Abstract
Background and aims
Year of release of a cultivar reflects the agricultural and breeding practices of its time; we hypothesize that there are differences in mycorrhizal responsiveness of new high yielding and old crop plants and landraces. We evaluated the importance of the year of release on mycorrhizal responsiveness, arbuscular mycorrhizal (AM) fungal root colonization and P efficiency. We also analyzed the effect of experimental treatments, P efficiency (P acquisition and P utilization efficiency) and AM fungal root colonization on a potential mycorrhizal responsiveness trend for year of release.
Methods
We conducted a meta-analysis on 39 publications working on 320 different crop plant genotypes.
Results
New cultivars were less intensely colonized but were more mycorrhiza-responsive (and possibly dependent) compared to ancestral genotypes. This trend was potentially influenced by the moderator variables density, pre-germination, plant, plant type and AMF species. AM root colonization was also important for the mycorrhizal responsiveness trend for year of release, but P efficiency was not.
Conclusions
With the data available we could find no evidence that new crop plant genotypes lost their ability to respond to mycorrhiza due to agricultural and breeding practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi are members of the Glomeromycota (Schüßler et al. 2001) and form symbiotic associations with the majority of land plant species (Fitter and Moyersoen 1996; Wang and Qui 2006). AMF can offer various benefits that potentially result in host biomass increase; these include improved P acquisition (Bolan 1991; Koide 1991), defense against pathogens (Borowicz 2001; Harrier and Watson 2004), improvement of water relations (Auge 2001), and stress tolerance (e.g., Al-Karaki et al. 2001; Rouphael et al. 2010; Smith et al. 2010).
The increase in biomass mediated by AMF is often expressed as mycorrhizal responsiveness. This is defined as the effect of mycorrhizal fungi on plant growth given a specific plant-available soil P concentration compared to non-mycorrhizal control plants (Janos 2007). The effect can be positive (Yao et al. 2001a; Yücel et al. 2009), neutral or negative (Hetrick et al. 1992; Hao et al. 2008). The extent of mycorrhizal responsiveness varies widely between plant species and even between plant genotypes. In order to find a pattern in this variability for crop plants, Hetrick et al. (1992), Hetrick and Wilson (1992) suggested that the cultivar year of release could be a decisive factor. The study of 20 wheat cultivars under greenhouse conditions revealed that cultivars released before 1950 profited more consistently from AM fungal inoculation in terms of biomass, while the response of cultivars released after 1950 was more variable. Additional greenhouse studies confirmed this general pattern (Hetrick et al. 1996; Zhu et al. 2001).
However, a study by Galvan et al. (2011) on onion cultivars and hybrids found no evidence that modern breeding practices changed growth responses, at least in onion. Sawers et al. (2010) also challenged the suggestion by Hetrick et al. (1992), Hetrick and Wilson (1992) by using linear regression models. The analysis of plant growth response to mycorrhiza in subsets of the publications of Hetrick et al. (1992) and Kaeppler et al. (2000) revealed that the trends (for plant biomass and year of release) were biased by non-linearity of the used response ratio (R′ = (M-NC)/NC) and thus suggested that new, old and ancestral genotypes have the same potential for an increase in mycorrhiza benefit (increase in biomass). As a result, it is currently difficult to make general statements regarding the effects of crop breeding on mycorrhizal responsiveness.
Breeding conditions have certainly changed over time, since the early beginnings of human agriculture to the present, and we suggest that the cultivar’s year of release represents the breeding practices of its time. 4000 years ago, humans finished the domestication of the major crops essential for their survival (Doebley et al. 2006). Throughout the millennia, genotypes were selected for positive traits like bigger fruits and more seeds. In the 19th century, the first artificial fertilizer, superphosphate, was used to improve yield. The re-discovery of the Mendelian theory in 1900 led to new technique of hybridization (Palladino 1993). Hybrid genotypes exhibited higher yield as compared to their inbred parental lines (heterosis). From then on, crop plants were bred to maximize yield and to respond better to fertilizer. In 1935, a dwarf wheat genotype, Norin 10, was bred in Japan (Reitz 1968). After 1950, this genotype was used by Norman Borlaug and colleagues to produce semi-dwarf varieties (Dalrymple 1985). Their characteristics were lower shoot biomass, but higher yield output and a reduced snapping of their shorter shoots. Besides wheat and rice, other crop plants were improved to high-yielding varieties in the following decades all over the world. The breeding of these new varieties in addition to improved agricultural techniques and management practices (already established in most parts of North America and Europe) increased food production around the world (Wissuwa et al. 2009). The increased food production is linked to higher water irrigation, pesticide and fertilizer use.
High fertilizer application means high concentrations of plant-available P in the soil. High P concentrations often cause a reduction in mycorrhizal responsiveness (Kaeppler et al. 2000; Hao et al. 2008). Additionally, breeding under high P input can influence the P efficiency of a cultivar (Manske et al. 2001; Huang et al. 2007; Wissuwa et al. 2009). P efficiency comprises both P utilization efficiency and P acquisition efficiency (Wang et al. 2010) and reflects the ability of a plant “to produce yield under a certain available P supply condition and/or to utilize it in the production of biomass or the harvestable organ” (Fernandez et al. 2009). P efficiency has a direct impact on mycorrhizal responsiveness, since P-efficient cultivars generally have lower mycorrhizal responsiveness than P-inefficient ones (Baon et al. 1993; Khalil et al. 1994; Tawaraya et al. 2001; Yao et al. 2001b). An improved P efficiency reduces the effectiveness of the interaction of plant and fungus, at least concerning the increased P supply by the fungus (Li et al. 2008b).
The effectiveness of the plant and fungus interaction is also influenced by the host plant. In the literature, mycorrhizal responsiveness trends based on the year of release differed by crop plant. Negative trends over time were found for members of the genus Triticum (Hetrick et al. 1992, 1996; Zhu et al. 2001) and positive trends for representatives of the genera Solanum and Avena (Koide et al. 1988; Bryla and Koide 1998).
Not only is the identity of the plant host important but the identity of the colonizing fungus as well. The right plant-fungus combination is critical for promoting optimal plant growth. AMF species are diverse in their effects on plant growth ranging from both extremes along a mutualism-parasitism continuum (Johnson et al. 1997; Sensoy et al. 2007), e.g. they can differ with their degree of P supply via the mycorrhizal pathway (Smith et al. 2003). Besides the intensity of root colonization, biomass increase, or P acquisition AMF species also have other influences on plant physiology, e.g. reducing expression of Pi-transporter and starvation-inducible genes (Burleigh et al. 2002). Despite the co-evolution of plant and AM fungi and the conservation of symbiosis-related features, it is rather astonishing that mycorrhizal Pi transporter genes diverged between, e.g. rice and potato (Paszkowski et al. 2002). Thus AMF need to be flexible in their interaction with different host plants, making it possible that physiological incompatibility can occur; this can result in a suboptimal plant growth reaction and mycorrhizal responsiveness, respectively.
Other factors can also decrease the mycorrhizal responsiveness of crop plant genotypes, e.g. plant density (Schroeder and Janos 2004), substrate volume (Daft 1991), type of growth substrate (Vierheilig and Ocampo 1991), experimental duration and country of origin of a cultivar (An et al. 2010). Year of publication is another, typically ignored factor and could also be indicative of changing scientific practices as demonstrated for herbivory and mycorrhizal colonization (Barto and Rillig 2010). Given the number of factors that contribute to mycorrhizal responsiveness, it is important to evaluate their effects on a potential mycorrhizal responsiveness trend for the year of release of crop plants.
To our knowledge only one study (An et al. 2010) tried to test multiple factors for their effect on AM fungal root colonization (but not biomass response) of different maize germplasms (inbred lines released between 1960 and 1999, hybrids and landraces) from different countries and with different pathogen resistances. Since this study contained no analysis on plant biomass performance, a synthesis of data on mycorrhizal responsiveness of plant genotypes with different year of release has not been performed to date.
Thus, we conducted a meta-analysis to quantitatively synthesize the data for mycorrhizal responsiveness in annual crop plants for different years of release and to test three hypotheses.
-
(i)
Due to changes in agricultural and breeding practices over time, we expect differences in mycorrhizal responsiveness between new high yielding and old crop plants and landraces. Although landraces were bred into parental lineages of old and new cultivars, they themselves are the product of mainly natural selection, are adapted to their local and natural environment, are more genetically and phenotypically diverse, but produce less yield than their hybrid offspring (Harlan 1975). In old genotypes, the hybridization was used actively to profit from the heterosis effect in the F1-generation of parental inbred lines resulting in higher yield and C translocation into shoot and ears causing higher nutrient demand. Finally, the dwarfing gave rise to genotypes with reduced shoot length but enhanced yield.
-
(ii)
For many abiotic and biotic factors, an influence on mycorrhizal responsiveness has been detected; thus we hypothesize that these factors also have an effect on any mycorrhizal responsiveness trend for the year of release of crop plants. Besides validation of the influence of factors (as reported in literature used in this meta-analysis) on mycorrhizal responsiveness in our dataset, in particular we need to test important factors for their effect on any mycorrhizal responsiveness trend for the year of release of crop plants. The flexibility in reaction to abiotic or biotic factors, respectively, was eventually co-influenced by changes in agricultural and breeding practices over time. Besides general biotic and abiotic factors such as plant density, soil volume, pH of growth substrate, seed pre-germination, duration of experiment, setting, P treatment and year of publication, we focus on the specific biotic factors AMF and plant species because of their importance for the quality of the symbiosis.
-
(iii)
We hypothesize that P efficiency and AM fungal root colonization affect mycorrhizal responsiveness. Furthermore, since these two factors were likely affected by breeding practices, we expect the year of release to influence both P efficiency and AM fungal root colonization.
Materials and methods
The focus of this meta-analysis was on publications dealing with AM fungi and multiple cultivars, genotypes or varieties of annual crop plants with different years of release.
The literature search started on 28 June 2010 and was performed with the Web of Science Citation Index Expanded database. The search strings used were mycorrhiza* AND cultiva*, mycorrhiza* AND genotyp*, mycorrhiza* AND variet*, mycorrhiza* AND accession*, and generated 969, 383, 319 and 26 publications, respectively.
Papers were screened for studies testing at least two different annual crop plant cultivars, genotypes or varieties under the same experimental conditions and using AMF as a treatment; thus a direct comparison of mycorrhizal and non-mycorrhizal plant growth performance was possible. We chose annual crop plants because most of the major food crops were annual (e.g. maize, wheat, barley, tomato, potato and soybean; see www.fao.org), and the greatest number of year of release dates were available for these major food crops.
To guarantee the independence of the extracted data, the plant genotypes were not allowed to be clones. Furthermore, root or shoot cultures were also not considered because of their highly artificial character and the low comparability with pot cultures or field trials. Therefore, experiments had to be performed in a soil substrate. In addition, the shoot, root or total dry weight biomass and the sample size (N) had to be reported.
Publications fitting these first criteria were further screened for the availability of the genotype’s year of release date, because only studies with at least one genotype with a YOR or YORgroup (for definitions of these terms see section “Effect Size and Moderator Variables”) were considered.
Determination of the year of release
For the determination of a crop plant’s year of release several sources were utilized: (i) Crop plant registration papers published by the Crop Science Journal (Crop Science Society of America) were searched for cultivar names via the online publication search function. (ii) The Germplasm Resources Information Network (GRIN) of the United States Department of Agriculture (USDA) provided information not only on a crop plant’s year of release but pedigrees and country of origin as well. (iii) Information about the year of release, the pedigree and the country of origin specifically for barley (Hordeum vulgare L.) was obtained by the lineage catalogue of barley cultivars of the Bayrische Landesanstalt für Landwirtschaft (LfL) and specifically for wheat (Triticum aestivum L.) by the online database “Wheat Pedigree and Identified Alleles of Genes” (http://genbank.vurv.cz/wheat/pedigree/). (iv) The crop plant’s name was searched using the GOOGLETM search engine or ISI Web of Science for publications about pedigrees. Studies analyzing pedigrees were a good source of information for the year of release. (v) Several papers contained information about year of release directly, but these dates were sometimes not reliable. However, if no data were available using other options (points i to iv), the data directly from the paper were used. If no year of release was available and the crop genotype was not a landrace, wild accession or wild crop relative, then the study was not included.
This final screening returned 39 papers fitting the above mentioned criteria and reporting YOR or YORgroup, respectively, for at least one annual crop plant genotype. The crop plants belonged to the families of Poaceae, Fabaceae, Pedaliaceae, Asteraceae and Cucurbitaceae. The 39 publications reported on 320 different crop plant genotypes (Online Resource 1) and for 120 genotypes a year of release could be determined. 270 of the 320 genotypes could be sorted into one of 3 year of release groups (ancestor, old or new).
Data recording
As in other meta-analyses (Curtis and Wang 1998; Lekberg and Koide 2005), several trials were extracted from each of the 39 publications. Multiple trials within each publication were treated as independent when they were drawn from systems differing in at least one of the following criteria: (i) setting (lab or field), (ii) Phosphorus treatment (yes or no), (iii) AMF species used as inoculum or (iv) plant genus used as experimental host plant. When systems only differed in duration of experiment, only the last harvest was included in the dataset.
Besides plant dry weight, AM fungal root colonization and P efficiency data were extracted from each publication. Biomass was recorded as mg of total, root and/or shoot dry weight excluding fruits, fruit seeds or flower dry weight. If the data were only available in graphs, the freeware Digitizeit 1.5.8a (by I. Bormann 2001–2006, http://www.digitizeit.de/de/) was used for data collection.
Effect sizes and moderator variables
The principal dependent variable (effect size) in this meta-analysis was mycorrhizal responsiveness (MR). The effect size was calculated by taking the natural logarithm of the response ratio of mycorrhizal to non-mycorrhizal plant biomass (MR = ln (biomass myc/biomass non-myc)). MR was calculated from total dry weight data. If not available, shoot or root dry weight data were used instead for calculations.
The usage of response ratios can be problematic (Righetti et al. 2007). As demonstrated (Online Resource 2), our response ratio fitted best the assumption of linearity and thus was reliable for interpretation of mycorrhiza effects.
P efficiency and AM fungal root colonization data were used to calculate the supplementary effect sizes to test for their role on mycorrhizal responsiveness. According to Wang et al. (2010) P efficiency can be divided into P utilization efficiency (PUE) and P acquisition efficiency (PAE). Additionally, for PUE (g shoot biomass/mg P) and PAE (mg P/g root biomass) standardized response ratios were calculated; resulting in the effect sizes mycorrhizal PAE (ln(PAE myc/PAE non-myc)) and mycorrhizal PUE (ln(PUE myc/PUE non-myc)). Data for PAE and PUE were reported only in 17 of the 39 papers. Therefore, the power of tests with these two effect sizes was low and results should be interpreted with caution.
For AM fungal root colonization (%AM), the percent of root length colonized by AMF was used to calculate the corresponding effect size by the mean difference of mycorrhizal and control plants (%AM =% root colonization myc–% root colonization non-myc). For one study only, the controls were contaminated with AM fungi. 35 of the 39 studies used a gridline intersect method, while only 4 studies randomly selected root fragments. Data of both methods were combined in our dataset in agreement with Lekberg and Koide (2005) who found no statistically significant difference in doing so.
The moderators used were year of release (YOR), year of release group (YORgroup), density (number of plants/kg soil), plant (e.g. Hordeum, Zea or Triticum), plant type (cereals, vegetables or legumes), pre-germination of seeds (yes or no), duration of experiment, setting (lab or field), year of publication, and experimental conditions such as AMF species used as inoculum, addition of P fertilizer (treatment P, yes or no), the applied P amount (treatment P concentration, in mg P/kg soil) and pH of growth substrate.
YOR and YORgroup were the principal independent variables (moderators) for answering questions in this meta-analysis. The YOR denoted the date when a crop plant became available on the market; it is not exactly the date when a crop plant was bred. YORgroup was related to the YOR moderator. This categorical moderator included three levels: ancestor, old and new. The “new” YORgroup contained all cultivars released after 1950, the “old” YORgroup were all released after 1900 and before 1950. The “Ancestor” YORgroup included all cultivars released before 1900 as well as the wild crop relatives and landraces, for which no YOR exist. This separation was made according to the studies of Hetrick et al. (1992), Hetrick and Wilson (1992) and to account for changes in plant breeding practices, i.e. cultivars bred before 1900 were more likely products of anthropogenic selection events (for criteria like size and taste), while cultivars bred after 1900 arose mainly from hybridization of inbred lines. Cultivars bred after 1950 comprised the high yielding varieties and Norin-10-based semi-dwarfs.
The moderator “plant” was dominated by members of the family Poaceae (Poaceae trials = 463, other plant trials = 113). Species of the Poaceae often have a fine and dense root system and thus are hypothesized to be less dependent on AMF (Newsham et al. 1995). To detect growth differences between Poaceae and non-Poaceace species, the moderator plant type was introduced. The moderator level “cereals” contained all study plants belonging to the family of the Poacecae, the level “legumes” all members of the family Fabaceae and the final level “vegetables” was formed by the remaining fruit and leaf vegetables. Trials for YORgroup “old” were only present in the plant type level “cereals”, i.e. for “legumes” and “vegetable” only data for “new” and “ancestral” genotypes were available.
The moderator setting was influenced by the high number of studies performed under controlled greenhouse conditions (lab trials = 562, field trials = 14). Therefore, the dataset is dominated by artificial growing systems.
The moderator P treatment (addition of P fertilizer, yes or no) was also dominated by the high number of P-deficient studies (P treatment no = 497, P treatment yes = 79). Thus, the dataset is dominated by potentially P-deficient growth substrates. This potential P deficiency was an important aspect of our analysis.
The moderator soil pH covered a range of acidic (5.5) to alkaline (8.7) pH levels.
Statistics
Only a small number of studies reported standard errors. Therefore, the sample size (N) was used to perform a non-parametric weighting of studies (Hedges et al. 1999). This non-parametric weight w ij was calculated as follows: For experiment j within study i, \( {w_{{ij}}} = \left( {{N_{{ij}}}E*{N_{{ij}}}C} \right)/\left( {{N_{{ij}}}E + {N_{{ij}}}C} \right) \), where N ij E is the sample size of mycorrhizal plants and N ij C is the sample size of non-mycorrhizal control plants. If N ij E = N ij C, then the formula was reduced to w ij = N²/2*N. This method has been widely used in the meta-analysis literature (Adams et al. 1997; Lekberg and Koide 2005; Hoeksema and Forde 2008).
The statistical analyses were performed with R version 2.12.1 (R Development Core Team 2010). The packages “meta“ (Schwarzer 2007), “metafor” v. 1.6-0 (Viechtbauer 2010), and a non-parametric bootstrap code were used. The code for the non-parametric bootstrap was based on the “error” bootstrap by Van den Noortgate and Onghena (2005). The bootstrap samples were simulated via a hierarchical system with two levels: vectors of level 1 residuals were nested within vectors of level 2 residuals. The R code is accessible in the electronic supplementary material (Online Resource 3).The “metafor” function was used for creating a random effects model testing the effect of a moderator on one effect size. The calculation of the P-value and 95% confidence interval was performed by using the non-parametric “error” bootstrap. To test for significance of moderator effects, a two-tailed test was used. The bootstrap was used to evaluate the influence of the moderators on the effect. The metagen function (“meta” package in R) was used for calculation of the mean effect size for each moderator level.
To deal with hypothesis (i) we tested the effect size MR against the moderator variables YOR and YORgroup. Additionally, we tested the effect of the moderators YOR and YORgroup on both mycorrhizal (lnM) and non-myorrhizal biomass (lnNC) to be able to interpret the moderator effect on MR (being a response ratio) correctly due to the problematic nature of response ratios (see above).
To address hypothesis (ii), we evaluated first the influence of the abiotic (density, pre-germination, duration of experiment, setting, year of publication, treatment P, treatment P concentration and soil pH) and the biotic moderator variables (plant, plant type, AMF species) on MR. Second, Pearson's Chi-squared test was performed on moderators to test for their independence. Specific subsets were produced to test non-independent moderators for their influence on the effect size MR and their importance for the MR trend for the year of release of crop plants. Only moderators with a sufficient number of trials could be tested by the bootstrap. The effects of chosen moderators on MR were examined in the subsets “Before 1950” and “After 1950”. The subset “Before 1950” contained all cultivars with the YORgroup levels “ancestor” and “old”. The “After 1950” subset included all “new” cultivars. Third, subsets for the biotic moderator variables plant, plant type and AMF species were produced for moderator levels with the highest number of trials: “Barley”, “Maize” and “Wheat” for plant, “Cereals”, “Legumes” and “Vegetables” for plant type and “Gl. mosseae” and “Gl. intraradices” for AMF species. In these subset populations, the effect of YOR and YORgroup on MR was re-evaluated.
Fourth, the plant genera, AMF species or experimental practices may change over time and may be detectable via correlation with the year of publication. Therefore, the method used by Barto and Rillig (2010) was used. The levels of the tested moderator were ranked by their mean year of publication. The level with the lowest mean received the first rank, the level with the second lowest mean rank two and so on. This modified moderator was correlated with the year of publication to determine whether or not there were temporal shifts in the moderator. If a moderator does not change over time, then there will be no correlation.
For the last hypothesis (iii), we tested first the correlation of mycorrhizal PAE (mPAE), mycorrhizal PUE (mPUE) and root colonization (%AM), respectively against MR. Additionally to the bootstrap, we used a weighted regression with a ranked dependent variable (following Kendall’s Tau rank correlation) for evaluation of potential relationships between the different effect sizes. Although both methods are based on regressions, the weighted, rank modified regression reported useful parameters, like R² and residual error, while these pieces of information were not delivered by the bootstrap. However, the bootstrap P-value was more trustworthy and was preferred. The correlation analysis was performed on the complete dataset. Second, we analyzed the effect of the moderator variables YOR and YORgroup on the effect sizes mPAE, mPUE and %AM by using the bootstrap.
Results
Question (i): Is there a mycorrhizal responsiveness trend for the year of release of crop plants?
We found a significant effect of YORgroup on MR in crop plants. Old and new cultivars were more responsive than ancestral accessions (Table 1). No effect was detectable for the moderator YOR.
Due to difficulties in interpretation of results of response ratios, we tested the influence of the year of release moderators on both lnM and lnNC (Table 2). Moderator YOR reported only non-significant effects thus we only presented results for moderator YORgroup. Moderator YORgroup had a negative effect on both lnM and lnNC, but the effect on lnM was not significant.
One of the major constraints of the dataset was the dominance of studies working with a potentially P-deficient soil substrate. Therefore, we tested the differences in the effect of the moderator YORgroup and both lnM and lnNC for P-deficient and sufficient studies (Table 2). In studies with potentially P-deficient soil substrate, the same negative effect of YORgroup on lnM and lnNC was detectable as for the complete dataset, but in this subset the effect on lnM was marginally significant. There were no significant differences for P-sufficient soil substrates, neither for lnM nor for lnNC.
Question (ii): What factors influence mycorrhizal responsiveness and the mycorrhizal responsiveness trend for the year of release in crop plants?
Testing for the importance of a variety of moderators revealed that MR was influenced by several factors (Table 3). For the moderator variables pre-germination and AMF species the effect on %AM was tested as well. The data are available in Online Resource 4. The pre-germination of seeds and the subsequent transplantation as seedlings caused a decrease in MR, as did a high plant density per soil weight (density).
For the moderators treatment P (application of phosphorus as a factor, yes or no) and treatment P concentration (applied P-level, when P was an experimental factor), no effect was observed on MR; neither in the complete dataset nor in the subsets “Before 1950” and “After 1950”. The moderator soil pH had a negative effect on MR: the more alkaline the soil the less plant biomass increased under AMF influence. However the relationship between MR and soil pH was more complex than was detectable by this simple model. Therefore, three subsets were produced: “Acidic” with soil pH levels smaller than 6, “Neutral” with soil pH levels between 6 and 7, and “Alkaline” with soil pH levels higher than 7. In the subset “Acidic”, soil pH had a positive effect on MR, in “Neutral” a weak negative effect was present, and in the subset “Alkaline”, soil pH had a negative effect on MR (data not presented). This indicated that the closer the soil pH was in the neutral pH range, the better plants were growing.
The duration of experiment also had a negative effect but a very flat slope (−0.0025). In addition, the more recently a paper was published the more positive was MR. The moderators plant, plant type and AMF species also had an influence on MR. The moderator plant type was more important than the moderator plant, and plant was more important than AMF species. The moderator setting (lab or field) was imbalanced by the low number of trials for the level “field” and of no use for interpretation.
Pearson’s Chi-squared test showed that none of the moderators were independent (Online Resource 5). Therefore, it was not possible to interpret the influence of one moderator on the effect size separately from the others. However, by analyzing moderators of interest in specific subsets, the extent of their importance could be evaluated.
To test for the impact of moderators on MR in old and ancestral accessions as well as in new cultivars, moderators were analyzed in the two subsets “Before 1950” and “After 1950” containing all genotypes of the YORgroup “ancestor”, “old”, and “new”, as appropriate. The results of the subset tests were similar to the overall analysis with two exceptions. In the subset “Before 1950”, soil pH was no longer significant (Table 4). In the subset “After 1950”, duration of experiment was no longer significant compared to the whole dataset. For the subset “After 1950” and the moderator soil pH, we tested if the same trend for acidic, neutral and alkaline pH is detectable as for the complete dataset. Therefore, the subset “After 1950” was subdivided into three subsets just like for the complete dataset. For the “After 1950- acidic”, soil pH had a positive effect on MR, but the number of trials was exceptionally low (15). For the other two pH subsets, no influence on MR was found (data not presented). Thus, the hump-shaped relationship present in the complete dataset was not detectable in the “After 1950” subset.
The moderator pre-germination was further analyzed in separate subsets to gain more insight into its effects. For this, the effect of plant type on MR was tested in the subsets “Preger YES” and “Preger NO”. For the first subset, the level “cereals” (monocots) had a lower MR that the levels “legumes” and “vegetables” (both dicots). For the latter subset, the opposite was true (Online Resource 6).
To test the specific influence of AMF species on MR trend for the year of release in crop plants, the importance of YOR and YORgroup was tested separately within the two subsets “Gl. intraradices” and “Gl. mosseae”, the two most often used AMF species in single cultures for this meta-analysis. Two opposing trends were found: The moderator YORgroup had a positive effect on MR of plants inoculated with Glomus intraradices isolates, but a negative effect on plants inoculated with Glomus mosseae isolates (Table 5). In other words, ancestral genotypes growing in Glomus mosseae single culture had a higher MR than new cultivars and the opposite was true for Glomus intraradices. No trends were detectable for the moderator YOR.
Furthermore, the moderators plant and plant type were also tested for influence on the MR trend for the year of release in crop plants. A positive influence of YORgroup on MR was detectable in subsets “Cereals” and “Legumes”, but no trend could be found for the moderator YOR (Table 5). For the complete dataset and the subset “Cereals” and “Legumes”, the same trend was present. For the plant subsets “Barley”, “Maize” and “Wheat”, an effect was observed only for the “Maize” subset and the moderator YORgroup. This trend had low statistical support due to low power of YORgroup levels (“ancestor”-trials = 2, “old”-trials = 10, “new”-trials = 62).
MR increased with year of publication (Table 3, Fig. 1a). This effect was mainly driven by studies published in the years 2000 to 2010 and 1990 to 1995. No clear shift in usage of AMF species was detected (Fig. 1b); Glomus mosseae, Glomus intraradices and Glomus etunicatum were all used in studies from 1990 to 1995 as well as from 2000 to 2010. The experimental plants shifted over time (Fig. 1c). In the years 1990 to 1995 legumes were most often used, while in the years 2000 to 2010 vegetables were the preferred study objects. Cereals were used regularly throughout the research history. The usage of pre-germinated and then transplanted seedlings was mainly found before 1995 (Fig. 1d). In studies published after 2000, plants were more often directly seeded into the substrate. There was no clear shift in YORgroup detectable; ancestral, old and new genotypes were used both in the years 1990 to 1995 and the years 2000 to 2010 (Fig. 1e). In contrast, YOR shifted clearly over time (Fig. 1f). Studies published before 1995 used older genotypes than studies published after 1995 indicating that researchers used more recently released cultivars as experimental plants in more recently published studies. This temporal shift may be biased by the availability of year of release dates for old genotypes because the moderator YOR contained 227 new and only 28 old genotypes.
Weighted correlation of mycorrhizal responsiveness (MR) and five moderators (AMF species, plant, pre-germination, YORgroup and YOR) and year of publication. Variables on the y-axis were ranked and sorted by their mean year of publication with the lowest mean located at the bottom of the figure. For better visualisation of overlapping data points, the data were jittered on the x- and y-axes. Relationship between year of publication and a) MR (R² = 0.1042, df = 574, P < 0.0001), b) AMF species used as single cultures (R² = 0.0807, df = 343, P < 0.0001, code: 1 = Gl. etunicatum, 2 = Gl. fasciculatum, 3 = Gl. manihotis, 4 = Gl. intraradices, 5 = Gl. mosseae, 6 = Gi. margarita, 7 = Gl. clarum, 8.5 = Ac. morrowiae/Gi. rosae), c) plants used as study object (R² = 0.3255, df = 574, P < 0.0001, code: 1 = Alfalfa, 2 = Oat, 3 = Pea, 4 = Sorghum, 5 = Groundnut, 6 = Tomato, 7 = Soybean, 8.5 = Wheat/Barley, 10 = Bean, 11 = Lettuce, 12 = Maize, 13.5 = Rice/Pepper, 15 = Cucumber), d) pre-germination of seeds (R² = 0.3781, df = 498, P < 0.0001), e) YORgroup (R² < 0.0001, df = 461, P = 0.954) and f) YOR (R² = 0.8356, df = 259, P < 0.0001)
Question (iii): What is the role of mycorrhizal responsiveness and the year of release for AM fungal root colonization and P efficiency in crop plants?
The correlation between MR and %AM, mPAE and mPUE was tested. %AM was positively correlated with MR (R² = 0.30, df = 395, P < 0.0001).
mPUE correlated negatively with MR (R² = 0.11, df = 122, P < 0.0001) and mPAE tended to correlate positively with MR (R² = 0.04, df = 122, P = 0.072). The correlation of mPUE and MR was mainly driven by the study of Khalil et al. (1994). After excluding this study from the dataset, the relationship was no longer significant (R² = 0.007, df = 100, P = 0.612). The same study also had a strong influence on mPAE. After the exclusion of this study, the correlation was negative but still weak (R² = 0.07, df = 90, P = 0.006).
A negative association between %AM and YORgroup could be detected. Ancestral genotypes showed a colonization of about 41%, old of 30% and new cultivars of about 32% root length (Table 6). Again, the number of trials for “old” genotypes was very low. No trend could be found for YOR.
For P efficiency, no trend could be detected for either YORgroup or for YOR (Table 6). However, the means of the YORgroup levels (ancestor, old, new) for mPAE were always positive, while those of mPUE were always negative. Overall, this indicated that the tested genotypes, when mycorrhizal, were efficient in P acquisition and inefficient in P utilization.
Discussion
Question (i): Is there a mycorrhizal responsiveness trend for the year of release of crop plants?
The analysis of MR trends in plant biomass revealed that new genotypes released after 1950 were more mycorrhiza-responsive than ancestral genotypes. The phenomenon where new genotypes had a higher MR than ancestral accessions was not related to a higher biomass of new cultivars when mycorrhizal. New cultivars grew less when mycorrhizal or non-mycorrhizal as compared to ancestral accessions, but this trend was more pronounced for non-mycorrhizal biomass by a steeper, negative slope.
New cultivars were bred to grow fast under high fertilizer input, but the majority of studies used in this meta-analysis grew their plants on potentially P-deficient soil substrate. The low P availability could have been responsible for the reduced biomass of new cultivars as compared to ancestral accessions. There were not enough trials to detect an effect of YORgroup on mycorrhizal or non-mycorrhizal biomass in P-sufficient soil. Thus, it could not be convincingly tested whether the effect of YORgroup on biomass (for the complete dataset) was mainly driven by the P deficiency or other factors (as demonstrated for MR; see Table 3).
Our findings for the effect of YORgroup on MR contradicted the hypothesis by Hetrick et al. (1992), Hetrick and Wilson (1992) but were supported by the findings of Koide et al. (1988) and Bryla and Koide (1998). However, this effect was not detectable for the moderator YOR. The lack of an effect of YOR could be explained by the low number of trials for old cultivars released between 1900 and 1950.
The positive relationship between MR and YORgroup would suggest that there was no negative effect of breeding under high fertilizer conditions on MR of modern crop plants compared to their ancestral relatives (An et al. 2010; Galvan et al. 2011; Jackson et al. 2002; Sawers et al. 2010; Wright et al. 2005). This hypothesis was further supported by the analysis of the moderator plant type. In cereals and legumes, new cultivars had higher MR than ancestral ones. This relation was also present in vegetables as a non-significant trend. Even if the focus was only on a specific plant type (cereals, legumes, vegetables) there was a positive effect of YORgroup on MR.
But why were ancestral accessions less mycorrhiza-responsive? Plants growing under nutrient limitation adapt to this condition (Chapin et al. 1986). Therefore, Koide et al. (1988) suggested that wild plant genotypes growing on natural, nutrient poor soil are better adapted to this nutrient limitation compared to new cultivars bred under high fertilizer input, and thus are less responsive to AM fungi. One way to adapt to nutrient limitation is to increase nutrient efficiency. Old genotypes and ancient accessions have greater root lengths, higher root to shoot ratios, and a more branched root system compared to their younger relatives produced under higher fertilizer input (Koide et al. 1988; Zhu et al. 2001, 2003). Although these root traits are genetically highly variable (Hao et al. 2008), there is no doubt that changes in root architecture and morphology can improve P efficiency (Gahoonia and Nielsen 2004). A large root system with long root hairs increases the root surface and thus P acquisition (Gahoonia et al. 1999). Plants also decrease the soil pH around their roots to dissolve immobile P via exudation of protons, organic acids or phosphatases (Dalal 1977; Schjorring 1986; Asmar et al. 1995; Gahoonia et al. 2000).
However, new cultivars could be more mycorrhiza-responsive because of an increased nutrient demand. New cultivars were bred to grow faster and produce more yield under fertilizer input. The selection for fast growth and high yield promoted the interaction of plant and AM fungi in P-deficient soil to satisfy the higher needs for nutrients, and thus resulted in an increased MR (as demonstrated in Table 2).
Question (ii): What factors influence mycorrhizal responsiveness and the mycorrhizal responsiveness trend for the year of release in crop plants?
The moderators density, soil pH, seed pre-germination, duration of experiment, year of publication, plant and AMF species all had an effect on MR.
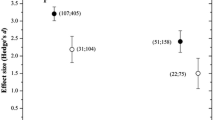
As expected, a high plant density per soil weight had a negative effect on MR (Schroeder and Janos 2004; Schroeder-Moreno and Janos 2008). A small substrate volume and high plant density are factors causing reductions in plant biomass and P acquisition due to nutrient and space limitations. In a large soil volume with high P concentration, root density correlates with P acquisition, but this is not the case in low soil volumes or soils with low P concentrations (Otani and Ae 1996). Furthermore, there are several possible explanations of why AMF did not ameliorate this reduction in biomass caused mainly by nutrient deficiency. (i) AMF might have reduced or disturbed the P acquisition pathway of the plant (Li et al. 2008a). (ii) The low P concentration in the growth substrate led to a conflict in plant and fungal P acquisition and to an overlap of the P depletion zones (Hayman 1983). (iii) Abbott and Robson (1984) reported that intraspecific density affected the development of intraradical AM fungal structures: higher density caused lower amounts of arbuscules per length of root colonized. Arbuscules are the P-exchange organs of AM fungi. Thus in P-deficient soil, plant and AMF are competing for nutrients and this might have caused conflicts in P exchange and/or plant PAE. Therefore, the plant might down-regulate C-translocation to the fungi and cause reduction in %AM (as demonstrated for our dataset; see Online Resource 4). MR and %AM are positively correlated (Fig. 2) and thus with decreasing %AM and increasing density, the MR decreases.
The effect of soil pH on MR was not surprising: the closer the soil pH was to the neutral pH range the better plants were growing. The negative effect of soil pH on MR was generated by genotypes released after 1950 (Table 4), although the hump-shaped relationship was no longer detectable in this subset. For genotypes released before 1950, no effect of soil pH on MR could be detected. AMF can support their host plants with nutrients and water and therefore reduce the stress of immobilized P caused by a strong acidic or alkaline pH (Cartmill et al. 2007, 2008; Cardarelli et al. 2010). For ancestral accessions and genotypes released before 1950 the lack of an influence of the soil pH moderator may be due to the better adaptation to P immobilization and a lower dependence on AM fungi compared with genotypes released after 1950. New cultivars would be more susceptible to alkalinity stress because of their higher nutrient demand due to higher yield production.
The moderator duration had a negative but weak effect on MR. This would mean that mycorrhizal plants grew less in long lasting experiments as compared to their non-mycorrhizal controls resulting in a smaller response ratio. This effect was statistically weak as compared to the other moderator variables; this moderator had a nearly flat slope (−0.0025). The fact that there was still a significant effect (P = 0.0164) was likely due to the high number of trials (471) and thus exceptionally high statistical power. The moderator duration lost its influence in the subset “After 1950”. Although the duration of experiments had a negative effect on plant growth of cultivars released before 1950, this effect was weak. The slope was again very flat (−0.0051) and the significance (P = 0.004) likely attributable to the large number of trials (139). Taking this fact into account we could state that the duration of experiments was not a strong factor influencing the MR trend for the year of release of crop plants.
Pre-germination and transplantation of seedlings caused a decrease in MR. During transplantation of seedlings, fine roots and root hairs can be damaged, and then plants experience stress due to new biotic and abiotic factors. This transplant shock can reduce overall plant biomass, leaf area and canopy photosynthesis as demonstrated in rice (Dingkuhn et al. 1980, 1991; Kotera et al. 2004) and could make the plant more susceptible to pathogens. In our dataset, pre-germination caused a reduction in MR of about 50% (Table 3). This leads to the assumption that pre-germination affected the plant and not the fungal symbiosis partner. Additionally, this is supported by the fact that %AM was not influenced by pre-germination (Online Resource 4). The importance of the moderator variable plant type on pre-germination (tested in the subsets “Preger Yes” and “Preger NO”) revealed that monocots (Poaceae) grew better when not pre-germinated while the opposite was true for dicots (Fabaceae, Pedaliaceae, Asteraceae and Cucurbitaceae). Thus, the negative effect of pre-germination can be explained partially by the dominance of the family Poaceae in our dataset, causing the high MR values for the pre-germination level “no”.
Treatment P (yes or no) and treatment P concentration unexpectedly had no influence on mycorrhizal responsiveness, even though a reduction in %AM and MR with increasing P input is often reported (Rajapakse et al. 1989; Raju et al. 1990; Jackson et al. 2002). An explanation may be the low number of trials for these moderators because only 8 of 39 studies worked with P application as a factor. Additionally, P application does not necessarily translate to P availability due to leaching or binding to soil ions.
The same problem existed for the moderator variable setting (lab or field): only one study reported data from field experiments causing a tremendous imbalance of the moderator levels (lab trials = 562, field trials = 14).
The strong positive effect of year of publication on MR, meaning that there was a tendency towards reporting increasing MR with newer publication date, was likely caused by the moderator variable plant and pre-germination, but not by the AMF species used as inoculum (Fig. 1). Pre-germination and plant type were moderators with strong effects on MR, thus the positive correlation of year of publication and the effect size can be explained by the positive impact of direct seeding and usage of specific plant genera.
There was a strong effect of AMF species on the MR trend for the year of release of crop plants. In the subset “Gl. mosseae“, YORgroup had a negative effect on MR and in the subset “Gl. intraradices” a positive effect (Table 5), i.e. new cultivars had a higher MR when growing with Glomus intraradices. Old and ancestral accessions grew more when colonized by Glomus mosseae. Although YORgroup had an effect on %AM (Table 6), there was no significant difference in %AM between the YORgroups (ancestor, old, new) in the subsets “Gl. mossaeae” and “Gl. intraradices” (Online Resource 4), i.e. in the two AMF species subsets, there were no differences in percent colonization by Glomus mosseae and Glomus intraradices, respectively, between ancestral, old and new genotypes. Glomus mosseae is an early-stage colonizer (Sykorova et al. 2007) and well adapted to highly disturbed systems like agricultural soils (Hijri et al. 2006, Oehl et al. 2004) or likewise pots inoculated with mixed soil or colonized root fragments. New cultivars were bred to grow fast, and therefore they need to quickly acquire nutrients. Most studies incorporated in this meta-analysis used a potentially P-deficient growth substrate and thus promoted the symbiosis. The lower MR of new cultivars growing with Glomus intraradices might indicate some physiological incompatibility between AMF and plant, e.g. the plant can down-regulate AMF colonization by reduced C translocation to the fungus (Ercolin and Reinhardt 2011) or the fungus can influence the level of gene transcription in the host plant as demonstrated for segregated lines (Angelard et al. 2010).
In plant subsets, the effect of YORgroup and YOR was tested on MR for the family Poaceae (the group with the highest number of trials). No trend was detectable for wheat and barley, but a negative effect for maize, i.e. new maize cultivars had lower MR as compared to ancestral maize accessions. This negative trend contradicted the finding that the plant type level “cereals” produced a positive effect for YORgroup on MR.
However, the statistical power of the moderator YORgroup in the maize subpopulation was very low and thus the reliability of this trend is not high. For barley, the number of trials was even smaller and the variability likely too high for a significant trend. The “Wheat” subset had a sufficient number of trials but no trend for MR and YORgroup was detectable either. The high variability in the wheat subpopulation might be due to the fact that plants (also being members of the same genus Triticum) differ dramatically in their physiological traits, like P efficiency, pathogen resistance and tolerance against influences like P deficiency or intraspecific density.
Summarized, the moderator variables density, pre-germination, plant, plant type and AMF species had an effect on both subsets “Before 1950” and “After 1950” thus possessing the potential to influence a MR trend for year of release in crop plants. In contrast, the moderator variables duration and soil pH were only important for genotypes released before or after 1950, respectively.
The analysis of the effect of AMF species and plant on MR revealed that the AM fungal genotype was more important than the plant identity; although this was only testable for three Poaceae genera (barley, maize, wheat). The analysis of Poaceae (“cereals”) and Fabaceae (“legumes”) as a subset showed that on a larger scale plant identity gained importance on the MR trend for year of release in crop plants.
Question (iii): What is the role of mycorrhizal responsiveness and the year of release for AM fungal root colonization and P efficiency in crop plants?
In our dataset, MR was positively correlated with %AM (Fig. 2) and this finding is consistent with those of Lekberg and Koide (2005). However, in the literature the opposite has also been reported (Hetrick and Wilson (1992); Kaeppler et al. 2000; Yücel et al. 2009). Each of these contradicting studies used about 30 trials, while our analysis and the meta-analysis of Lekberg and Koide 2005 used about 400 and 290 trials, respectively. This large number of studies (containing even those reporting the opposite effect) likely helped uncover the positive relationship of MR and %AM, although the relationship was not strong (R² = 0.30).
The relationship between MR and P efficiency was inconsistent. Most of the studies used for the analysis of P efficiency worked with potentially low P soil. For this soil fertility level, it was suggested that PAE is more important than PUE (Wang et al. 2010). However, in our dataset mPAE had no significant effect on MR, but the negative effect of mPUE was highly significant. Therefore, plant genotypes with high MR acquired more P when mycorrhizal and utilized more efficiently the acquired P when non-mycorrhizal. This was notably the case for the maize and soybean genotypes of the Khalil et al. (1994) study. The exclusion of this study was able to turn the correlation of mPAE with MR from positive to negative, and additionally to nullify the effect of mPUE on MR. Some of the plant genotypes used in that study were those with the highest mPAE and lowest mPUE of the whole dataset, i.e. when those genotypes were mycorrhizal, they took up more P than non-inoculated control plants. They were highly inefficient in P acquisition, while non-mycorrhizal genotypes utilized P to a higher degree, i.e. they were P utilization efficient. These P acquisition inefficient and P utilization efficient genotypes were all highly mycorrhizal responsive.
The other genotypes in the dataset had a higher mPAE and a lower mPUE, but showed a high variability in MR. High P efficiency may cause an increased P supply and thus an increased plant P level. The high plant P level reduces the intensity of the AMF and plant interaction, as in %AM and biomass accumulation (Baon et al. 1993; Gao et al. 2007). For single studies and genotypes this might be true, but in general, variability in MR was too high and too dependent on other factors, like soil pH, plant density and substrate volume, plant species and AMF species, to expect a direct relationship between MR and P efficiency.
Analyzing the influence of the moderator YORgroup on %AM revealed that ancestral accessions were more intensely colonized than new cultivars (Table 6). This decrease in colonization from ancestral to new genotypes is consistent with the literature (Hetrick and Wilson (1992); Kaeppler et al. 2000; Zhu et al. 2001). An explanation for a reduction in %AM in new cultivars could be an increase in pathogen resistance. Toth et al. (1990) suggested that genotypes with a reduction in pathogen susceptibility tend to be less colonized by mycorrhizal fungi as well. However, no correlation between genotype age and pathogen susceptibility was evident (An et al. 2010; Steinkellner et al. 2011).
The moderator variables YORgroup and YOR had no influence on P efficiency, neither on mPAE nor on mPUE (Table 6). New cultivars were not more P-efficient or inefficient than old or ancestral accessions. This result is supported by the inconsistent findings in the literature. Thus, P-efficient cultivars can be found among old varieties and landraces (Wissuwa and Ae 2001) as well as among new cultivars (Zhu et al. 2003; Wright et al. 2005). The ability of a genotype to acquire and utilize P is not related to any changes in agricultural and breeding practices (at least for this dataset) but is influenced by other factors such as root parameters (Gahoonia et al. 1999), nutrient supply (Wang et al. 2010), pathogenic state, plant species (Fernandez et al. 2009) and associated AMF species (Khalil et al. 1994).
Summarized, %AM was important for the MR trend for the year of release of crop plants but P efficiency was not (for our dataset). A possible re-evaluation of the influence of P efficiency on this trend would need a higher number of trials for PAE and/or PUE. It would be of great interest if agricultural and breeding practices had an influence on cultivars over time and thus on their potential to respond to AMF. Breeding for higher yield by introducing valuable traits of landraces into parental inbred lines is a one-way street, and limited by nutrient availability. Breeding for higher responsiveness without higher dependence (Janos 2007; Galvan et al. 2011) and/or breeding for higher P efficiency (Wissuwa et al. 2009), and thus better P acquisition and/or better conversion of P into yield, is of greater importance for future agriculture.
Conclusions
In general, new cultivars were less intensely colonized but were more mycorrhiza-responsive compared to ancestral genotypes, although the response was not always consistent across all conditions. This MR trend for year of release in crop plants was confirmed by the moderator plant type and potentially influenced by the moderator variables density, pre-germination, plant, plant type and AMF species, while duration and soil pH were only important for genotypes released before or after 1950, respectively. %AM was also important for the MR trend for year of release but P efficiency was not (at least in our dataset). Therefore, we state that new crop plant genotypes did not lose their ability to respond positively to AMF for plant growth due to agricultural and breeding practices, but this statement is only true under certain conditions; plants need to grow on P-deficient soil, with AMF species like Glomus mosseae, and the comparison needs to be done between ancestral and new genotypes.
Additionally, the MR trend for year of release was detected in a dataset dominated by lab studies, i.e. studies performed under controlled and mostly artificial greenhouse environments and thus an extrapolation of the results of this meta-analysis to the field situation is not recommended. More field studies testing the effect of AMF inoculation on new, old and ancestral genotypes need to be done before more reliable predictions can be made. The fact that this MR trend for the year of release was present under potentially P-deficient conditions highlighted the potential of the combined use of new cultivars and specific AMF for sustainable agriculture. Even though our discussion is based on the concept of mycorrhizal responsiveness, it is very possible that we can also make a statement about mycorrhizal dependence: more modern cultivars grew less well without mycorrhiza in likely P-limited conditions, thus the pattern we observed could also be interpreted as increased mycorrhizal dependence. We cannot conclude this with absolute certainty because we lack detailed data on actual P availability (which would need to be very low in order for this statement to be solidly supported).
The low impact of the moderator variable YOR (representing the year of release dates) was due to the fact that year of release dates were only available for new and old cultivars, and the latter ones were under-represented in our dataset. Although old genotypes hold the potential to outperform new cultivars in terms of MR, additional work needs to be done with this year of release class. Most studies focused on the comparison of ancestral and new genotypes and thus the number of old genotypes released between 1900 and 1950 was quite low, which is problematic in terms of establishing clear patterns.
Additionally, it is highly recommended that in future studies a measure of the variance of sample means, like standard error, is included to permit parametric weighting methods. Then it would be possible to test with higher statistical power the influence of agricultural and breeding practices on plant growth promotion by AM fungi.
For this study and under these data constraints, new crop plant genotypes did not lose their ability to respond to mycorrhiza due to agricultural and breeding practices. Therefore, plant breeders focusing on sustainable, organic agriculture can include new cultivars in their germplasms.
References
Abbott LK, Robson AD (1984) The effect of root density, inoculum placement and infectivity of inoculum on the development of vesicular-arbuscular mycorrhizas. New Phytol 97:285–299
Adams DC, Gurevitch J, Rosenberg MS (1997) Resampling tests for meta-analysis of ecological data. Ecology 78:1277–1283
Al-Karaki GN, Hammad R, Rusan M (2001) Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 11:43–47
An GH, Kobayashi S, Enoki H, Sonobe K, Muraki M, Karasawa T, Ezawa T (2010) How does arbuscular mycorrhizal colonization vary with host genotype? An example based on maize (Zea mays) germplasms. Plant Soil 327:441–453
Angelard C, Colard A, Niculita-Hirzel H, Croll D, Sanders IR (2010) Segregation in mycorrhizal fungus alters rice growth and symbiosis-specific gene transcription. Curr Biol 20:1216–1221
Asmar F, Gahoonia TS, Nielsen NE (1995) Barley genotypes differ in extracellular phosphatase activity and depletion of organic phosphorus from rhizosphere. Plant Soil 172:117–122
Auge RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Baon JB, Smith SE, Alston AM (1993) Mycorrhizal response of barley cultivars differing in P efficiency. Plant Soil 157:97–105
Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98:745–753
Bolan NS (1991) A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134:189–207
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068
Bryla DR, Koide RT (1998) Mycorrhizal response of two tomato genotypes relates to their ability to acquire and utilize Phosphorus. Ann Bot 82:849–85
Burleigh SH, Cavagnaro T, Jakobsen I (2002) Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J Exp Bot 53:1593–1601
Cardarelli M, Rouphael Y, Rea E, Colla G (2010) Mitigation of alkaline stress by arbuscular mycorrhiza in zucchini plants grown under mineral and organic fertilization. J Plant Nutr Soil Sc 173:778–787
Cartmill AD, Alarcon A, Valdez-Aguilar LA (2007) Arbuscular mycorrhizal fungi enhance tolerance of Rosa multiflora cv. Burr to bicarbonate in irrigation water. J Plant Nutr 30:1517–1540
Cartmill AD, Valdez-Aguilar LA, Bryan DL, Alarcon A (2008) Arbuscular mycorrhizal fungi enhance tolerance of vinca to high alkalinity in irrigation water. Sci Hortic 115:275–284
Chapin FS, Vitousek PM, Van Cleve K (1986) The nature of nutrient limitation in plant communities. Am Nat 127:48–58
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Daft MJ (1991) Influence of genotypes, rock phosphate and plant densities on mycorrhizal development and the growth responses of five different crops. Agr Ecosyst Environ 35:151–169
Dalal R (1977) Soil organic phosphorus. Adv Agron 29:83–117
Dalrymple DG (1985) The development and adoption of high-yielding varieties of wheat and rice in developing-countries. Am J Agr Econ 67:1067–1073
Dingkuhn M, Schnier HF, De Datta SK, Wijangco E, Dorffling K (1980) Diurnal and developmental changes in canopy gas exchange in relation to growth in transplanted and direct-seeded flooded rice. Aust J Plant Physiol 17:119–134
Dingkuhn M, Schnier HF, De Datta SK, Dorffling K, Javellana K (1991) Relationships between ripening-phase productivity and crop duration, canopy photosynthesis and senescence in transplanted and direct-seeded lowland rice. Field Crop Res 26:327–345
Doebley JF, Gaut BS, Smith BD (2006) The molecular genetics of crop domestication. Cell 127:1309–1321
Ercolin F, Reinhardt D (2011) Successful joint ventures of plants: arbuscular mycorrhiza and beyond. Trends Plant Sci 16:356–362
Fernandez MC, Belinque H, Gutierrez Boem FH, Rubio G (2009) Compared phosphorus efficiency in soybean, sunflower and maize. J Plant Nutr 32:2027–2043
Fitter AH, Moyersoen B (1996) Evolutionary trends in root-mircobe symbiosis. Philos T Roy Soc B 351:1367–1375
Gahoonia TS, Nielsen NE (2004) Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 260:47–57
Gahoonia TS, Nielsen NE, Lyshede OB (1999) Phosphorus acquisition of cereal cultivars in the field at three levels of P fertilization. Plant Soil 211:269–281
Gahoonia TS, Asmar F, Giese H, Nielsen GG, Nielsen NE (2000) Root released organic acids and phosphorus uptake of two barley cultivars in laboratory and field experiments. Eur J Agron 12:281–289
Galvan GA, Kuyper TW, Burger K, Keizer LCP, Hoekstra RF, Kik C, Scholten OE (2011) Genetic analysis of the interaction between Allium species and arbuscular mycorrhizal fungi. Theor Appl Genet 122:947–960
Gao X, Kuyper TW, Zou C, Zhang F, Hoffland E (2007) Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 290:283–291
Hao L, Zhang J, Chen F, Christie P, Li X (2008) Response of two Maize inbred lines with contrasting Phosphorus efficiency and root morphology to mycorrhizal colonization at different soil Phosphorus supply levels. J Plant Nutr 31:1059–1073
Harlan JR (1975) Our vanishing genetic resources. Science 188:618–621
Harrier L, Watson CA (2004) The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag Sci 60:149–157
Hayman DS (1983) The physiology of vesicular-arbuscular endomycorrhizal symbiosis. Can J Bot 61:944–963
Hedges LV, Guevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hetrick BAD, Wilson GWT (1992) Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can J Bot 71:512–518
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Todd TC (1996) Mycorrhizal response in wheat cultivars: relationship to phosphorus. Can J Bot 74:19–25
Hijri I, Sykorova Z, Oehl F, Ineichen K, Mäder P, Wiemken A, Redecker D (2006) Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol 15:2277–2289
Hoeksema JD, Forde SE (2008) A meta-analysis of factors affecting local adaptation between interacting species. Am Nat 171:275–290
Huang ML, Deng XP, Zhao YZ, Zhou SL, Inanaga S, Yamada S, Tanaka K (2007) Water and nutrient efficiency in diploid, tetraploid and hexaploid wheats. J Integr Plant Biol 49:1672–9072
Jackson LE, Miller D, Smith SE (2002) Arbuscular mycorrhizal colonization and growth of wild and cultivated lettuce in response to nitrogen and phosphorus. Sci Hortic 94:205–218
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low Phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364
Khalil S, Loynachan TE, Tabatabai MA (1994) Mycorrhizal dependency and nutrient uptake by improved and unimproved corn and soybean cultivars. Agron J 86:949–958
Koide RT (1991) Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386
Koide RT, Li M, Lewis J, Irby C (1988) Role of mycorrhizal infection in the growth and reproduction of wild vs. cultivated plants I. Wild vs. cultivated oats. Oecologia 77:537–543
Kotera A, Nawata E, Van Chuong P, Giao NN, Sakuratani T (2004) A model for phenological development of Vietnamese rice influenced by transplanting shock. Plant Prod Sci 7:62–69
Lekberg Y, Koide RT (2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol 168:189–204
Li H, Smith FA, Dickson S, Holloway RE, Smith SE (2008a) Plant growth depressions in arbuscular mycorrhizal symbiosis: not just caused by carbon drain? New Phytol 178:852–862
Li H, Smith SE, Ophel-Keller K, Holloway RE, Smith FA (2008b) Naturally occurring arbuscular mycorrhizal fungi can replace direct P uptake by wheat when roots cannot access added P fertiliser. Funct Plant Biol 35:124–130
Manske GGB, Ortiz-Monasterio JI, van Ginkel M, Gonzalez RM, Fischer RA, Rajaram S, Vlek PGL (2001) Importance of P uptake efficiency versus P utilization for wheat yields in acid and calcareous soils in Mexico. Eur J Agron 14:261–274
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411
Oehl F, Sieverding E, Mäder P, Dubois D, Ineichen K, Boller T, Wiemken A (2004) Impact of long-term conventional and organic farming on diversity of arbuscular mycorrhizal fungi. Oecologia 138:574–583
Otani T, Ae N (1996) Sensitivity of phosphorus uptake to changes in root length and soil volume. Agron J 88:371–375
Palladino P (1993) Between craft and science: plant breeding, Mendelian genetics, and British universities, 1900–1920. Technol Cult 34:300–323
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. PNAS 99:13324–13329
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajapakse S, Zuberer DA, Miller JC (1989) Influence of phosphorus level on VA Mycorrhizal colonization and growth of cowpea cultivars. Plant Soil 114:45–52
Raju PS, Clark RB, Ellis JR, Duncan RR, Maranville JW (1990) Benefit and cost analysis and phosphorus efficiency of VA mycorrhizal fungi colonization with sorghum (Sorghum bicolour) genotypes grown at varied phosphorous levels. Plant Soil 124:199–204
Reitz LP (1968) Origin, history, and use of Norin 10 wheat. Crop Sci 8:686–689
Righetti TL, Sandrock DR, Strik B, Vasconcelos C, Moreno Y, Ortega-Farias S, Banados P (2007) Analysis of ratio-based responses. J Am Soc Hortic Sci 132:3–13
Rouphael Y, Cardarelli M, Mattia ED, Tullio M, Rea E, Colla G (2010) Enhancement of alkalinity tolerance in two cucumber genotypes inoculated with an arbuscular mycorrhizal biofertilizer containing Glomus intraradices. Biol Fert Soils 46:499–509
Sawers RJH, Gebreselassie MN, Janos DP, Paszkowski U (2010) Characterizing variation in mycorrhiza effect among diverse plant varieties. Theor Appl Genet 120:1029–1039
Schjorring JK (1986) Nitrate and ammonium absorption by plants growing at a sufficient or insufficient level of phosphorus in nutrient solution. Plant Soil 91:313–318
Schroeder MS, Janos DP (2004) Phosphorus and intraspecific density alter plant responses to arbuscular mycorrhizas. Plant Soil 264:335–348
Schroeder-Moreno MS, Janos DP (2008) Intra- and inter-specific density affects plant growth responses to arbuscular mycorrhizas. Botany 86:1180–1193
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Schwarzer, G. (2007) meta: Meta-Analysis. R package version 0.8-2
Sensoy S, Demir S, Turmen O, Erdine C, Savur OB (2007) Response of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci Hortic 113:92–95
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Smith SE, Facelli E, Pope S, Smith FA (2010) Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326:3–20
Steinkellner S, Hage-Ahmed K, Garcia-Garrido JM, Illna A, Ocampo JA, Vierheilig H (2011) A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f. sp. lycopersici. Mycorrhiza in press (doi: 10.1007/s00572-011-0393-z)
Sykorova Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from field, from bait plants transplanted to the field and from greenhouse trap experiment. Mycorrhiza 18:1–14
Tawaraya K, Tokairin K, Wagatsuma T (2001) Dependence of Allium fistulosum cultivars on the arbuscular mycorrhizal fungus, Glomus fasciculatum. Appl Soil Ecol 17:119–124
Toth R, Toth D, Starke D, Smith DR (1990) Vesicular-arbuscular mycorrhizal colonization in Zea mays affected by breeding for resistance to fungal pathogens. Can J Bot 68:1039–1044
Van den Noortgate W, Onghena P (2005) Parametric and nonparamentric bootstrap methods for meta-analysis. Behav Res Meth 37:11–22
Viechtbauer W (2010) Conducting meta- analyses in R with the metafor package. J Stat Soft 96:1–48
Vierheilig H, Ocampo JA (1991) Susceptibility and effectivness of vesicular-arbuscular mycorrhizae in wheat cultivars under different growing conditions. Biol Fert Soils 11:290–294
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306
Wissuwa M, Ae N (2001) Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breeding 120:43–48
Wissuwa M, Mazzola M, Picard C (2009) Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 321:409–430
Wright DP, Scholes JD, Read DJ, Rolfe SA (2005) European and African maize cultivars differ in their physiology and molecular responses to mycorrhizal infection. New Phytol 167:881–896
Yao Q, Li X, Feng G, Christie P (2001a) Influence of extramatrical hyphae on mycorrhizal dependency of wheat genotypes. Commun Soil Sci Plan 32:3307–3317
Yao Q, Li X, Feng G, Christie P (2001b) Factors affecting arbuscular mycorrhizal dependency of wheat genotypes with different phosphorus efficiencies. J Plant Nutr 24:1409–1419
Yücel C, Özkan H, Ortas I, Yagbasanlar T (2009) Screening wild emmer wheat accessions (Triticum turgidum subsp. diccoides) for mycorrhizal dependency. Turk J Agric For 33:513–523
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Zhu YG, Smith FA, Smith SE (2003) Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 13:93–100
Acknowledgements
We thank the Dahlem Center of Plant Sciences (DCPS) at Freie Universität Berlin for funding, and reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thom W. Kuyper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lehmann, A., Barto, E.K., Powell, J.R. et al. Mycorrhizal responsiveness trends in annual crop plants and their wild relatives—a meta-analysis on studies from 1981 to 2010. Plant Soil 355, 231–250 (2012). https://doi.org/10.1007/s11104-011-1095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1095-1