q. acutissima in mixture probably experienced P deficiency.
However, there are physiological processes by which plants can enhance their acquisition of P by using N, and as such, higher soil N levels become beneficial in P-limited environments (Treseder and Vitousek 2001; Marklein and Houlton 2012). In mixture, the leaf N:P ratio of Q. acutissima with N addition was significantly lower than that in the control group. Nutrients in leaves can be obtained from within the plants and from the soil (Lukac et al. 2010; Liu et al. 2013a), and explicitly mirror the short-term nutrient demands of plants. Therefore, we can conclude that N addition probably mitigated the P deficiency of Q. acutissima in mixture caused by the strong competitiveness of R. pseudoacacia. Liu et al. (2013a) also found that N addition significantly lowered the leaf N:P ratios of two tree species and suggested that N addition could help the plants to alleviate P limitation. With increased N availability, plants are able to invest excess N to structure extracellular phosphatases (Treseder and Vitousek 2001), which mineralize soil organic P and release phosphate for plants (Pant and Warman 2000). Since they are anchored on the root surface, most of the released phosphate can be obtained by the same plant (Treseder and Vitousek 2001). A meta-analysis by Marklein and Houlton (2012) also suggested that across a wide range of biomes, N fertilization substantially improved the activity of phosphatase on plant roots, thus postponing the negative effects of P deficiency on plant productivity.
As a N2-fixing species, R. pseudoacacia is able to reduce atmospheric N2 to available N through symbiotic rhizobia (Cierjacks et al. 2013). The estimated N fixation rates of this species vary from 23 to 300 kg ha−1 year−1 (Danso et al. 1995; Noh et al. 2010; Cierjacks et al. 2013), which is comparable with or even much higher than the simulated N deposition rate in our experiment. As a result, short-term addition of N may not promote the growth of R. pseudoacacia. Compared with Q. acutissima, the leaf N:P ratio of R. pseudoacacia in mixture did not show an obvious trend with increasing N addition. Therefore, we could not infer the nutrient status of R. pseudoacacia from the results of our experiment. If the growth of R. pseudoacacia was also restricted by soil P availability, increased N addition may not alleviate the P limitation because of the high P requirement for rapid growth and N2-fixation (Uliassi and Ruess 2002; Vitousek et al. 2010). As a result, the growth of R. pseudoacacia could not be promoted either. Nevertheless, the reason why increased N addition did not stimulate the growth of R. pseudoacacia was unclear and the underlying mechanisms remain to be explored.
In short, because of its widely recognized invasiveness and extensive use in reforestation, the relationships between R. pseudoacacia and native plants remain a matter of concern in the context of future global change. In this study, seedlings were only treated with N deposition for 3 months; therefore, the results may not reflect the responses of mature trees to long-term N deposition accurately. Since greenhouse studies cannot simulate the complicated biotic and abiotic environmental factors in natural conditions, our results should not be extrapolated to the field arbitrarily.
Conclusion
In summary, R. pseudoacacia adopted an active strategy for resource absorption and utilization. In competition with Q. acutissima, R. pseudoacacia achieved success in resource acquisition through a high growth rate in the early stage as well as a high root biomass. In return, it was able to invest more resources into photosynthesis and growth, and thus obtain a higher biomass and greater competitive advantage. However, the competitive effects of R. pseudoacacia on Q. acutissima were alleviated with increasing N deposition. Like N, P is also an important nutrient element for plant growth and is often limited in the soil. Since P deficiency may limit or alter the responses of plants to increased N availability, in the future, pot experiments with both elevated N and P availability should be conducted for R. pseudoacacia and native plants. In addition, to reflect the effects of R. pseudoacacia on native forests accurately, field experiments are also necessary.
References
Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, Huang JH, Zhang LX, Han XG (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Glob Chang Biol 16:358–372. doi:10.1111/j.1365-2486.2009.01950.x
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman JW, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59. doi:10.1890/08-1140.1
Boring LR, Swank WT (1984) Symbiotic nitrogen fixation in regenerating black locust (Robinia pseudoacacia L.) stands. For Sci 30:528–537
Bradford MA, Schumacher HB, Catovsky S, Eggers T, Newingtion JE, Tordoff GM (2007) Impacts of invasive plant species on riparian plant assemblages: interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 152:791–803. doi:10.1007/s0042-007-0697-z
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443. doi:10.1890/1540-9295(2004)002[0436:Nwisat]2.0.Co;2
Callaway RM, Bedmar EJ, Reinhart KO, Silvan CG, Klironomos J (2011) Effects of soil biota from different ranges on Robinia invasion: acquiring mutualists and escaping pathogens. Ecology 92:1027–1035. doi:10.1890/10-0089.1
Cierjacks A, Kowarik I, Joshi J, Hempel S, Ristow M, von der Lippe M, Weber E (2013) Biological flora of the British Isles: Robinia pseudoacacia. J Ecol 101:1623–1640. doi:10.1111/1365-2745.12162
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For Ecol Manag 247:120–130. doi:10.1016/j.foreco.2007.04.023
Danso S, Zapata F, Awonaike K (1995) Measurement of biological N2 fixation in field-grown Robinia pseudoacacia L. Soil Biol Biochem 27:415–419. doi:10.1016/0038-0717(95)98612-R
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. doi:10.1046/j.1365-2745.2000.00473.x
Ding WJ, Wang RQ, Yuan YF, Liang XQ, Liu J (2012) Effects of nitrogen deposition on growth and relationship of Robinia pseudoacacia and Quercus acutissima seedlings. Dendrobiology 67:3–13
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139. doi:10.1016/S0169-5347(98)01554-7
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Evol Syst 41:59–80. doi:10.1146/annurev-ecolsys-102209-144650
Fang YT, Gundersen P, Mo JM, Zhu WX (2009) Nitrogen leaching in response to increased nitrogen inputs in subtropical monsoon forests in southern China. For Ecol Manag 257:332–342. doi:10.1016/j.foreco.2008.09.004
Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit LYP, Zheng YL (2009) Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc Natl Acad Sci U S A 106:1853–1856. doi:10.1073/pnas.0808434106
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081. doi:10.1038/Nature05719
Godoy O, Valladares F, Castro-Díez P (2011) Multispecies comparison reveals that invasive and native plants differ in their traits but not in their plasticity. Funct Ecol 25:1248–1259. doi:10.1111/j.1365-2435.2011.01886.x
Gorchov DL, Trisel DE (2003) Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol 166:13–24. doi:10.1023/A:1023208215796
Guo X, Guo WH, Luo YJ, Tan XF, Du N, Wang RQ (2013) Morphological and biomass characteristic acclimation of trident maple (Acer buergerianum Miq.) in response to light and water stress. Acta Physiol Plant 35:1149–1159. doi:10.1007/s11738-012-1154-0
Han WX, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385. doi:10.1111/j.1469-8137.2005.01530.x
Hanba Y, Miyazawa SI, Terashima I (1999) The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Funct Ecol 13:632–639. doi:10.1046/j.1365-2435.1999.00364.x
He WM, Montesinos D, Thelen GC, Callaway RM (2012) Growth and competitive effects of Centaurea stoebe populations in response to simulated nitrogen deposition. PLoS One 7(4):e36257. doi:10.1371/journal.pone.0036257
Hellmann C, Sutter R, Rascher KG, Máguas C, Correia O, Werner C (2011) Impact of an exotic N2-fixing Acacia on composition and N status of a native Mediterranean community. Acta Oecol 37:43–50. doi:10.1016/j.actao.2010.11.005
Hidaka A, Kitayama K (2009) Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. J Ecol 97:984–991. doi:10.1111/j.1365-2745.2009.01540.x
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42. doi:10.1093/Aob/Mcf140
Jiang LF, Luo YQ, Chen JK, Li B (2009) Ecophysiological characteristics of invasive Spartina alterniflora and native species in salt marshes of Yangtze River estuary, China. Estuar Coast Shelf Sci 81:74–82. doi:10.1016/j.ecss.2008.09.018
Jordan NR, Larson DL, Huerd SC (2008) Soil modification by invasive plants: effects on native and invasive species of mixed-grass prairies. Biological Invasions 10:177–190. doi:10.1007/s10530-007-9121-1
Jose S, Merritt S, Ramsey CL (2003) Growth, nutrition, photosynthesis and transpiration responses of longleaf pine seedlings to light, water and nitrogen. For Ecol Manag 180:335–344. doi:10.1016/S0378-1127(02)00583-2
Kawaletz H, Mölder I, Annighöfer P, Terwei A, Zerbe S, Ammer C (2013a) Back to the roots: how do seedlings of native tree species react to the competition by exotic species? Ann For Sci 71:337–347. doi:10.1007/s13595-013-0347-z
Kawaletz H, Mölder I, Zerbe S, Annighöfer P, Terwei A, Ammer C (2013b) Exotic tree seedlings are much more competitive than natives but show underyielding when growing together. J Plant Ecol 6:305–315. doi:10.1093/Jpe/Rts044
Killingbeck K (1993) Inefficient nitrogen resorption in genets of the actinorhizal nitrogen fixing shrub Comptonia peregrina: physiological ineptitude or evolutionary tradeoff? Oecologia 94:542–549. doi:10.1007/bf00566970
Lamarque LJ, Delzon S, Lortie CJ (2011) Tree invasions: a comparative test of the dominant hypotheses and functional traits. Biol Invasion 13:1969–1989. doi:10.1007/s10530-011-0015-x
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. doi:10.1016/j.tree.2007.10.008
Lee CS, Cho HJ, Yi H (2004) Stand dynamics of introduced black locust (Robinia pseudoacacia L.) plantation under different disturbance regimes in Korea. For Ecol Manag 189:281–293. doi:10.1016/j.foreco.2003.08.012
Liu JX, Huang WJ, Zhou GY, Zhang DQ, Liu SZ, Li YY (2013a) Nitrogen to phosphorus ratios of tree species in response to elevated carbon dioxide and nitrogen addition in subtropical forests. Glob Chang Biol 19:208–216. doi:10.1111/Gcb.12022
Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS (2013b) Enhanced nitrogen deposition over China. Nature 494:459–462. doi:10.1038/Nature11917
Lü CQ, Tian HQ (2007) Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J Geophys Res 112: D22S05. doi:10.1029/2006jd007990
Lu XK, Mo JM, Gundersern P, Zhu WX, Zhou GY, Li DJ, Zhang X (2009) Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere 19:189–198. doi:10.1016/S1002-0160(09)60108-9
Lukac M, Calfapietra C, Lagomarsino A, Loreto F (2010) Global climate change and tree nutrition: effects of elevated CO2 and temperature. Tree Physiol 30:1209–1220. doi:10.1093/treephys/tpq040
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704. doi:10.1111/j.1469-8137.2011.03967.x
Maskell LC, Smart SM, Bullock JM, Thompson K, Stevens CJ (2010) Nitrogen deposition causes widespread loss of species richness in British habitats. Glob Chang Biol 16:671–679. doi:10.1111/j.1365-2486.2009.02022.x
Morris TL, Esler KJ, Barger NN, Jacobs SM, Cramer MD (2011) Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers Distrib 17:898–910. doi:10.1111/j.1472-4642.2011.00802.x
Noh NJ, Son Y, Koo JW, Seo KW, Kim RH, Lee YY, Yoo KS (2010) Comparison of nitrogen fixation for north- and south-facing Robinia pseudoacacia stands in central Korea. J Plant Biol 53:61–69. doi:10.1007/s12374-009-9088-9
Olsen SR, Watanabe FS, Cosper HR, Larson W, Nelson L (1954) Residual phosphorus availability in long-time rotations on calcareous soils. Soil Sci 78:141–152. doi:10.1097/00010694-195408000-00008
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425. doi:10.1111/j.0269-8463.2004.00847.x
Pant HK, Warman PR (2000) Enzymatic hydrolysis of soil organic phosphorus by immobilized phosphatases. Biol Fertil Soils 30:306–311. doi:10.1007/s003740050008
Perkins LB, Nowak RS (2013) Native and non-native grasses generate common types of plant-soil feedbacks by altering soil nutrients and microbial communities. Oikos 122:199–208. doi:10.1111/j.1600-0706.2012.20592.x
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737. doi:10.1111/j.1365-2486.2011.02636.x
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1:430–437. doi:10.1038/Ngeo230
Reich PB (2009) Elevated CO2 reduces losses of plant diversity caused by nitrogen deposition. Science 326:1399–1402. doi:10.1126/science.1178820
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770
Scherer-Lorenzen M, Venterink HO, Buschmann H (2007) Nitrogen enrichment and plant invasions: the importance of nitrogen-fixing plants and anthropogenic eutrophication. In: Nentwig W (ed) Biological invasions. Springer, Berlin, pp 163–180
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16:326–331. doi:10.1046/j.1365-2435.2002.00626.x
Stevens C, Duprè C, Gaudnik C, Dorland E, Dise N, Gowing D, Bleeker A, Alard D, Bobbink R, Fowler D, Vandvik V, Corcket E, Mountford JO, Aarrestad PA, Muller S, Diekmann M (2011) Changes in species composition of European acid grasslands observed along a gradient of nitrogen deposition. J Veg Sci 22:207–215. doi:10.1111/j.1654-1103.2010.01254.x
Stone EL, Kalisz PJ (1991) On the maximum extent of tree roots. For Ecol Manag 46:59–102. doi:10.1016/0378-1127(91)90245-Q
Thomsen MA, Corbin JD, D’Antonio CM (2006) The effect of soil nitrogen on competition between native and exotic perennial grasses from northern coastal California. Plant Ecol 186:23–35. doi:10.1007/s11258-006-9109-4
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954. doi:10.1890/0012-9658(2001)082[0946:Eosnao]2.0.Co;2
Uliassi DD, Ruess RW (2002) Limitations to symbiotic nitrogen fixation in primary succession on the Tanana River floodplain. Ecology 83:88–103. doi:10.2307/2680123
Vasquez E, Sheley R, Svejcar T (2008) Nitrogen enhances the competitive ability of cheatgrass (Bromus tectorum) relative to native grasses. Invasive Plant Sci Manag 1:287–295. doi:10.1614/Ipsm-08-062.1
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. doi:10.1111/j.1461-0248.2011.01628.x
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15. doi:10.1890/08-0127.1
Von Holle B, Joseph KA, Largay EF, Lohnes RG (2006) Facilitations between the introduced nitrogen-fixing tree, Robinia pseudoacacia, and nonnative plant species in the glacial outwash upland ecosystem of Cape Cod, MA. Biodivers Conserv 15:2197–2215. doi:10.1007/s10531-004-6906-8
Wang XC, Wang YL (1996) Study on succession of Robinia pseudoacacia forest in Yaoxiang Forest Farm. J Henan For Sci Technol 16:22–24
Wang RQ, Zhou GY (2000) The vegetation of Shandong Province. Shandong Science and Technology Publisher, Jinan
Werner C, Zumkier U, Beyschlag W, Máguas C (2010) High competitiveness of a resource demanding invasive acacia under low resource supply. Plant Ecol 206:83–96. doi:10.1007/s11258-009-9625-0
Yang L, Liu N, Ren H, Wang J (2009) Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. For Ecol Manag 257:1786–1793. doi:10.1016/j.foreco.2009.01.033
Yuan YF, Guo WH, Ding WJ, Du N, Luo YJ, Liu J, Xu F, Wang RQ (2013) Competitive interaction between the exotic plant Rhus typhina L. and the native tree Quercus acutissima Carr. in Northern China under different soil N:P ratios. Plant Soil 372:389–400. doi:10.1007/s11104-013-1748-3
Zhang CH, Zheng YQ, Liu N, Zong YC, Jiao M, Guo HL (2008) Invasion of Robinia pseudoacacia and impacts on native vegetation. J Bejing For Univ 30:18–23. doi:10.3321/j.issn:1000-1522.2008.03.004
Zhang Y, Dore AJ, Liu X, Zhang F (2011) Simulation of nitrogen deposition in the North China plain by the FRAME model. Biogeosciences 8:3319–3329. doi:10.5194/bg-8-3319-2011
Zhang R, Zhou ZC, Luo WJ, Wang Y, Feng ZP (2013) Effects of nitrogen deposition on growth and phosphate efficiency of Schima superba of different provenances grown in phosphorus-barren soil. Plant Soil 370:435–445. doi:10.1007/s11104-013-1644-x
Acknowledgements
We thank two anonymous reviewers and the editor for their insightful and helpful comments. We are grateful to Professor Liu Chunsheng for his help in soil chemical analyses. The research was supported by the Ministry of Science and Technology of China (No. 2011FY110300), National Science Foundation of China (No. 31270374) and Science and Technology Project of Shandong Province (No. 2012GSF11609).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Luo, Y., Guo, W., Yuan, Y. et al. Increased nitrogen deposition alleviated the competitive effects of the introduced invasive plant Robinia pseudoacacia on the native tree Quercus acutissima . Plant Soil 385, 63–75 (2014). https://doi.org/10.1007/s11104-014-2227-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2227-1