Abstract
Plant Zn uptake from low Zn soils can be increased by Zn-mobilizing chemical rhizosphere processes. We studied whether inoculation with arbuscular mycorrhizal fungi (AMF) can be an additional or an alternative strategy. We determined the effect of AMF inoculation on growth performance and Zn uptake by rice genotypes varying in Zn uptake when nonmycorrhizal. A pot experiment was conducted with six aerobic rice genotypes inoculated with Glomus mosseae or G. etunicatum or without AMF on a low Zn soil. Plant growth, Zn uptake and mycorrhizal responsiveness were determined. AMF-inoculated plants produced more biomass and took up more Zn than nonmycorrhizal controls. Mycorrhizal inoculation, however, significantly increased Zn uptake only in genotypes that had a low Zn uptake in the nonmycorrhizal condition. We conclude that genotypes that are less efficient in Zn uptake when nonmycorrhizal are more responsive to AMF inoculation. We provide examples from literature allowing generalization of this conclusion on a trade off between mycorrhizal responsiveness and nutrient uptake efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low zinc (Zn) availability is a constraint to plant production on about one-third of agricultural soils worldwide. In addition, Zn deficiency affects over two billion people (Welch and Graham 2002), mostly in developing countries where diets are cereal-based and low in meat, fish and vegetables. Increase of Zn levels in cereal grains through increase of Zn uptake is therefore needed.
Total Zn in soils with low bioavailable Zn is generally high enough to support high yields for many years. Consequently, genotypes with a high Zn-mobilizing capacity can perform relatively well (Rengel and Marschner 2005). Thus, plant Zn uptake from low Zn soils can, apart from Zn fertilization (Rengel et al. 1999), be enhanced by engineering Zn efficiency in plants (Ramesh et al. 2004), selecting genotypes that inherently can take up Zn efficiently (Hacisalihoglu and Kochian 2003) or by inoculation with arbuscular mycorrhizal fungi (AMF). In a previous paper (Gao et al. 2005) we showed large variation in Zn uptake among nonmycorrhizal aerobic rice genotypes. In the current study we investigate whether inoculation of these genotypes with AMF could further increase Zn uptake. We thereby address the question whether efficient chemical nutrient-mobilizing rhizosphere processes can be combined with high mycorrhizal responsiveness in one genotype.
A beneficial effect of mycorrhizal colonization on Zn uptake has been shown for maize (Faber et al. 1990; Liu et al. 2000; Sharma et al. 1992), pigneonpea (Wellings et al. 1991), wheat (Khare et al. 1998; Ryan and Angus 2003) and wetland rice (Purakayastha and Chhonkar 2001). Upon colonization, the mycelium of the AMF increases the nutrient absorbing surface area of the symbiosis, enhances exploration of a larger soil volume and thereby increases uptake, specifically of immobile nutrients such as P and Zn (Smith and Read 1997).
Variation in responsiveness to inoculation with AMF among plant genotypes has been often documented but the genetic basis for this variation remains poorly understood. Studies in wheat (Hetrick et al. 1995) and maize (Kaeppler et al. 2000) indicated that there is a genetic basis for dependency on or responsiveness to AMF. Hetrick et al. (1992) suggested that modern breeding practices have produced cultivars that are highly dependent on fertilizers and show a reduced dependency on and responsiveness to the mycorrhizal symbiosis. It was then implied that there is considerable potential for redesigning crops that show an enhanced dependency on and responsiveness to AMF (Ryan and Graham 2002). Zhu et al. (2001) also highlighted the importance of including mycorrhizal responsiveness in breeding programs for maximizing nutrient uptake efficiency. While this suggestion that modern breeding practices have limited the mycorrhizal contribution to nutrient uptake is intuitively plausible, it has been challenged, however, by Kaeppler et al. (2000) who alternatively proposed that selection for environmental stability may automatically be manifested in a decreased mycorrhizal response.
Aerobic rice varieties are currently developed for the new water-saving aerobic cultivation system (Bouman et al. 2005) by crossing high-yielding lowland with traditional upland varieties. So far, breeding has mainly been focused on yield and drought resistance of aerobic genotypes. Previously we have shown that there is considerable variation in Zn uptake from low Zn soils among newly bred genotypes (Gao et al. 2005). We are unaware of previous reports on genotypic variation in mycorrhizal responsiveness based on Zn uptake in any plant species. Here we test if there is variation in mycorrhizal responsiveness based on growth and Zn uptake among aerobic rice genotypes, and how this variation is related to Zn uptake in the nonmycorrhizal condition.
We test the following hypotheses: (i) AMF-colonized rice plants are more efficient in mobilizing Zn from a low Zn soil than nonmycorrhizal plants; (ii) significant genotypic variation in mycorrhizal responsiveness exists among rice genotypes; (iii) high mycorrhizal responsiveness based on Zn uptake does not combine with inherent plant factors involved in efficient Zn mobilization in the rhizosphere. We discuss previous papers on other plant species and nutrients to evaluate if our conclusion on this third hypothesis can be generalized.
Materials and methods
Experimental design and conditions
A pot experiment was conducted in a greenhouse of China Agricultural University, Beijing. Treatments included six rice genotypes and three mycorrhizal treatments in a factorial design with three replicates.
Six aerobic rice genotypes were selected because of their previously shown variation in Zn uptake and Zn efficiency (Gao et al. 2005). Han 72, Han 44, Han 297 and 91B-8-30-3 are newly bred varieties in China Agricultural University. K 150 is a variety bred by Liaoning Academy of Agricultural Science, North China. Hongkelaoshuya is a traditional upland variety from Yunnan Province, South China. The genotypes had similar root surface area and similar root length at low soil Zn levels. Zn uptake, however, calculated as the difference between plant Zn content after 28 days of growth and seed Zn content, ranged from 5.2 μg pot−1 to 30.4 μg pot−1 (Gao et al. 2005).
Mycorrhizal treatments included a nonmycorrhizal control (−AMF) and inoculation with the mycorrhizal fungus Glomus mosseae (BEG167) or Glomus etunicatum (BEG168). Both were originally isolated from a high pH soil (\( {\hbox{pH}}_{{\rm{H}}_{\rm{2}} {\rm{O}}} \,8.2 \)) in North China. The inoculum consisted of colonized root segments and attached rhizosphere soil from maize grown under P and Zn deficient conditions in a glasshouse for 2 months.
A clay soil was collected from Shou city, Anhui province, China. Major characteristics: \( {\hbox{pH}}_{{\rm{H}}_{\rm{2}} {\rm{O}}} \,6.5 \), organic matter 1.7%, DTPA-extractable Zn 0.3 mg kg−1, and P-Olsen 18.5 mg kg−1. The Zn status is well below the critical level (0.8 mg kg−1) and the P status is high (>10 mg kg−1; Dobermann and Fairhurst 2000). In a previous experiment, this soil induced Zn deficiency symptoms in most of the genotypes used (Gao et al. 2005). Growth of all genotypes responded to Zn application. The soil was sterilized by autoclaving at 120°C for 2 h, and air-dried. DTPA-extractable Zn was only slightly increased due to autoclaving but was on average still 0.3 mg kg−1.
Eight seeds of one genotype were sown per pot containing 2 kg soil. At sowing time, each pot received a basal application of 150 mg kg−1 N as Ca(NO3)2 and 83 mg kg−1 K as KCl. In the + AMF treatments, inoculum (200 g) was mixed uniformly with the soil. In the −AMF treatment, an equivalent amount of sterilized inoculum together with the filtrate (<0.25 μm) of unsterilized soil was added to provide a similar microflora apart from the mycorrhizal fungus. The plants were thinned to four seedlings per pot one week after emergence. The pots were watered daily with deionized water, maintaining water content at 15% (w/w). The temperature in the greenhouse was 30 ± 3°C during the day and 23 ± 3°C during the night. Plants were grown under natural day length and light intensity in July 2005.
Harvest and analyses
Plants were harvested 2 months after germination, at tillering stage. Shoots were cut off at ground level and soil was washed from the roots with tap water. Shoots and roots were rinsed in deionized water. Roots were cut into 1 cm segments and mixed thoroughly. A subsample of 0.2 g fresh weight per pot was taken to determine mycorrhizal root colonization as described by Phillips and Haymann (1970). Briefly, 1 cm root samples were cleared in 10% KOH, acidified in 2% HCl and stained with 0.05% trypan blue in lactophenol. Thirty 1 cm root segments were randomly selected and mounted parallel to each other on a slide. Each root was observed under a microscope (200–400×) and rated according to the range of classes indicated by Trouvelot et al. (1986). The computer program MYCOCALC (www.dijon.inra.fr/mychintec/Mycocalc-prg/download.html) was used to calculate the percentage of root length colonized by mycorrhizal fungi.
The shoots and remaining roots were oven-dried at 70°C for 48 h, and weighed. Dried and ground plant samples were digested in acid mixture (HNO3 + HClO4) for Zn analysis (Jackson 1973). Zinc in plant digests was analyzed with an atomic absorption spectrophotometer (Pye Unicam SP 9 800, Cambridge, UK). Zinc analyses were checked using the certified Zn values in standard samples obtained from Wageningen Evaluating Programmes for Analytical Laboratories (WEPAL, Wageningen University, the Netherlands). Zn uptake was calculated as the difference between plant Zn content and seed Zn content.
Mycorrhizal responsiveness (MR) was calculated as:
Mycorrhizal Zn responsiveness (MZnR) was calculated similarly:
Statistical analysis
All data were tested and met the requirements on normality (Kolmogorov–Smirnov) and homogeneity of variance (Levene’s test). Analysis of variance was done on data on shoot and root dry weight, shoot Zn mass fractions and Zn uptake. Means were compared with Tukey’s Honestly Significant Differences test at the 5% level of probability. All analyses were performed with SAS Release 8.02 (SAS Inc.).
Results
Plant growth and mycorrhizal responsiveness
Inoculation with either G. mosseae or G. etunicatum significantly increased shoot dry weight of aerobic rice (Tables 1, 2). The average mycorrhizal responsiveness based on plant dry weight of six rice genotypes was 48% for G. mosseae and 27% for G. etunicatum (Table 2). G. mosseae-colonized plants had significantly higher root dry weights than G. etunicatum-colonized and nonmycorrhizal plants.
There was an interaction (P = 0.002) between genotype and AMF treatment (Table 1). Genotypes Han 72 and 91B-8-30-3 did not increase shoot dry weight upon inoculation with either of the two AMF species (Table 2). Genotypes Han 44 and Hongkelaoshuya responded differently depending on the AMF species. Shoot dry weight of genotypes K 150 and Han 297 increased upon inoculation with either of the two AMF species.
Obvious symptoms of Zn deficiency (chlorotic leaves with brown necrotic spots on leaves) showed at the end of the experiment. There was no difference in the severity of symptoms among AMF treatments. The genotypes Han 44, 91B-8-30-3 and Hongkelaoshuya showed less symptoms than the other three genotypes.
Zn uptake and mycorrhizal Zn responsiveness
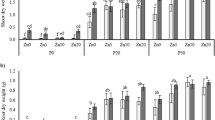
Shoot Zn mass fraction of all plants ranged between 13.2 mg kg−1 and 22.7 mg kg−1 (Fig. 1). Neither G. mosseae nor G. etunicatum affected shoot Zn mass fraction (Table 1; Fig. 1). Zn uptake was significantly increased by inoculation with AMF (Tables 1, 3). For G. mosseae and G. etunicatum mycorrhizal Zn responsiveness was on average 54% and 23%, respectively.
There was genotypic variation (P < 0.0001) in Zn uptake among the six rice genotypes (Tables 1, 3). Also, the interaction between genotype and AMF treatment was significant for Zn uptake. Inoculation with G. mosseae significantly increased Zn uptake of genotypes K 150, Han 72 and Han 297 with 117%, 88% and 112%, respectively. For the other three genotypes, no significant effect of inoculation on Zn uptake was found (Table 3). Inoculation with G. etunicatum significantly increased Zn uptake of genotype K 150. Mycorrhizal Zn responsiveness varied between 19% and 117% for G. mosseae and between −7% and 170% for G. etunicatum (Table 3). On this Zn deficient soil, the genotypes with low Zn uptake when nonmycorrhizal (K 150, Han 72 and Han 297) were more responsive with respect to Zn uptake than the three genotypes with higher Zn uptake when nonmycorrhizal (Han 44, 91B-8-30-3 and Hongkelaoshuya). Plant Zn uptake by nonmycorrhizal plants was negatively correlated with MZnR (P = 0.006) and MR (P = 0.04) for both mycorrhizal fungi (Fig. 2).
Mycorrhizal root colonization
No root colonization by AMF was observed in the uninoculated plants. Variation in root colonization was found (P < 0.0001) among the six rice genotypes. There was no interaction between genotype and AMF treatment (P = 0.1; Table 4). Root colonization of AMF-inoculated plants ranged from 28% to 58% (Table 5). On average, root colonization by G. mosseae was slightly but significantly higher than by G. etunicatum. Neither MR (P = 0.9) nor MZnR (P = 0.6) correlated with root colonization.
Discussion
We demonstrated a beneficial effect of AMF inoculation on Zn uptake by aerobic rice (Table 3). The effect in our study was equal to (G. mosseae) or smaller (G. etunicatum) than reported for lowland rice (Purakayastha and Chhonkar 2001), confirming the potential of AMF to increase Zn uptake from low Zn soils. Also in a heavy metal-contaminated soil, two upland rice varieties inoculated with G. mosseae took up more Zn than uninoculated plants (Zhang et al. 2005). This suggests that the mycorrhizal effect on Zn uptake is independent on the Zn status of the soil. All studies done so far were pot studies, in which rooting density is high compared to a field condition. Verification under field conditions is necessary.
A beneficial effect of AMF inoculation on biomass is frequently attributed to increased P uptake. In our experiment, however, it is highly unlikely that increased P uptake explains increased biomass production and thus increased Zn uptake. Firstly, the P status of the soil was high and the Zn status was low (Dobermann and Fairhurst 2000). So Zn, and not P, was the growth-limiting nutrient, which is confirmed by the appearance of Zn deficiency symptoms. P deficiency symptoms were not recorded. Secondly, Zn uptake (Table 3) and biomass production (Table 2) were significantly correlated (P < 0.05 for both G. mossae and G. etunicatum). Thirdly, Zn mass fractions for all samples but three (Fig. 1) were below the level required for sufficient growth (20 mg kg−1; Dobermann and Fairhurst 2000) and similar to those found in a previous field experiment where a significant response to Zn application was shown (Gao et al. 2006). Earlier studies (Baon et al. 1993; Zhu et al. 2001) showed a negative correlation between mycorrhizal response and P utilization efficiency (PUE—the inverse of P mass fraction). An increase in P mass fraction (and hence a decrease in PUE) upon mycorrhizal inoculation is likely to be explained by luxury uptake of P and differential nutrient limitation by mycorrhizal and nonmycorrhizal plants. In the present study, Zn mass fractions were low but hardly differed between nonmycorrhizal plants and mycorrhizal plants (Table 1), indicating that plants under both treatments were Zn-limited. Therefore, no other nutrients but Zn limited the growth of both nonmycorrhizal and mycorrhizal plants, indicating that the growth response of rice plants was completely explained by the mycorrhizal contribution to Zn uptake.
There was large genotypic variation in MZnR (Table 3), which was not related to variation in root colonization. Zinc uptake under nonmycorrhizal conditions was significantly negatively related to MR and MZnR for both mycorrhizal fungi (Fig. 2). This correlation indicates that genotypes with high responsiveness to AMF colonization are inherently less efficient to take up Zn from low Zn soils. This confirms our hypothesis that mycorrhizal Zn responsiveness correlates negatively with Zn uptake in the nonmycorrhizal condition.
A negative correlation between mycorrhizal responsiveness and nutrient uptake in the nonmycorrhizal condition has been reported repeatedly. Koide et al. (1988) showed that cultivated oats had a lower P uptake and were more responsive to mycorrhizal inoculation than wild oats. A tomato genotype with a lower P uptake when nonmycorrhizal was also more responsive to mycorrhizal colonization than a more efficient genotype (Bryla and Koide 1998). The authors concluded that plants that possess mechanisms for acquiring phosphorus efficiently, may be less dependent on (and responsive to) mycorrhizal colonization. For barley, Baon et al. (1993) observed that the cultivar with the lowest P uptake when nonmycorrhizal (Shannon) showed the largest mycorrhizal responsiveness. Wright et al. (2005), who compared an African landrace with a European high-yielding variety of maize, showed that the African landrace combined a higher nutrient uptake when nonmycorrhizal with a lower mycorrhizal response. And Kaeppler et al. (2000) showed that P uptake of maize inbred lines was negatively correlated with mycorrhizal responsiveness. These latter authors pointed out that variation in mycorrhizal responsiveness could be due to variation of plants to efficiently acquire nutrients and grow well under conditions of low nutrient availability. If there is substantial variation in that latter character (due to other mechanisms through which plants could perform well on nutrient-deficient soils), plants without these other mechanisms perform poorly in the nonmycorrhizal condition and hence will derive more benefit from mycorrhizal inoculation. Under that interpretation a low responsiveness to mycorrhizal inoculation is an almost unavoidable consequence of the possession of other, sometimes more important nutrient acquiring mechanisms of a plant. It would then be important to determine the relative contribution of plant and mycorrhizal fungus to nutrient uptake efficiency, to target those processes that make the larger contribution to nutrition (Smith et al. 1992). A dominant focus on mycorrhizal symbiosis and mycorrhizal responsiveness could then lead to selection of plants that are less able to cope with low soil nutrient levels. In the case of aerobic rice, the variation in rhizosphere mobilization potential outweighs a mycorrhizal contribution (Table 3). Even though AMF do contribute to Zn uptake, selecting and breeding plants on the basis of maximum mycorrhizal response will likely not lead to the most efficient cultivars.
Inoculation with AMF did not significantly increase Zn uptake beyond levels that were found in genotypes with an inherently high uptake (Table 3). This indicates that mechanisms other than mycorrhizal formation were more efficient in Zn mobilization. Root-induced chemical changes in rhizosphere could play a major role in Zn uptake. These rhizosphere effects may involve Fe oxidation and acidification of the rhizosphere (Kirk and Bajita 1995) and exudation of Zn chelators such as phytosiderophores (Tolay et al. 2001) or citrate (Hoffland et al. 2006). In this case, the mycorrhizal symbiosis is apparently just one of several nutrient-acquiring specializations (Pate 1994).
References
Baon JP, Smith SE, Alston AM (1993) Mycorrhizal responses of barley cultivars differing in P efficiency. Plant Soil 157:97–105
Bouman BAM, Peng S, Castaneda AR, Visperas RM (2005) Yield and water use of irrigated tropical aerobic rice systems. Agr Water Manage 74:87–105
Bryla DR, Koide RT (1998) Mycorrhizal response of two tomato genotypes relates to their ability to acquire and utilize phosphorus. Ann Bot 82:849–857
Dobermann A, Fairhurst T (2000) Rice: nutrient disorders and nutrient management. International Rice Research Institute, Manila, Philippines
Faber BA, Zasoski RJ, Burau RG, Uriu K (1990) Zinc uptake by corn as affected by vesicular-arbuscular mycorrhizae. Plant Soil 129:121–130
Gao XP, Zou CQ, Fan XY, Zhang FS, Hoffland E (2006) From flooded to aerobic conditions in rice cultivation: consequences for zinc uptake. Plant Soil 280:41–47
Gao XP, Zou CQ, Zhang FS, Van der Zee SEATM, Hoffland E (2005) Tolerance to zinc deficiency in rice correlates with zinc uptake and translocation. Plant Soil 278:253–261
Hacisalihoglu G, Kochian LV (2003) How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol 159:341–350
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Gill BS, Cox TS (1995) Chromosome location of mycorrhizal responsive genes in wheat. Can J Bot 73:891–897
Hoffland E, Wei CZ, Wissuwa M (2006) Citrate exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India, New Delhi
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364
Khare AK, Rawat AK, Dubey SB, Patel KS, Rathore GS, Thompson JP, Takkar PN (1998) Role of native vesicular-arbuscular mycorrhizal fungi in wheat (Triticum aestivum)-based cropping sequence for efficient use of phosphorus and zinc in black soils of Madhya Pradesh. Indian J Agr Sci 68:247–250
Kirk GJD, Bajita JB (1995) Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytol 131:129–137
Koide R, Li M, Lewis J, Irby C (1988) Role of mycorrhizal infection in the growth and reproduction of wild vs. cultivated plants. I. Wild vs. cultivated oats. Oecologia 77:537–543
Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL (2000) Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Pate JS (1994) The mycorrhizal association—just one of many nutrient acquiring specializations in natural ecosystems. Plant Soil 159:1–10
Phillips JM, Haymann DS (1970) Improved procedures for cleaning and staining parasitic and vesicular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc 55:158–160
Purakayastha TJ, Chhonkar PK (2001) Influence of vesicular-arbuscular mycorrhizal fungi (Glomus etunicatum L.) on mobilization of zinc in wetland rice (Oryza sativa L.). Biol Fertil Soils 33:323–327
Ramesh SA, Choimes S, Schachtman DP (2004) Over-expression of an arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol Biol 54:373–385
Rengel Z, Batten GD, Crowley DE (1999) Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res 60:27–40
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Ryan MH, Angus JF (2003) Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant Soil 250:225–239
Ryan MH, Graham JH (2002) Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 244:263–271
Sharma AK, Srivastava PC, Johri BN, Rathore VS (1992) Kinetics of zinc uptake by mycorrhizal (VAM) and non-mycorrhizal corn (Zea mays L.) roots. Biol Fertil Soils 13:206–210
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London
Smith SE, Robson AD, Abbott LK (1992) The involvement of mycorrhizas in assessment of genetically dependent efficiency of nutrient uptake and use. Plant Soil 146:169–179
Tolay I, Erenoglu B, Romheld V, Braun HJ, Cakmak I (2001) Phytosiderophore release in Aegilops tauschii and Triticum species under zinc and iron deficiencies. J Exp Bot 52:1093–1099
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un systèmeradiculaire. Récherche de methodes d’ estimation ayant une signification fonctionelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA Press, Paris, pp 217–221
Welch RM, Graham RD (2002) Breeding crops for enhanced micronutrient content. Plant Soil 245:205–214
Wellings NP, Wearing AH, Thompson JP (1991) Vesicular-arbuscular mycorrhizae (VAM) improved phosphorus and zinc nutrition and growth of pigeonpea in a Vertisol. Aust J Agr Res 42:835–845
Wright DP, Scholes JD, Read DJ, Rolfe SA (2005) European and African maize cultivars differ in their physiological and molecular responses to mycorrhizal infection. New Phytol 167:881–896
Zhang XH, Zhu YG, Chen BD, Lin AJ, Smith SE, Smith FA (2005) Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal contamination in soil. J Plant Nutr 28:2065–2077
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Acknowledgements
This research is part of the programme “From Natural Resources to Healthy People” sponsored by the Interdisciplinary Research and Education Fund (INREF) of Wageningen University, The Netherlands, Project 948 (2003-Z53) in China and the National Natural Science Foundation of China (No. 30571106). We thank Dr. Xiaolin Li (CAU) and Dr. Gu Feng (CAU) for their constructive comments on experimental design and for kindly providing the inoculum. Dr. Sjoerd van der Zee (WU) is thanked for his comments on a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Kuyper, T.W., Zou, C. et al. Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 290, 283–291 (2007). https://doi.org/10.1007/s11104-006-9160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9160-x