Abstract
Aims
We determined whether nitrogen (N) deposition on phosphorus (P)-limited soil could increase Schima superba growth or alter root formation or P efficiency. The effects of N deposition on S. superba were also used to investigate the N/P requirements of plants of different provenances.
Methods
One-year-old S. superba seedlings from eight geographic areas were grown in P-limited soil and treated with N (0, 50, 100, and 200 -kg N ha−1 year−1; i.e., control, N50, N100, or N200, respectively). Seedling growth, root development, phosphorus acquisition efficiency (PAE), and phosphorus utilization efficiency (PUE) were measured.
Results
S. superba responded positively to N supplementation. Seedling growth and dry biomass were highest with N100 treatment and lowest with N200. Root biomass and acquisition of soil P were greatest with N100. Significant differences were observed among plants of different geographical provenances. PAE and PUE had a strong relationship with root growth in plants subjected to N100 treatment.
Conclusion
A threshold for N and P requirements related to different genetic conditions and soil nutrients may exist for S. superba. Root growth and PAE can be divided into three categories based on soil nitrate levels. Nutrients were found to control root morphology and to enhance aboveground differences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The macronutrient phosphorus (P) is essential to plant growth and development, but it is usually present at only low levels in subtropical soils, particularly forest soils (Holford 1997; He et al. 2003). Overall soil P levels are roughly < 0.3 mg g−1 across most areas of southern China, which is well below the global average (National Soil Office of China 1998; Han et al. 2005). P deficiency is a major factor limiting the primary productivity of forests (Darrell and Fownes 1995; Feller et al. 1999; Davidson et al. 2004; Wassen et al. 2005; Elser et al. 2007). The P concentration in plants is maintained by the balance between by P uptake and the plant’s growth rate. Phosphorus acquisition efficiency (PAE) and phosphorus utilization efficiency (PUE) are two important indices of plant growth. PAE here refers to the ability of the plant to acquire P from the soil, and PUE is the amount of biomass produced per unit of acquired P (Huang et al. 2011). PAE and PUE may be affected by several environmental and genetic factors (Pearse et al. 2006).
The rate of deposition of nitrogen (N) is high in subtropical forests in southern China, and it is expected to increase further in the coming decades (Xu et al. 2001; Zheng et al. 2007; Mo et al. 2008; Hu et al. 2010; Ti and Yan 2010). N deposition leads to soil acidity, causes depletion of base cations, and mobilizes Al. The concentration of Al3+ has been found to increase the levels of Al-P compounds and so reduce the content of soluble phosphate (Pi) available in the soil. High N input into forests has been linked to nutritional disorders in plants and to reduced growth and productivity (Vitousek et al. 1997; Emmett 2007; Moore and Houle 2009). However, moderate amounts of added N have been found to increase the net photosynthetic rate and biomass production of seedlings (Nakaji et al. 2001, 2002; Mo et al. 2008). Nitrogen deposition affects the concentration of P in the soil, which in turn influences PAE and PUE. The differential responses of root growth and morphology to N (which are related to different genotypes), suggest that N plays an important role in nutrient uptake (Sas et al. 2002; Wang et al. 2006; Zhang et al. 2007; Ge et al. 2008; Magaña et al. 2009; Richardson et al. 2009).
Schima superba is a representative, widely distributed species of broadleaf tree found in the subtropical forests of southern China. This species is valued commercially for its timber. It can also prevent the spread of fire and thus help protect forests (Luo 2009; http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200014158). In general, S. superba grows in relatively nutrient-poor soils; and its root system, PAE, and PUE vary greatly across regions (Lin et al. 2009; Lin and Zhou 2009). However, the changes in the PAE and PUE of S. superba under different N-deposition and P-limitation conditions have not been documented.
This study focuses on the effects of N deposition and P limitation on S. superba plants of different provenances and addresses the following questions: (a) What is the combined effect of increased N deposition and P limitation on seedling growth, root system growth, PAE, and PUE of S. superba? (b) Is there any difference in the response to N and P availability among S. superba from different places?
Materials and methods
Experimental conditions
This study was executed from March to November 2011 in a glass house. The glass house was not climate controlled. The temperature in the glass house ranged from 5 to 15 °C from December to the following February and from 26 to 34 °C from July to September. S. superba seeds from eight provenances were germinated in normal forest soil (see below) in March 2011. The seeds were collected from Hangzhou (HZ) and Longquan (LQ) in Zhejiang province and at altitudes of 100 m, 300 m, and 600 m in Jian’ou (JO100, JO300 and JO600, respectively); Gutian (GT) in Fujian Province; Guiyang (GY) in Hunan Province; and Xinfeng (XF) in Jiangxi Province (Fig. 1). When the seedlings produced two cotyledons between 2 and 3 cm in height, they were planted in plastic containers 15 cm in diameter and 18 cm in height in April 2011, two seedlings per container. The seedlings were watered every other day. If both seedlings in a given container survived, one was removed to maintain the one seedling/container planting density. A double layer of root-cloth was laid inside the container to prevent the roots from growing out of the drainage hole. The soil used was regular forest soil (P limited) from Zhejiang province. The soil pH was 5.06 (potentiometric method, LY/T 1239–1999), total nitrogen was 0.41 g kg−1 (semi-micro Kjeldahl method, LY/T 1228–1999), hydrolyzable nitrogen was 46.43 mg kg−1 (alkaline hydrolysis diffusion method, LY/T 1229–1999), available phosphorous was 13 mg kg−1 (hydrochloric acid-sulfuric acid extraction method, LY/T 1233–1999), available potassium was 130.08 mg kg−1 (1 mol L−1 ammonium acetate extraction-flame photometry method, LY/T 1236–1999), and organic matter was 6.68 g kg−1 (potassium dichromate oxidation-external heating method, LY/T 1237–1999).
The factorial design of the experiment included S. superba seedlings of eight provenances and four levels of N addition using NH4NO3 (0, 50, 100, and 200 kg N ha−1 year−1; N deposition mainly contained NH4 + and NO3 − forms under natural conditions). There were 18 replicates per treatment with a total of 576 individuals. The four levels of N addition included very low levels of N deposition (0 kg N ha−1 year−1, N0), which served as a control treatment, low-to-medium deposition (50 kg N ha−1 year−1, N50), high deposition (100 kg N ha−1 year−1, N100), and very high deposition (200 kg N ha−1 year−1, N200). These levels were determined based on current and historical deposition levels in southern China (He 1993; Zheng et al. 2007; Ti and Yan 2010; Hu et al. 2010). The added N was applied once every 2 weeks for a month, and then once a month for the next 6 months, beginning when the leaves started to bud (from the April 20, 2011 to the October 20, 2011). Whole plants were sprayed using dissolved NH4NO3, and the concentrations were 0.0016, 0.0031, and 0.0062 mol L−1, for the N50, N100, and N200 treatments, respectively. Seedlings were watered with distilled water until harvest during the first week of November 2011. Pests and weeds were controlled manually.
Measurements
Seedling height and stem increments were measured each month. At the final harvest, roots were carefully separated from the soil and rinsed with water for later measurement. Root images were acquired using a scanner. Morphological and architectural parameters of the roots, such as root length and root volume, were quantified using computer image analysis software (WinRhizo Pro STD1600+, Regent Inc. Canada). The biomass of roots, stems, and leaves was determined. The plant materials were dried at 105 °C for 1 h, followed by 80 °C for 48 h. They were then cooled in a desiccator filled with silicon dioxide gel until they reached a constant mass. To determine the mineral composition, plant materials were dried, ground up, and passed through a 1 mm sieve. They were then digested with H2SO4 and H2O2 was added as a catalyst. Then they were cooled and diluted with deionized water to 50 ml. Total nitrogen content was determined using the standard Kjeldahl method (Bremner and Mulvaney 1982). Total phosphorous was determined using Mo-Sb antispetrophotography method (Anderson and Ingram 1993).
Statistical analysis
Eighteen plants were used to determine average seedling height and stem base diameter (SBD). Nine seedlings were used to measure the average dry mass of roots, stems, and leaves, calculate the root:aboveground ratio (RAR, root mass divided by aboveground mass), determine root length, diameter, and volume. Six were used to evaluate average PAE and PUE. The data were analyzed using the SAS statistical program (version V8, SAS Institute, Cary, NC, U.S.). Two-way ANOVA was performed at a significance level of 0.05, with provenance and nitrogen levels as the independent factors. All variables were normally distributed and did not need to be transformed. A Duncan’s multiple-range test was used to determine whether significant (P < 0.05) differences existed between and among treatments.
Results
Overall response of aboveground and root growth to addition of N
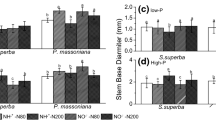
The growth of S. superba from all eight provenances was generally faster in the presence of added N. Early on, plant height and SBD were the highest among specimens subjected to N50 treatment. At the end of the growing season, however, these parameters were highest among plants subjected to N100 treatment (14.4 cm and 3.1 mm, respectively). These values were 40 % and 23 % higher, respectively, than those of plants exposed to no added N (10.3 cm and 2.5 mm, respectively). The stimulatory effect was lower among plants subjected to N200 than among plants subjected to N50 or N100. The height and SBD of plants subjected to the N200 treatment were higher than those of controls. The differences were 14 % and 9 %, respectively (Fig. 2).
Nitrogen addition significantly increased the growth in root diameter. The growth rate was significantly higher among plants subjected to N100 and N200 treatments than among controls (Fig. 3). RAR was significantly lower among plants subjected to N100 and N200 than among controls.
Overall response of biomass allocation, PAE, and PUE to the addition of N addition
Increased accumulation of dry biomass was found to be associated with the addition of N. Leaf, root, and total dry biomass among plants subjected to N50 treatment were higher than among plants exposed to no added N (Fig. 4). Dry biomass was 1.9 g for leaves, 1.6 g for roots, and 4.1 g for whole plants subjected to N100 treatment (the highest among all treatments; Figs. 3d and 4). These values were smaller with the N200 treatment (1.7 g, 1.5 g, and 3.7 g for leaves, roots, and total dry biomass, respectively).
N addition had a positive effect on PAE and PUE (Tables 1 and 2). PAE was greater among plants subjected to N100 treatment than among controls. This indicated that the addition of N promoted P acquisition. The PUE of the stems and leaves of plants subjected to N100 treatment increased by 92 % and 51 %, respectively, relative to controls. However, the PUE of the roots increased steadily as the amount of N added increased, peaking at 1.86 g mg−1 with N200 treatment.
Seedling and root growth in plants of various provenances
Provenance was found to significantly affect the characteristics of seedling growth in this experiment (Table 3). For example, the increase in seedling height and SBD of HZ, GT, XF, and GY provenances were the greatest among plants subjected to N50 treatment (Fig. 5a, b). JO100 and LQ had the highest seedling heights and SBD values of any provenance when subjected to N100 treatment, but the highest values for JO300 and JO600 were observed with the N200 treatment. The standard deviations for seedling height and SBD from the different provenances in the N treatment groups were greater than those for the controls (Fig. 5a, b).
Differences in seedling growth traits of S.superba from provenances under N addition and P-limitation treatments. Each column represents the mean value in a given treatment within the same provenance. Vertical bars represent standard deviations from the mean (n = 18 for A and B, n = 9 for C, D, E and F). Different letters above the columns indicate significant differences among provenances (p < 0.05). Lowercase letters denote controls; lowercase letters within brackets denote N50 treatment; uppercase letters denote N100 treatment; and uppercase letters within brackets denote N200 treatment
Nitrogen addition clearly stimulated the growth of root length and volume in plants from the JO100 (N100), JO300 (N50), GT (N200), and GY (N50) provenances more than in plants from other provenances (Fig. 5e, f). The length and the volume of root systems of the JO600 group subjected to N200 treatment and of the LQ group subjected to N50 treatment, conversely, were smaller than those of controls. In the N treatment groups, the standard deviations of root length and volume were lower than among the controls, but they increased as the level of N increased. The root:aboveground ratios for the JO100, HZ, and GT groups were greater with the N50 treatment than in the controls, but the opposite was true of plants from the other provenances (Fig. 5d).
Biomass allocation, PAE, and PUE in different provenances
N addition significantly affected biomass growth rate, and the JO100, JO300, and GT groups benefited the most from treatment. The JO100 group showed the highest biomass growth rate of any provenance or treatment group when subjected to the N100 treatment, 2.6 times that of the controls. The next highest rate was observed in the GT group subjected to N200 treatment and the JO300 group subjected to N100 treatment, both 2.1 times that of the controls. Although S. superba from the LQ provenance showed the highest biomass value, its growth rate when exposed to added N was the lowest among all the provenances (Fig. 5c). Nitrogen addition changed the biomass allocation among roots, stems, and leaves. The stem and leaf biomass of the LQ, GT, and GY groups exposed to N100 treatment increased by more than 10 % relative to the controls. GT and GY groups showed the highest increase in stem biomass of any provenance or treatment group when subjected to N100 treatment (Fig. 6).
Root-, stem- and leaf-weight ratios of S.superba from eight 8 provenances under N addition and P-limitation treatments. The relative root-weight ratio is the percentage of the dry biomass of roots to the total seedling dry biomass; the relative stem-weight ratio is the percentage of the dry biomass of stems to the total seedling dry biomass; and the leaf-weight ratio is the percentage of the dry biomass of leaves to the total seedling dry biomass. Each column represents the mean value for a given treatment within the same provenance. Vertical bars represent standard deviations from the mean (n = 9)
The plant PAE of S. superba from the JO600, XF, and GY groups was greater than that of controls among plants subjected to N50 treatment. The PAE of the JO300 and HZ groups was greater than that of controls among plants subjected to the N100. The PAE of the JO100, LQ, and GT groups was greater than that of controls among plants subjected to the N200 treatment. The plant PAE of S. superba from the HZ, LQ, and GT provenances showed the lowest values with the N50 treatment. (Table 1). The PUE of the LQ and GY groups, especially the stem PUE at the N100 level (PUEstem LQ = 3.24 and PUEstem GY = 2.73), was higher than that of plants from the other provenances. Five provenances, HZ, LQ, GT, XF, and GY, showed the highest values of leaf PUE of any provenance or treatment when subjected to N100 treatment. Plants from the JO300, JO600, HZ, GT, and XF groups showed the highest values for root PUE of any provenance or treatment when subjected to N200 treatment (Table 2).
Discussion
Our results indicated that N addition had a positive effect on the height, SBD, root system development, and biomass production of S. superba; but a decreasing trend was observed when N treatment was increased to N200 levels (200 kg N ha−1 year−1) (Figs. 2 and 3c, d). These results suggested that N was probably a limiting factor for the growth of S. superba seedlings in P-deficient soils (Hermans et al. 2006). Our findings are consistent with the results of a seedling experiment performed on evergreen conifer species (Cryptomeria japonica) and evergreen broadleaved species (Cryptocarya concinna), which showed that the height and SBD of seedlings increased with N5 (5 g N m−2 year−1) treatment and decreased with increasing levels of N from N5 to N30 (30 g N m−2 year−1) (Nakaji et al. 2001; Mo et al. 2008).
In our study, aboveground growth was found to be more sensitive to added N than root growth was. When levels of added N were low (N50), the growth and PUE of the aboveground tissues increased, peaking under moderate N addition (N100) (Table 2 and Fig. 6). Roots showed a delayed response to N addition, and their growth and PUE increased at higher levels of added N (N200) (Table 2 and Fig. 3a, b).
Nitrogen addition was found to increase the number, diameter, and length of roots (Hermans et al. 2006; Wang et al. 2006; Magaña et al. 2009). It also enhanced root uptake of nutrients (Wang et al. 2006; Magaña et al. 2009). Our study showed the relationships between root growth and PAE of S. superba of different geographic provenances after N addition to be complicated. For example, root development promoted the PAE at the N100 level in plants from the JO100, JO300, and GT provenances. However, they were less well correlated among plants subjected to N200 treatment (Table 1 and Fig. 5e, f). These results suggested that the provenances could be divided into three categories based on the relationships among plants exposed to high levels of added N (Fig. 7). The first category was characterized by efficient absorption; it included JO100 and LQ provenances. These plants maintained a high rate of phosphorus acquisition when exposed to high levels of added N but did not show pronounced root development. The second category manifested higher rates of root growth and lower P absorption; it included plants of JO300, HZ, and GT provenances. The root growth from these provenances was not found to increase plant PAE. This may be because soil nitrate was insufficient for growing conditions in these provenances. Although an extra supply of N may promote root development, it can also fix soil-available phosphorus (Liu et al. 2006). In addition, the N:P ratios in the plants were reported to be strongly correlated with the N:P supply ratio (Koerselman and Meuleman 1996; Güsewell 2004). This shows that the plant regulates its metabolic progress to maintain a high N:P ratio by increasing N acquisition, reducing P acquisition, or both (Hermans et al. 2006; Marschner 2012). The third category is characterized by negative root growth and lower PAE; it includes plants from JO600, XF, and GY. This may be because soil nitrate was saturated with respect to growing conditions in these provenances, and even a small amount of N addition can harm the roots. In this way, N and P requirements may be affected by different genetic combinations and levels of available soil nutrients (Güsewell 2004; Hammond et al. 2004; Zhang et al. 2004; Schulze et al. 2006; Liu et al. 2006; Ristvey et al. 2007; Miller and Smith 2008).
Significant differences in seedling growth, root system growth, and phosphorus efficiency were found among plants of different provenances exposed to added N in P-limited soil. When no N was added, the LQ provenance exhibited greater seedling and root growth, PAE, and PUE in P-limited soil; but the HZ and XF groups had lower values for these same parameters. When N was added, plants of all provenances except LQ and GT, gave a positive response and showed high growth rates (Fig. 5). This suggested that the LQ and GT groups possessed a more pronounced ability to resist external disturbances. Similar results were observed in water- and phosphorus-limited experiments with S. superba (Lin and Zhou 2009). The addition of nitrogen produced a positive effect on aboveground growth and biomass, which induced growth in plants from all provenances except LQ; this approached a level similar to that of the controls in the LQ provenance and significantly reduced the difference among provenances (Fig. 5).
One of the findings of the present work was that the standard deviation in seedling and root growth showed a regular pattern with all levels of N exposure. Nitrogen deposition augmented the standard deviation of aboveground seedling growth while minimizing standard deviations of the root system (Fig. 5a, b, e, and f). This signified that root growth was more homogeneous and the aboveground growth more heterogeneous among individuals exposed to added N. This also implies that sufficient nutrient supply in the soil may control root morphology and architecture and that excess nutrients (such as N) are probably converted to storage compounds in the aboveground tissues, especially in stems (Limpens and Berendse 2003). PUE also confirmed that the stems and leaves utilized more P than the roots for equivalent biomass production. The difference between individuals with respect to aboveground growth was augmented by the addition of N, which facilitated the selection of seedlings with higher growth rates and biomass yield under N deposition conditions.
Summary
The effect of N deposition on Schima superba from eight different geographic areas was compared to determine whether N deposition on P-limited soil could increase S. superba growth or alter root formation or P efficiency. We found that S. superba responded positively to the addition of N. Seedling growth and dry biomass were the highest in plants subjected to N100 and lowest in plants subjected to N200. Root biomass was greatest, and plants acquired and utilized the most soil P with the N100 treatment. There was a significant difference among geographical provenances with respect to root growth, PAE, and PUE. PAE and PUE from plants of different provenances had a stronger relationship with root growth under N100 treatment. A threshold for nitrogen and phosphorus requirements (N:P ratios) related to different genetic combinations and to the availability of soil nutrients may exist for S. superba. Root growth and PAE can be divided into three categories based on soil nitrate levels.
Abbreviations
- PAE:
-
Phosphorus Acquisition Efficiency
- PUE:
-
Phosphorus Utilization Efficiency
- RAR:
-
Root:Aboveground Ratio
- SBD:
-
Stem Base Diameter
References
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. 2th edn. CAB International, Wallingford press
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR, (eds) Methods of soil analysis. Part 2: chemical and microbial properties, Agronomy Monograph 9. Agronomy Society of America, Madison, 595–624
Darrell AH, Fownes JH (1995) Phosphorus limitation of forest leaf area and net primary production on a highly weathered soil. Biogeochemistry 29:223–235
Davidson EA, Carvalho CJR, Vieira ICG, Figueiredo RO, Moutinho P, Ishida FY, Santos MTP, Guerrero JB, Kalif K, Sabá RT (2004) Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol Appl 14:150–163
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Emmett BA (2007) Nitrogen saturation of terrestrial ecosystems: some recent findings and their implications for our conceptual framework. Water Air Soil Pollut Focus 7:99–109
Feller IC, Dennis FW, John PON, Karen LM (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80:2193–2205
Ge TD, Tang DM, Lu B, Xia HY, Song SW, Huang DF (2008) Influnece of organic and inorganic nitrogen supply on the composition of Tomato seedling root exudates, xylem and phloem sap grown in hydroponic culture. Acta Horticulturae Sinica 35:39–46
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hammond JP, Broadley MR, White PJ (2004) Genetic responses to phosphorus deficiency. Ann Bot 94:323–332
Han WX, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385
He YQ (1993) The status and utilization approach of forest soil fertility in tropical and subtropical china. Red soil ecological research, vol II. Jiangxi Science and Technology Press, Nanchang, China, pp 16–22
He Y, Liao H, Yan XL (2003) Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant Soil 248:247–256
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617
Holford ICR (1997) Soil phosphorus: its measurement and its uptake by plants. Aust J Soil Res 35:227–239
Hu ZH, Li HM, Yang YP, Chen ST, Li CZ, Shen SH (2010) Effects of simulated nitrogen deposition on soil respiration in northern subtropical deciduous broad-leaved forest. Environ Sci 31:1726–1732
Huang CY, Shirley N, Genc Y, Shi BJ, Langridge P (2011) Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol 156:1217–1229
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Limpens J, Berendse F (2003) Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: the role of amino acid nitrogen concentration. Oecologia 135:339–345
Lin L, Zhou ZC (2009) Effects of soil moisture condition and phosphorus supply on the seedlings growth and phosphorus efficiency of Schima superba provenances. Chin J Appl Ecol 20:2617–2623
Lin L, Zhou ZC, Fan HH, Jin GQ, Chen YL, Hong GM (2009) Provenance difference in stable carbon isotope discrimination of Schima superba. Chin J Appl Ecol 20:741–746
Liu PW, Ivanov II, Filleur S, Gan YB, Remans T, Forde BG (2006) Nitrogen regulation of root branching. Ann Bot 97:875–881
Luo WJ (2009) Forest germplasm resources conservation and utility. Zhejiang Science and Technology Press, China, pp 238–242
LY/T 1228–1999 (1999) Determination of total nitrogen in forest soil. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–4
LY/T 1229–1999 (1999) Determination of hydrolyzable nitrogen in forest soil. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–3
LY/T 1233–1999 (1999) Determination of available phosphorus in forest soil. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–4
LY/T 1236–1999 (1999) Determination of available potassium in forest soil. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–3
LY/T 1237–1999 (1999) Determination of organic matter in forest soil and calculation carbon-nitrogen ratio. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–4
LY/T 1239–1999 (1999) Determination of pH value in forest soil. Department of forest soil, Research Institute of Forestry, CAF. State Forestry Administration, CSIC-LY. P.R.China 1–3
Magaña RH, Adamowicz S, Pagès L (2009) Diel changes in nitrogen and carbon resource status and use for growth in young plants of tomato (Solanum lycopersicum). Ann Bot 103:1025–1037
Marschner P (2012) Marschner’s Mineral Nutrition of Higher Plants. 3th edn. Academic Press
Miller AJ, Smith SJ (2008) Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Ann Bot 101:485–489
Mo JM, Li DJ, Gundersen P (2008) Seedling growth response of two tropical tree species to nitrogen deposition in southern China. Eur J For Res 127:275–283
Moore JD, Houle D (2009) Soil solution and sugar maple response to NH4NO3 additions in a base-poor northern hardwood forest of Québec, Canada. Environ Monit Assess 155:177–190
Nakaji T, Fukami M, Dokiya Y, Izuta T (2001) Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees 15:453–461
Nakaji T, Takenaga S, Kuroha M, Izuta T (2002) Photosynthetic response of Pinus densiXora seedlings to high nitrogen load. Environ Sci 9:269–282
National soil survey office of China (1998) Soil of China. Chinese Agriculture Press, Beijing, China, pp 483–486
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139
Richardson AE, Barea JM, McNeill AM, Combaret CP (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Ristvey AG, Lea-Cox JD, Ross DS (2007) Nitrogen and phosphorus uptake efficiency and partitioning of container-grown azalea during spring growth. J Am Soc Hortic Sci 132:563–571
Sas L, Rengel Z, Tang CX (2002) The effect of nitrogen nutrition on cluster root formation and proton extrusion by Lupinus albus. Ann Bot 89:435–442
Schulze J, Temple G, Temple SJ, Beschow H, Vance CP (2006) Nitrogen fixation by White Lupin under phosphorus deficiency. Ann Bot 98:731–740
Ti CP, Yan XY (2010) Estimation of atmospheric nitrogen wet deposition in China mainland from based on N emission data. J Agro Environ Sci 29:1606–1611
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wang L, Mou PP, Jones RH (2006) Nutrient foraging via physiological and morphological plasticity in three plant species. Can J For Res 36:164–173
Wassen MJ, Venterink HO, Lapshina ED, Tanneberger F (2005) Endangered plants persist under phosphorus limitation. Nature 437:547–550
Xu YG, Zhou GY, Lou TS, Wu ZM, He ZC (2001) Soil solution chemistry and element budget in the forest ecosystem in Guangzhou. Acta Ecol Sin 21:1760–1861
Zhang FC, Kang SZ, Zhang JH, Zhang RD, Li FS (2004) Nitrogen fertilization on uptake of soil inorganic phosphorus fractions in the Wheat root zone. Soil Sci Soc Am J 68:1890–1895
Zhang JY, Wang JG, Xu YL, Li HG (2007) Effect of nitrogen on the species and contents of organic acids in root exudates of different soybean cultivars. Plant Nutr Fertilizer Sci 13:398–403
Zheng LX, Liu XJ, Zhang FS (2007) Atmospheric deposition of organic nitrogen: a review. Acta Ecol Sin 27:3828–3834
Acknowledgements
We would like to thank Dr. Xiaomei Tan, Dr. Qing Yang, Dr. Yi Zhang, and Dr. Hongwei Pan for field assistance. This study was supported by the Special Fund for Forestry-Scientific Research in the Public Interest (20110400107) and the National Key Technology R&D Program (2012BAD01B04). We would also like to thank the two anonymous reviewers for their comments. The clarity and strength of this article greatly benefited from their suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip John White.
Rights and permissions
About this article
Cite this article
Zhang, R., Zhou, Z., Luo, W. et al. Effects of nitrogen deposition on growth and phosphate efficiency of Schima superba of different provenances grown in phosphorus-barren soil. Plant Soil 370, 435–445 (2013). https://doi.org/10.1007/s11104-013-1644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1644-x