Abstract
Aims
Our aim was to explore the way that root system type affects mycorrhizal growth response of plants.
Methods
An extensive meta-analysis with 943 peer-review publications was conducted to test the difference in mycorrhizal responses between taproot plants and plants with a fibrous root system.
Results
We found that taproot plants showed greater growth response (biomass, P and N uptake) to colonization by arbuscular mycorrhizal fungi (AMF) than do plant species with fibrous root systems. This response pattern was dependent on stress types, AMF identity and species richness, and particularly the type of stress (abiotic vs. biotic). Taproot plants respond more to AMF than plants with a fibrous root system; but no difference was shown under biotic stress. The interaction effect seen for AMF and biotic stress was significantly higher for plants with fibrous root system, but was not significant between taproot plants and abiotic stress. Difference in biomass response was only found for Glomeraceae and Gigasporaceae between the two types of plants, while difference was found in P uptake response for Glomeraceae and Claroideoglomeraceae. However, plants with fibrous root system showed higher growth response than taproot plants under nematode stress.
Conclusions
Taproot plants might be more dependent on mycorrhiza than plants with fibrous root system. This indicates that environmental conditions can modify the relative abundance of taproot plants and plants with fibrous root system through mycorrhizal functioning, which will regulate plant community dynamics and processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi (AMF) are ecologically important soil microbes and form obligate symbiosis with roots of most terrestrial plants (Smith and Read 2008). In the symbiosis, host plants provide carbon for AMF in return for several benefits, i.e., promoting nutrient uptake, tolerating drought and salt stress, resisting pathogens and herbivores, etc. (Smith and Read 2008). AMF also can redistribute resources (i.e., C, N and P) between plants and alter their competitive interactions (van der Heijden et al. 2003; Wagg et al. 2011), and thus drive plant population dynamics and community processes (Koide and Dickie 2002van der Heijden et al. 1998).
Roots form intimate associations with AMF (Smith and Read 2008). Previous studies showed that AMF could modify plant root morphology and architecture (Atkinson et al. 1994; Berta et al. 1990, 1993, 1995; Schellenbaum et al. 1991). However, other studies have found that root system type potentially modifies how plant growth responds to colonization by AMF. As early as 1970, Baylis found that Coprosma robusta with coarse root architecture did not grow without mycorrhiza, but other plant species with fine root system were not affected without colonization by AMF. Based on this study, a frequently cited hypothesis is formed that plant species with coarse root system will accrue more benefits from symbioses with AMF. Wilson and Hartnett (1998) argued that root morphology was an extreme determinant for mycorrhizal responsiveness. Hetrick et al. (1992) determined that mycorrhizal dependence of plants was highly correlated to root fibrousness. Plants possibly have higher mycorrhizal dependency when they have thick and less branched roots and few root hairs; lower mycorrhizal dependence might occur in those plants with many fine roots and root hairs (Wilson and Hartnett 1998). Thus, highly branched roots and mycorrhizal fungi might be alternative strategies for absorbing nutrients (Wilson and Hartnett 1998). Schweiger et al. (1995) argued that root hairs could promote uptake for immobile nutrients, thereby affecting mycorrhizal responsiveness of plants.
Generally, plants have 1 of 2 types of root system: taproot system and fibrous system (Torrey and Clarkson 1975; Ye 2007). The taproot system consists of a tap root and coarse lateral roots, in which the tap root is first developed from radical and becomes the most prominent root, and then many smaller branch roots grow from this tap root. The fibrous root system forms with numerous fine roots about similar size developing from radical, in which the radical is short-lived. The fibrous system has higher root number than taproot system (Torrey and Clarkson 1975). These morphological differences might determine functional differentiation. Sullivan et al. (2000) found that tap root and fibrous root systems have different surface areas and differential ability to take up nutrients. Fibrous root system had larger belowground area and thus higher nitrate uptake rate than tap root system. This would potentially affect mycorrhizal responsiveness of plants. For example, Medicago sativa L. with taproot system is highly mycorrhizal dependent, while Hordeum vulgare L. with fibrous root system does not show strong positive growth response to colonization by AMF (Azcón et al. 1991; Grace et al. 2009).
Many factors affect plant growth responses to AMF, including environmental conditions, AMF identity and diversity, etc. Some studies found that abiotic stress could enhance mycorrhizal responses while opposite results were also reported. For example, salt stress increased growth response of maize to Glomus mosseae (Sheng et al. 2008). However, Wu et al. (2007) reported that drought stress reduced mycorrhizal responsiveness of Citrus tangerine. Barto and Rillig (2010) conducted a meta-analysis and found that herbivory inhibited AMF to some extent, and thus affected mycorrhizal responses of plants. Mycorrhizal effects on plant growth also depended on AMF species identity and diversity. Klironomos (2003) reported high variation in plant responses to different AMF species; van der Heijden et al. (1998b) found that plant productivity increased with AMF species richness. However, we do not know whether the effects of these factors on mycorrhizal responses of plant growth depend on different root system types.
Recently, Maherali (2014) conducted a meta-analysis to test the Baylis hypothesis. In that study, the author combined published literatures and phylogenic information of host plants to test the correlation between root architectural traits and mycorrhizal growth response. This work utilized novel and attractive methods for data analysis of root traits and provided exciting conclusions. Root traits (i.e., specific root length, root diameter, root hair length and root hair density, etc.) might be direct determinants for mycorrhizal responsiveness of plants (Baylis 1970). Thus, it might be more accurate to use these data to meta-analyze the effects of root system on mycorrhizal functioning than other root data. However, studies that reported the relationship between root trait and mycorrhiza are so limited that Maherali (2014) had to only select 12 publications to carry out his research. Nonetheless, if we use other root data (i.e., root system type) as an approximation for root traits, there might be sufficient data to test the same hypothesis as Maherali (2014). Therefore, in this study, we utilized taproot vs. fibrous system data as an approach complementary to that of Maherali (2014). An intensive meta-analysis with 943 peer-review publications was conducted to reveal the growth response patterns of plants with either root system to AMF. We hypothesized that plants with fibrous root system respond less to AMF than plants with taproot systems. Our questions are as follows: (1) How do plants with tap or fibrous root systems respond to AMF? (2) Is the response pattern context-dependent? (3) How does AMF taxonomic family identity affect the response pattern? (4) How does AMF species richness affect the response pattern?
Methods
Literature search
Different means were used to retrieve relevant papers for this meta-analysis. First, literature searches were conducted on May 10, 2012 in Web of Science (http://apps.webofknowledge.com/) and Google Scholar (http://scholar.google.com.hk/) with following key terms: (biomass OR phosphorus OR nitrogen) AND (abuscular AND mycorrhiza*). Papers published in Chinese were searched in CNKI database (http://www.cnki.net/). Publication year was not restricted. Second, we extracted all papers used for meta-analysis by Hoeksema et al. (2010), Veresoglou et al. (2012), Borowicz (2001) and Koricheva et al. (2009). However, publication years were restricted between 1970 and 1998 for Borowicz (2001) and before 2007 for Koricheva et al. (2009). In order not to miss the references published after these three meta-analsysis studies, we thereby searched Web of Science with original terms of (abuscular AND mycorrhiza*) AND (pathogen OR nematode) for Borowicz (2001) with publication year limited between 1999 and 2012. Searches were also conducted with original terms of (arbuscular AND mycorrhiza*) AND herbivor* for Koricheva et al. (2009) with restriction of publication year between 2008 and 2012.
Data selection
We included studies satisfying the following criteria in our meta-analysis: (1) studies should include pair-wise control and experimental treatments; (2) AMF identity should be identified to species level, data of mixed AMF species were included while fungicide-treatments were excluded; (3) for the same host plant or AMF species in different publications, each study was treated as an independent data record; (4) for the same AMF-host pair from different sites, habitats or experimental approaches, each observation was considered as an independent data record; (5) for study reported AMF interacting with other non-pathogen microbes, such as rhizobia and P solubilizing bacteria, data was only collected for sole AMF without any interactions.
In total, 943 peer-review publications satisfied our selection criteria. Previous meta-analysis studies only randomly selected a small subset of all satisfied publications for further analysis (Hoeksema et al. 2010; Veresoglou et al. 2012). However, in order to provide more solid evidence, we included all these papers for our analysis.
Data collection
We extracted mean, standard deviation (SD) or standard error (SE) and sample size (N) of plant growth parameters (biomass, P content and N content) from each pair of control and experimental study. Here, we collected P/N content (mg · plant−1), not concentration (mg · g−1), because it is an absolute metric to directly reflect mycorrhiza functioning (Li et al. 2006). Root length colonization (RLC; %) data was also extracted from experimental studies. For data presented in table form, it was directly extracted. For data presented with graphs, it was digitized with GetData software (http://getdata-graph-digitizer.com/). For experiments along stress gradients, data were only collected at both ends; for the same AMF-host pairs in different years, the most recent data were collected. SE was transformed to SD with SD = SE*sqrt (N) when studies only provided SE value. When SD or SE was missing, we estimated SD following van Groenigen et al. (2011). We first calculated coefficient of variation (CV) for each dataset and then averaged all CVs. The missing SD was estimated by multiplying the reported mean value by the average CV.
Data category
Plants were classified as roots with either ‘taproot system’ or ‘fibrous system’ through the following criteria: (1) all monocotyledons are categorized as fibrous root system; (2) all woody dicotyledons are categorized as taproot system (Torrey and Clarkson 1975; Ye 2007); (3) because some herbaceous dicotyledons are characteristic of fibrous root system, the root system information of these plants was obtained by searching Google, original publications or contacting authors. Although this root system category might be confounded with phylogenetic information of plants (Maherali 2014), the phylogeny does not determine functioning per se, but possibly through root structural and functional traits.
AMF was classified into four groups at family level: Glomeraceae, Gigasporaceae, Claroideoglomeraceae and Acaulosporaceae. We categorized AMF into family groups by searching Index Fungorum (http://www.indexfungorum.org/names/Names.asp). In order to compare the effects of AMF family identity on mycorrhizal response of plants with different root systems, we divided the biomass dataset into eight groups: Tap-Glomeraceae, Tap-Gigasporaceae, Tap-Claroideoglomeraceae, Tap-Acaulospora- ceae, Fibrous-Glomeraceae, Fibrous-Gigasporaceae, Fibrous-Claroideoglomeraceae, Fibrous-Acaulosporaceae.
Experimental context was categorized as: normal, abiotic stress and biotic stress. For the trials which did not report any stress treatment, we assumed that these trials were conducted under no stressful conditions and defined it as “normal”. “Abiotic stress” category included drought, salinity, heavy metal, low pH, high pH, low vs. high temperature, shade, N and P deficiency, anoxia and chemical pollution. Here, we did not consider the stressful extent. According to the original paper, as long as the trials were conducted under abiotic stress with control treatments, we included the data in this group. Low pH stress refers to soil pH < 5.5; alkaline stress refers to soil pH > 8.5 (Heijne et al. 1996; Mardukhi et al. 2011). Low temperature refers to <15 °C, while high temperature refers to >35 °C (Hasanuzzaman et al. 2014; Wu 2011; Zhang et al. 2013). Anoxia stress refers to the trials were conducted in submerged conditions. Although different abiotic stressors potentially exert distinct effects on mycorrhizal functioning, here, we only wonder to give a general pattern for plants with different root system type. Thus, all these abiotic stressors were generally assigned into one group. “Biotic stress” referred to nematode, pathogen and herbivore. In order to compare the mycorrhizal response of taproot and fibrous root plants among different types of stress, we divided our biomass dataset into six groups: Tap-no, Tap-abiotic, Tap-biotic, Fibrous-no, Fibrous-abiotic and Fibrous-biotic.
Meta-analysis
We selected Hedge’s d to calculate individual effect size for biomass, P and N response variables. Hedge’s d is an estimate of standardized mean differences that is not biased by small size (Hedges and Olkin 1985). From each pair of mean values (\( \overline{X} \)), the individual effect size of Hedge’s d was calculated as (Rosenberg et al. 2000):
Here, \( {\overline{X}}^E \) is mean of experimental study, \( {\overline{X}}^C \) is mean of control study. S is the pooled standard deviation, and J is a weighting factor based on sample size (N) per study. J is calculated as (Rosenberg et al. 2000):
The variance of Hedge’s d, V(d) was calculated as:
Meta-analyses were performed in MetaWin 2.0 (Rosenberg et al. 2000). We selected random effect model for all these analyses. However, in random effect model, each entry is treated as a distinct study (Rosenberg et al. 2000), this might cause some issues under specific conditions. Thus, in this study, we assumed that across trial differences are of the same magnitude as across study differences. Weighted mean effect size (d +) was calculated for each response variable. 95 % confidence interval (CI) was used to determine whether d + was significantly different from zero with 9999 iterations. If 95 % CIs did not overlap zero, they were considered significantly different from zero. Comparison between categories was examined by P between (P b) associated to Q between statistics (Q b).
In order to address whether the mycorrhizal response pattern of plants with different root system is context-dependent, we conducted two-way factorial meta-analysis with the method of Morris et al. (2007). We calculated an effect size (Hedge’s d) of each study for the growth response of plants with different root system type to AMF, stress (abiotic vs. biotic) and their interactions. Mean effect size (d +) was then computed with weighted mean from individual studies with random effect model, where the weights were the inverse of sampling variance. 95 % CIs, Q b and P b were also calculated to compare difference among groups of studies with a mixed-effect model.
Meta-analysis assumes that studies are independent of each other (Gurevitch and Hedges 1999). For our analysis, if we included multiple pairs of data from one paper, there might be pseudo-replication. In order to examine whether potential pseudo-replications would affect our conclusions, we randomly selected one study per paper to perform meta-analyses (Vilà et al. 2011). The reduced datasets showed similar mean effect size and 95 % CIs to the whole datasets. Thus, we are confident for our analysis with these whole datasets.
Generally, positive or negative data in ecology studies was easier to be published than neutral data. However, one important assumption for meta-analysis is including data without publication biases (Rosenberg et al. 2000). Thus, we examined whether our datasets were publication-biased (Peters et al. 2006). First, we performed Spearman correlation analysis between the standardized effect size of raw data and sample size. If no correlation was found, we would consider the dataset with no publication bias; or else, we need to estimate fail-safe number to confirm whether publication bias will influence the final conclusions. Fail-safe number is the number of studies that would have to be added to change the results of meta-analysis from significant to non-significant (Rosenberg et al. 2000). Here, we used Rosenthal’s fail-safe calculation of 5 N + 10, where N is the original number of studies.
AMF richness effects
In order to investigate whether AMF species richness differs between taproot plants and plants with fibrous root system, another meta-analysis was performed with literature about AMF molecular diversity in plant roots. According to Veresoglou et al. (2014), sequencing depth is an important factor for species delineation in microbial community. For example, Sanger-sequencing studies generally reported considerably lower AMF species richness than 454-prysequencing studies. Thus, in this study, we only selected Sanger-sequencing studies for our meta-analysis to avoid the bias from different sequencing depth.
In total, we collected 165 host plant species from 90 publications, including 102 plants in which AMF communities were targeted with SSU gene, 29 with ITS gene and 34 with LSU gene. Ohsowski et al. (2014) found that AMF communities in natural conditions largely consist of uncultured species, thus, we can not assign each sequence to an AMF species identity at present. Generally, researchers preferentially assigned AMF DNA sequences into operational taxonomic unit (OTU) under the same taxonomy resolving level (i.e., 97 % similarity).
In order to better delineate the AMF OTUs, we extracted SSU, ITS or LSU rDNA sequences from GenBank with accession numbers published in these papers. For taxonomy of SSU sequences, we used a fasta-format file to blast MaarjAM database (Öpik et al. 2010). SSU sequences were assigned to virtual taxa (VT) with phylogentics and 97 % similarity. For ITS and LSU, both sequence datasets were aligned in Clustal X, respectively (Larkin et al. 2007). Both alignments were submitted into Geneious version 4.8.3, manually edited and saved as fasta-format. Because AMF ITS and LSU sequences have high variation (Yang et al. 2012), these fasta-formated alignments was then clusterd into operational taxonomic unit (OTU) based on 90 and 95 % similarity, respectively. Here, we refered OTU of ITS as ITS-OTU0.10, and OTU of LSU as LSU-OTU0.05. The suffex cutoff value was used to define respective OTU. This was done in Mothur version 1.19.0 (Schloss et al. 2009). AMF richness was calculated as AMF taxa in root of each host. Host associating with above 3 AMF taxa was included in our analysis. For each dataset, each host plant was used as an independent sample. Student t-test was used to compare AMF richness between tap and fibrous rooted plants.
To investigate how AMF speciess richness affects the mycorrhizal responsiveness of fibrous and tap-rooted plants, we defined three AMF richness groups: low, median and high. Low AMF richness referred to single AMF species inoculum; median AMF richness referred to inoculum with 2–4 fungal speciess; high AMF species referred to inoculum with >5 fungal species. In fact, it would have been better if we had treated AMF species richness as continuous variable. However, at some specific AMF species richness, there are limited samples so that the effects of insufficient sampling will exceed the effects of root system type. Thus, we categorized our data into these goups in order to ensure sufficient sampling for each group when conducting statistic analysis. The whole dataset was then classified into six groups: low-fibrous, low-tap, median-fibrous, median-tap, high-fibrous, high-tap. Meta-analysis was performed with the random effect model in MetaWin 2.0 to compare the differences among these groups.
Results
Publication bias test
Publication bias was examined for each dataset of different parameters. Spearman correlation analysis showed significant correlation between standardized effect size and sample size for biomass, P and N content datasets (Spearman r = −0.04, P = 0.02 for biomass, r = 0.11, P < 0.00 for P content and r = 0.22, P < 0.00 for N content). Statistics suggested that there was slight publication bias for these datasets.
Fail-safe number was much larger than 5 N + 10 for these datasets: fail-safe number were 16744849.3, 1451191.6, 154992.9 and 95931.1 for biomass, P and N content while the respective 5 N + 10 values were 18260, 3720 and 1435. It indicated that although there was slight publication bias for these datasets, it would not change the overall meaning of the results.
Plant growth responses to AMF
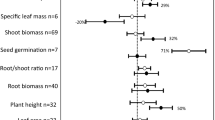
Overall, AMF significantly enhanced plant biomass, N and P uptake at no stress, abiotic stress and biotic stress (Fig. 1a). However, mycorrhizal effects differed between taproot plants and plants with fibrous root systems under different types of stresses. Taproot plants showed higher biomass response to AMF than plants with fibrous root system at no and abiotic stress (Q b = 36.15, P < 0.00 at no stress; Q b = 20.48, P b < 0.00 at abiotic stress), but no difference was found between the two types of plants at biotic stress (Q b = 2.68, P b = 0.10) (Fig. 1a; Table 1). P content was higher in taproot plants than that in plants with fibrous roots under no stress and abiotic stress (Q b = 21.55, P b < 0.00 at no stress; Q b = 12.63, P b < 0.00 under abiotic stress) (Fig. 1b; Table 1). N content was higher in taproot plants than that in plants with fibrous roots under abiotic stress but not significantly different under no stress (Q b = 13.27, P b < 0.00; Q b = 4.40, P b = 0.08) (Fig. 1c; Table 1).
Effect size of plant growth response to AMF: (a) Biomass at no stress, abiotic stress and biotic stress; (b) P content at no and abiotic stress; (c) N content at no and abiotic stress; (d) biomass at nematode and fungal pathogen attacks. Error bars represents ± 95 % CI. The number in the parenthesis represents (# study; # trial)
Two-way factorial meta-analysis showed that mycorrhizal growth response was context-dependent for plants with tap- and fibrous-rooted systems (Fig. 2). Taproot plants had greater biomass response to AMF than plant with fibrous root systems (Fig. 2). However, the two types of plants showed distinctive patterns when responding to abiotic and biotic stress. Abiotic stress showed negative and significant effects on plant biomass, but were not different between the two types of plants (Q b = 0.31, P b = 0.58; Table 1). Biotic stress only had significantly negative effect on taproot plants, and significant difference was found between the two types of plants (Q b = 16.86, P b < 0.00; Table 1). In addition, the interaction effects between AMF and stress showed distinct patterns between taproot and fibrous root plants. AMF interacting with abiotic stress showed small, negative and significant effects on taproot plants, but not significant effects on plants with fibrous root systems. Meanwhile, the interaction effect was not different between the two types of plants (Q b = 0.33, P b = 0.56; Table 1). However, the effect of AMF interacting with biotic stress was significantly negative on taproot plants but positive on plants with fibrous root systems (Q b = 7.17, P b < 0.00; Table 1).
Mycorrhizal effects on plants with different types of root system were dependent on fungal taxonomic family identity (Fig. 3; Table 1). When inoculated with Glomeraceae and Gigasporaceae, taproot plants showed higher biomass than plants with fibrous roots (d ± 95 % CI: 1.19 ± 0.03 vs. 0.96 ± 0.05 for Glomeraceae; 0.66 ± 0.07 vs.0.43 ± 0.14 for Gigasporaceae). The biomass of the two types of plants was not different when inoculated with Acaulosporaceae and Claroideoglomeraceae (d ± 95 % CI: 0.63 ± 0.13 vs. 0.7 7 ± 0.15 for Acaulosporaceae; 0.69 ± 0.07 vs. 0.70 ± 0.11 for Claroideoglomeraceae) (Fig. 3a). For P content, significant differences were found between plants with tap and fibrous roots when inoculated with Glomeraceae and Claroideoglomeraceae (d ± 95 % CI: 2.32 ± 0.085 vs.1.42 ± 0.13 for Glomeraceae; 1.54 ± 0.24 vs. 0.97 ± 0.27 for Claroideoglomeraceae). No difference was found for Acaulosporaceae and Gigasporaceae (d ± 95 % CI: 1.33 ± 0.38 vs. 1.39 ± 1.83 for Acaulosporaceae; 2.55 ± 0.25 vs. 2.34 ± 1.03 for Gigasporaceae) (Fig. 3b).
AMF species richness effects
Mycorrhizal responsiveness of taproot plants and plants with fibrous root system varied with AMF species richness (Fig. 4a). At low and median AMF species richness, taproot plants had higher mycorrhizal growth response than plants with fibrous root system. However, no difference was found between the two types of plants at high AMF species richness. In addition, median AMF species richness had the lowest growth promotion while high AMF richness exhibited the highest growth promotion for the two types of plants.
AMF richness effects: (a) Effect size of tap- and fibrous-rooted plants to low, median and high AMF richness. Error bars represents ±95 % CI. (b) Comparison of AMF richness between plants with tap and fibrous root systems. Error bars represents mean ± SE. (c) Difference of root length colonization (RLC, %) between plants with tap roots and fibrous root systems. Error bars represents mean ± SE. The number in the parenthesis represents (# study; # trial)
Student t-test revealed that AMF richness was not different between taproot plants and plants with fibrous root system (Fig. 4b). SSU-VT richness for the two types of plants was 10.35 ± 0.87 and 10.67 ± 1.38. ITS-OTU0.10 richness was 8.41 ± 1.71 and 9.5 ± 1.63, respectively. LSU-OTU0.05 richness was 18.50 ± 3.30 and 16.50 ± 2.93, respectively. Libshuff analysis showed that AMF composition was not different between tap- and fibrous- rooted plants (dCXYScore = 0.02, P = 0.44 for SSU-VT; dCXYScore = 0.02, P = 0.35 for ITS-OTU0.01; dCXYScore = 0.01, P = 0.92 for LSU-OTU0.05). However, AMF internal abundance is significantly higher in taproot plants than plant with fibrous root system (tap: 44.22 ± 0.65; fibrous: 41.21 ± 1.06; P = 0.02).
Discussion
Previous case studies suggested that root architecture was one determinant for mycorrhizal functioning (Hetrick et al. 1992; Sikes and CottenieK 2009). Our extensive meta-analysis provided evidence that AMF effects on plant growth possibly depends on root system type. Taproot plants benefited more from AMF than plants with fibrous root (about 1.43, 1.31 and 1.27 times for biomass data at no stress, abiotic stress and biotic stress, respectively) (Fig. 1a-c). This response pattern might be caused by difference in root surface absorptive area. Generally, plants with fibrous root system have more highly branched fine roots and active root hairs than taproot plants. This indicates that plants with fibrous root system might possess larger absorptive surface area. For example, one tuft of ryegrass can be associated with about 15000 adventitious roots, and their total surface area nearly equals to the size of one standard volleyball playground (Ye 2007). Some plants developed such extensive root systems as a strategy for direct nutrient acquisition, and thus were possibly less mycorrhizal dependent (Hetrick et al. 1988). However, taproot plants developed coarse and less branched root systems (Sullivan et al. 2000). Coarse root system has a limited intrinsic ability to absorb nutrients (Bates and Lynch 2001). Thus, direct nutrient uptake from coarse roots might not meet the demands of plant growth. AMF possibly perform complementary functioning of roots to explore more soil volume for nutrients (Koide 2000). In brief, AMF and fine roots might be alternative strategies of plants for acquisition of soil nutrients (Wilson and Hartnett 1998). Taproot plants and plant with fibrous root system may thus have different absorptive strategies for nutrients from soils.
However, our study also found that it was context-dependent for mycorrhizal responses of plants with different types of root systems (Fig. 2). For AMF functioning under abiotic stress, there was small but negative effect on growth of plants with tap roots but no significant effect on plants with fibrous root system (Fig. 2a). For AMF functioning under biotic stress, negative response occurred for taproot plants but positive effect was shown for the growth of plants with fibrous root systems (Fig. 2b). These stress dependent effects suggested that AMF might magnify the detrimental effects of abiotic and biotic stress on taproot plants but enhance tolerance of plants with fibrous root system towards biotic stress. As stated previously, taproot plants showed more dependence on mycorrhizae for nutrient acquisition (Hetrick et al. 1988; Fig. 1). When taproot plants encountered stress, growth of plants themselves would be limited or inhibited; meanwhile, AMF growth would also be negatively affected (Wu et al. 2007) and thus the mycorrhizal functioning would be inhibited. However, even under such stressful environments, the obligate nature of mycorrhizal symbiosis suggested that taproot plants would have to provide carbon to the fungal symbionts. Therefore, stress not only inhibits plant growth, but also limits carbon consumption and mycorrhizal functioning, generating negative effects on growth performance of plants with taproot systems. However, nutrient acquisition is not the main functioning for mycorrhizal symbiosis of plants with fibrous root systems, as other roles may be more important, such as biotic stress tolerance and resistance (Sikes and CottenieK 2009). Abiotic stress might cause death of finer roots and root hairs of plants with fibrous root systems, thus, these plants may preferentially allocate carbon to root hair recruitment but not the fungal symbionts (Wilson and Hartnett 1998). Under these circumstances, plants might shut off carbon flow to AMF due to abiotic stress. However, studies suggested that AMF were effective biocontrol agents, especially for plants with fibrous root systems (Azcón-Aguilar and Barea 1997; Sikes and CottenieK 2009). Thus, AMF might ameliorate biotic stress.
AMF family identity may affect mycorrhizal functioning mediated by root architecture (Sikes and CottenieK 2009). In this study, we found that mycorrhizal growth response of plants with different root systems depended on AMF family identity (Fig. 3). It holds that taproot plants showed higher growth response to Glomeraceae and Gigasporaceae. However, no difference was found between the two types of plants for Claroideoglomeraceae and Acaulosporaceae. Our results showed that Claroideoglomeraceae promoted higher P uptake for taproot plants, thus the no-difference pattern in plant growth for Claroideoglomeraceae might be caused by other symbiotic functioning for plants with fibrous root system, i.e., biocontrol, or more likely the lack of ability to transport N which often limits plant growth. For example, Sikes and CottenieK (2009) found that Glomus etunicatum (Claroideoglomeraceae) was efficiently inhibited root disease caused by fungal pathogen Fusarium oxysporum for Setaria glauca with fibrous root system. No difference between the two types of plants for Acaulosporaceae might be determined by life history strategies of this group of fungi. Maherali and Klironomos (2007) reported that Acaulosporaceae had low root colonization and few external hyphae, thus was less competitive. Under natural conditions, Acaulosporaceae might make smaller contribution to growth of plants with both types of roots compared to other AMF families.
Studies suggested that AMF species richness had great effects on plant growth (Maherali and Klironomos 2007; van der Heijden et al. 1998). Our results showed that higher growth response to AMF for plants with taproot plants was still held at low and median AMF species richness. However, no difference was found for the two types of plants at high AMF species richness. The latter pattern could be cautiously explained by two possibilities: one is insufficient sampling. As shown in Fig. 4, at high AMF species richness, plants with fibrous root systems only had 7 samples, but taproot plants had 51 samples. This discrepancy in sampling depth possibly led to wider 95 % CI of plant with fibrous root systems, thus causing no statistical difference between the two types of plants. Another possibility is that higher AMF species richness possibly shows high functional diversity (Cavagnaro et al. 2005). Although plants with fibrous root systems showed less mycorrhizal dependence for nutrients, this type of plants possibly obtained other ecological benefits from mycorrhizal symbiosis, i.e., pathogen protection (Sikes and CottenieK 2009; Wilson and Hartnett 1998). Thus, high AMF species richness might provide greater ecological functioning, further increase growth performance of plants with fibrous root system and thus eliminate the difference between the two types of plants.
Conclusions
AMF effects on plant growth depended on the types of root system. Taproot plants had higher mycorrhizal responsiveness than plants with fibrous roots. This pattern was affected by stress types, AMF identity and richness. Especially, interaction effects were observed between AMF and stress (abiotic vs. biotic). These results suggest that in future climate change scenarios, environmental disturbance might alter the relative abundance of taproot plants and plant with fibrous root system, and thus shift plant community composition.
References
Atkinson D, Berta G, Hooker JE (1994) Impact of mycorrhizal colonisation on root architecture, root longevity and the formation of growth regulators. Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems. Springer, pp. 89–99.
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and rhizobium-meliloti strains, and their effects on growth, N2-fixation (N15) and nutrition of Medicago sativa L. New Phytol 117:399–404
Azcón-Aguilar C, Barea JM (1997) Arbuscular mycorrhizas and biological control of soil-born plant pathogens- An overview of the mechanisms involved. Mycorrhiza 6:457–464
Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98:745–753
Bates T, Lynch J (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236:243–250
Baylis GTS (1970) Root hairs and phycomycetous mycorrhizas in phosphorus- deficient soil. Plant Soil 33:713–716
Berta G, Fusconi A, Trotta A, Scannerini S (1990) Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol 114:207–215
Berta G, Fusconi A, Trotta A (1993) VA mycorrhizal infection and the morphology and function of root systems. Environ Exp Bot 33:159–173
Berta G, Trotta A, Fusconi A, Hooker JE, Munro M, Atkinson D, Gianinazzi S (1995) Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol 15:281–293
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068
Cavagnaro TR, Smith FA, Smith SE, Jakobsen I (2005) Functional diversity in arbuscular mycorrhizas, exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ 28:642–650
Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2009) Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol 181:938–949
Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analyses. Ecology 80:1142–1149
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2014) Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium- supplemented brassica napus seedlings confers tolerance to high temperature stress. Biol Trace Elem Res 161:297–307
Hedges LV, Olkin I (1985) Statistical methods for meta-Analysis. Accedemic press, New York
Heijne B, Vandam D, Heil GW, Bobbink R (1996) Acidification effects on vesicular-arbuscular mycorrhizal (VAM) infection, growth and nutrient uptake of established heathland herb species. Plant Soil 179:197–206
Hetrick BAD, Kitt DG, Wilson GT (1988) Mycorrhizal dependence and growth habit of warm-season and cool-season tallgrass prairie plants. Can J Bot 66:1376–1380
Hetrick B, Wilson GWT, Todd TC (1992) Relationships of mycorrhizal symbiosis, rooting strategy, and phenology among tallgrass prairie forbs. Can J Bot 70:1521–1528
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Koide RT (2000) Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol 147:233–235
Koide R, Dickie I (2002) Effects of mycorrhizal fungi on plant populations. Plant Soil 244:307–317
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172:536–543
Maherali H (2014) Is there an association between root architecture and mycorrhizal growth response? New Phytol 204:192–200
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Mardukhi B, Rejali F, Daei G, Ardakani MR, Malakouti MJ, Miransari M (2011) Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. C R Biol 334:564–571
Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Maron JL, Mitchell CE, Parker IM, Power AG, Torchin ME, Vázquez DP (2007) Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology 88:1021–1029
Ohsowski BM, Zaitsoff PD, Öpik M, Hart MM (2014) Where the wild things are: looking for uncultured Glomeromycota. New Phytol 204:171–179
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison MJ, Kalwij JM, Reier Ü, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–680
Rosenberg MS, Adams DC, Gurevitch J (2000) MetaWin: statistical software for meta-analysis, version 2.0. Sinauer Associated, Sunderland
Schellenbaum L, Berta G, Ravolanirina F, Tisserant B, Gianinazzi S, Fitter AH (1991) Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann Bot-Lond 68:135–141
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Weber CF (2009) Introducing mothur: open-source, platform-independent, community- supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schweiger P, Robson AD, Barrow NJ (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol 131:247–254
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Sikes BA, CottenieK KJN (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic press Inc, San Diego
Sullivan WM, Jiang ZC, Hull RJ (2000) Root morphology and its relationship with nitrate uptake in Kentucky bluegrass. Crop Sci 40:765–772
Torrey JG, Clarkson DT (1975) The development and function of roots. Academic, New York, p 618
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sander IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578
van Groenigen KJ, Osenberg CW, Hungate BA (2011) Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216
Veresoglou SD, Menexes G, Rillig MC (2012) Do arbuscular mycorrhizal fungi affect the allometric partition of host plant biomass to shoots and roots? A meta-analysis of studies from 1990 to 2010. Mycorrhiza 22:227–235
Veresoglou SD, Powell JR, Davison J, Lekberg Y, Rillig MC (2014) The Leinster and Cobbold indices improve inferences about microbial diversity. Fungal Ecol 11:1–7
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Wagg C, Jansa J, Stadler M, Schmid B, van der Heijden MGA (2011) Mycorrhizal fungal identity and diversity relaxes plant-plant competition. Ecology 92:1303–1313
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Wu QS (2011) Mycorrhizal efficacy of trifoliate orange seedlings on alleviating temperature stress. Plant Soil Environ 57:459–464
Wu QS, Zou YN, Xia RX, Wang MY (2007) Five Glomus species affect water relations of Citrus tangerine during drought stress. Bot Stud 48:147–154
Yang H, Zang Y, Yuan Y, Tang J, Chen X (2012) Selectivity by host plants affects the distribution of arbuscular mycorrhizal fungi: evidence from ITS rDNA sequence metadata. BMC Evol Biol 12:50
Ye CX (2007) Botany. Higher Education Press, Beijing, p 89
Zhang GL, Zhang ST, Xiao LT, Wu XJ, Xiao YH, Chen LY (2013) Effects of high temperature stress on physiological characteristics of anther and pollen traits of rice at flowering stage. Acta Agron Sin 39:177–183
Acknowledgments
This study was funded by the Natural Science Foundation of China (No. 31400373), Natural Science Foundation of Jiangsu Province (No. BK20140689) and China Postdoctoral Science Foundation (No. 2014 M561659).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Erik J. Joner.
Rights and permissions
About this article
Cite this article
Yang, H., Zhang, Q., Dai, Y. et al. Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: a meta-analysis. Plant Soil 389, 361–374 (2015). https://doi.org/10.1007/s11104-014-2370-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2370-8