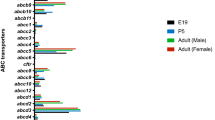Abstract
The human placenta fulfills a variety of essential functions during prenatal life. Several ABC transporters are expressed in the human placenta, where they play a role in the transport of endogenous compounds and may protect the fetus from exogenous compounds such as therapeutic agents, drugs of abuse, and other xenobiotics. To date, considerable progress has been made toward understanding ABC transporters in the placenta. Recent studies on the expression and functional activities are discussed. This review discusses the placental expression and functional roles of several members of ABC transporter subfamilies B, C, and G including MDR1/P-glycoprotein, the MRPs, and BCRP, respectively. Since placental ABC transporters modulate fetal exposure to various compounds, an understanding of their functional and regulatory mechanisms will lead to more optimal medication use when necessary in pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnant women are often under medication for medical conditions such as asthma, epilepsy, hypertension, viral, fungal or bacterial infections or for pregnancy related conditions such as nausea, vomiting, preeclampsia or gestational hypertension/diabetes. In a study that monitored drug use in pregnancy, 578 pregnant women were interviewed; the results indicated that 95.8% of these pregnant women used at least one prescription drug (including prenatal vitamins), 92.6% used over-the-counter medications and 45.2% used herbal medications during pregnancy (1). Drug use during all stages of pregnancy and especially during the first trimester is of great concern due to potential fetal toxicity that may result from transplacental transfer of drugs. Several drugs including anticonvulsants such as valproic acid and phenytoin used during pregnancy are classified as Food and Drug Administration (FDA) category D drugs (drugs that exhibit fetal toxicity reported from marketing survey or studies in humans, however, use of the drug in pregnancy may be acceptable due to potential benefit). Notably, valproic acid is FDA category X (unacceptable risk) when used for migraine prophylaxis.
The placenta serves as the link between the mother and the fetus and acts as a multifunctional organ responsible for the supply of nutrients and transfer of waste products from the fetal circulation to the maternal circulation. It may limit the transfer of toxic xenobiotics to the fetus and secretes physiological compounds such as hormones and prostaglandins (2,3). The human placenta consists of a multinucleated, polarized syncytiotrophoblast layer that separates the fetal circulation from the maternal circulation (Fig. 1) (4). The apical (brush border or maternal) surface of the syncytiotrophoblast is bathed in maternal blood and acts as the main barrier to the passage of drug molecules between the mother and the fetus. The basal (or fetal) surface is in contact with the discontinuous cytotrophoblast or the fetal connective tissue. Fetal capillaries that are relatively leaky are embedded in the fetal connective tissue and are present in the center. Thus transfer of nutrients or xenobiotics across the placenta from the mother to the fetus would require translocation across the apical and basolateral membranes of the syncytiotrophoblast and across the fetal capillary endothelium (5).
Cross sectional schematic view of the human placental chorionic villous tissue [Adapted from reference (4)].
Transplacental exchange of endogenous substrates and xenobiotics can occur by passive diffusion, active transport, facilitated diffusion, phagocytosis or pinocytosis. Of these processes, phagocytosis and pinocytosis are slow processes that do not have a significant effect on the extent of transfer of most drugs. Facilitated diffusion is known to be involved in the placental transfer of cephalosporins and glucocorticoids (6,7), but the overall contribution of facilitated diffusion towards placental drug transfer is likely to be much lower than passive diffusion or active transport (8).
Passive diffusion is a major pathway for placental transfer of drugs down their concentration gradient and the rate of passive diffusion is governed by the physicochemical properties of the drug, concentration of the drug in the maternal circulation, and the structural features as well as physiologic parameters of the placenta. Drugs that have a molecular weight <500 Da, are lipophilic, and are predominantly unionized at physiologic pH, easily diffuse across the placenta (9). Maternal-fetal differences in plasma protein binding to albumin (acidic drugs) or α1-acid glycoprotein (basic drugs), and binding of drugs to the placental tissue could also affect the distribution of drugs in the maternal-placental-fetal unit. Furthermore, passive diffusion would depend upon the surface area available for exchange (3.4–12.6 m2), membrane thickness (4–100 μm) and the uterine blood flow (50–600 mL/min) (5). Nonetheless, these parameters may differ with gestational age.
Active transport of drugs against a concentration gradient across the placenta, is mediated by influx or efflux pumps that are driven by energy from ATP-hydrolysis or the transmembrane electrochemical gradient provided by Na+, Cl− or H+ (10). Active transport pumps in the placenta include, but are not limited to, various ATP binding cassette (ABC) transporters as well as monoamine transporters (norepinephrine transporter and serotonin transporter), organic cation and anion transporters, monocarboxylate and dicarboxylate transporters (10). In addition, the placenta also expresses a plethora of nutrient transporters.
ABC Transporters in the Placenta
The placental syncytiotrophoblast has several active transporters on the apical and basal side that may regulate fetal exposure to xenobiotics (10,11). These efflux transporters include ABC transporters such as P-glycoprotein (P-gp) or multidrug resistance protein (MDR1), gene symbol ABCB1; breast cancer resistance protein (BCRP) or mitoxantrone resistance protein or placental ABC, gene symbol ABCG2 and multidrug resistance associated proteins (MRP)-1, -2 or -3, gene symbols ABCC1, ABCC2, or ABCC3, respectively. These are integral membrane proteins that depend on the energy derived from ATP to drive the transport of substances from the inside of the cell or from the cell membrane out of the cell (12–14). Recently, the mRNA and/or protein expression of these transporters in the fetal membranes; yolk sac and amniotic membranes, and decidua have also been characterized (15–17). While further studies are required to establish localization and functional activity of these ABC transporters expressed in the placenta, localization and substrate selectivities of some ABC transporters has been established (see Table I).
Schinkel & Jonker reviewed the ABC transporter superfamily, providing structural characteristics and tissue localizations (46). The prototypical putative ABC transporter structure (P-gp) comprises an intracellular N-terminus, 6 transmembrane segments (transmembrane domain 1), a nucleotide binding domain, followed by another 6 transmembrane segments (transmembrane domain 2), then a second nucleotide binding domain, ending with a cytoplasmic C-terminus. MRP4 and MRP5 are similar, but MRP1, MRP2, and MRP3 have an additional 5 transmembrane segments on the N-terminus. In contrast, BCRP is described as a half-transporter, comprising a cytoplasmic N-terminus, then a single nucleotide binding domain and a single transmembrane domain; thus it appears to be half of the P-gp structure turned backwards, and may dimerize to become functional (46). Additionally, outside of their expression in the placenta (discussed below), these transporters are expressed to varying extents in the liver, kidney, small intestine, and blood–brain barrier (46).
The ABC transporters located on the apical surface of the placental syncytiotrophoblast are suitably positioned to efflux xenobiotics toward the maternal circulation (Fig. 1). These transporters have wide substrate specificity and are involved in excretion of endogenous compounds in the bile or urine, or provide protection against xenobiotics in other tissues such as the liver, kidney, lung, gastrointestinal tract, blood–brain barrier and blood-testis barrier. The following sections review available literature on the expression, structure, substrate specificity, biochemical function, polymorphism and hormonal regulation for the major ABC transporters in the placenta.
ABCA Subfamily
ABCA1
ABCA1 was first cloned in 1999 (47), and its expression was found in various human tissues with high levels of expression found in the placenta, liver, adrenal glands, small intestine, and fetal tissues. Furthermore, it was shown that ABCA1 expression was upregulated in macrophages (47). Soon after, a complete genomic sequence of the mouse and ABCA1 genes, including their promoter and regulatory elements was described (48). A fragment of the ABCA1 gene promoter was found to contain a cholesterol regulatory element that modulates ABCA1 expression in macrophages (48). Both these discoveries suggested a possible role of ABCA1 in lipid transport. Schmid et al. performed the first studies in humans that demonstrated cholesterol transport from maternal to fetal compartment using BeWo cells as a model of placental monolayer function (49). However, these studies did not precisely determine the involvement of ABCA1 in efflux of cholesterol from placenta. Mutations in ABCA1 were first linked to genetic disorders of high-density lipoprotein (HDL) deficiency and Tangier disease (50–52). Furthermore, loss of ABCA1 function in knockout mice, results in a marked reduction of lipid transport, placental malformation, impaired steroidogenesis, kidney glomerulonephritis, and heart failure (53). ABCA1 not only transports cholesterol but also the phospholipids sphingomyelin, phosphatidylcholine, and phosphatidylserine (54).
Recent studies have shown that ABCA1 expression is linked to disorders associated with abnormal placentation, such as pre-eclampsia and antiphospholipid syndrome (APS) (55). Investigations on the placentae from patients with these conditions revealed that ABCA1 mRNA expression (normalized to β-actin) was significantly decreased in APS (approx. 5-fold); ABCA1 protein expression was also decreased, as opposed to women with pre-eclampsia. These results suggested that the etiology of APS but not pre-eclampsia may be related to abnormalities of ABCA1 function in the placenta (55).
Nikitna et al studied the cellular and subcellular localization of ABCA1 in different types of isolated primary placental cells. While after 12 h of cultivation the primary cytotrophoblast cells displayed intensive localization of ABCA1 in the membranes and cytoplasm, after 24 h with progressive syncytium formation the levels of ABCA1 were reduced and it was more dispersed in the cytoplasm of the newly formed syncytial layer. In other cell lines such as the amnion epithelial cells, the placental macrophages and the mesenchymal cells, the ABCA1 transporter was mostly expressed at the cell membranes and in the cytoplasm, particularly in the endoplasmic reticulum. The ABCA1 staining intensity in these cell lines was independent of the cultivation time (18). Bhattacharjee et al showed that ABCA1 is present in the first trimester and term placenta with minimal difference in their mRNA and protein levels. Immunohistochemical studies showed that ABCA1 was localized in the villous and extravillous cytotrophoblasts and also in the stromal and endothelial cells. ABCA1 is predominantly localized on the basolateral side of the cytotrophoblast layer of the first trimester of pregnancy, suggesting its potential role in the efflux of cholesterol to the fetal side (19). While findings by Albrecht et al were similar to that by Bhattacharjee et al, Albrecht et al also found localization of ABCA1 in early pregnancy in the possible Hofbauer cells in the villous stroma (20). Furthermore, while Albrecht et al found variable but confirmed staining of ABCA1 in the cytoplasm of villous syncytiotrophoblasts by immunohistochemistry (20), Bhattacharjee et al had shown ABCA1 staining by immunofluorescence only on the apical side of a few villous, syncytiotrophoblasts (19). Baumann et al showed that the ABCA1 mRNA expression is dependent on gestational age and displayed significantly higher ABCA1 mRNA levels in preterm compared to term placenta. However, these levels were downregulated in conditions involving preeclampsia. Further protein expression in their studies was also downregulated in the apical membrane of villous syncytiotrophoblasts in preeclampsia. Consequently, the placental content of levels of phospholipid had increased which were partially related with reduced levels of ABCA1. This also suggested that the lipid transport capacity of ABCA1 might be compromised in preeclampsia (21). Plosch et al showed that ABCA1 gene showed increased expression from 2nd trimester to preterm. In addition, the mRNA expression of ABCA1 was increased in early onset preeclampsia. Further when the JAR trophoblast cells and first trimester human placental explants were subjected to hypoxic conditions (1% oxygen) the expression of ABCA1 mRNA was shown to be increased. Liver X receptor (LXR), a nuclear receptor that regulates cholesterol metabolism and ABCA1, also displayed elevated expression levels during normal pregnancy and under low oxygen tension. The expression of ABCA1 was also increased in presence of an LXR agonist suggesting that the LXR pathway is pharmacologically activated. The authors concluded that the LXR-ABCA1 system could be deregulated under hypoxic conditions such as in the case of preclampsia which could consequently lead to alterations in placental cholesterol transfer (22). Recent evidence shows that the fetus, during the early phase of pregnancy, obtains cholesterol from the mother and thus altered cholesterol transfer could hamper the fetal development (56).
An oxidized low density lipoprotein (Ox-LDL) binds to oxidized LDL receptor (LOX-1) to produce endothelial dysfunction, a key feature of preclampsia. Chigusa et al. found a decrease in LOX-1 expression in preeclamptic placentae and concluded that this might be the cause of elevated serum levels of Ox-LDL that could play a role in pathogenesis of preeclampsia (57). In a subsequent study, the same group also showed that the decreased LOX-1 expression could lead to insufficient uptake of Ox-LDL and subsequently leading to decreased LXR activation and thereby decreasing ABCA1 expression. These findings indicate that ABCA1 expression is decreased in preclamptic placentae and also provides new insights in understanding pathophysiology of preeclampsia (58). However, these results are different with those reported by Plosch et al, indicating an upregulation of ABCA1 in early onset preeclamptic placentae but not significant elevation in late onset preeclampsia (22).
Guay et al reported that ABCA1 epipolymorphism is linked to the HDL particle profile as well as cornonary artery disease in subjects with familial hypercholesterolemia. This finding indicated that the ABCA1 DNA methylation profiles could be altered by the in utero environment and this in turn could have an impact on the lipid profile and onset of cardiovascular diseases in newborn (59). Hence, Houde et al conducted a study to assess the relationship between the maternal metabolic profile and levels of DNA methylation of ABCA1 in placenta and cord blood (60). Studies were performed on human placentae, included those from women with impaired glucose tolerance. The studies showed that the DNA methylation levels (high) of ABCA1 on the maternal side of placenta negatively correlated with maternal high density lipoprotein cholesterol (HDL-C) levels (low) and positively correlated with glucose levels (high) 2 h post oral glucose tolerance test in first trimester and second trimester of pregnancy, respectively. The higher DNA methylation was accompanied by a decrease in ABCA1 expression. DNA methylation levels (high) of ABCA1 on the fetal side of the placenta positively correlated with HDL-C (high), and negatively with triglyceride levels (low) in cord blood and with the maternal glucose levels. The ABCA1 DNA methylation variability on both sides of placenta was negatively associated with ABCA1 mRNA levels. These results suggest that in response to the in utero environment and fetal stimuli, the DNA methylation profile of ABCA1 changes to ensure optimal delivery of cholesterol to the fetus. Nonetheless it was also suggested that they may trigger the long-term susceptibility of the newborn to dyslipidemia and cardiovascular diseases due to alterations in lipid transport.
ABCB Subfamily
P-glycoprotein (MDR1, ABCB1)
Introduction
The earliest discovered and most widely known ABC transporter is MDR1, gene symbol ABCB1. This integral membrane protein has 12 putative transmembrane domains and two ABCs. It exports a wide variety of mainly lipophilic compounds out of cells, and causes resistance to many chemotherapeutic agents in cancer cells overexpressing MDR1. Although most MDR1 substrates are cationic and lipophilic, there are several MDR1 substrates which are either anionic or neutral. MDR1 is expressed not only in the liver, kidney, intestine, and brain, but also in the testes and the placenta, giving rise to the formation of “pharmacologic sanctuaries” due to its protective and excretory functions.
Placental P-gp Activity In Vivo
Generation of P-gp knockout mice (Mdr1a/b(-/-)) was an important milestone in demonstrating the in vivo role of placental P-gp in excluding drugs from the fetal compartment (61–63). Initially, a direct correlation was observed between cleft palate induction in fetal mice transplacentally exposed to an avermectin analog L-652,280 and their genotype for the mouse homologs of MDR1 (Mdr1a/1b). Cleft palate was found in 30/30 mice devoid of Mdr1a/1b, about 30% of 70 mice that were heterozygous, and none of 70 mice that were homozygous positive for Mdr1a/1b (64). Thus, Mdr1a/1b was effective at preventing the exposure of fetal mice to an agent that can induce cleft palate. Another study showed 2.4- to 16-fold increases in fetal tissue levels of prototypical P-gp substrates such as digoxin, saquinavir and paclitaxel in mice devoid of Mdr1a/1b compared to the wild type (65). These data again illustrate the protective function of Mdr1a/1b in mice. In the same paper Smit et al observed that accumulation of digoxin, a P-gp substrate, in the brain is 7-fold higher in presence of the P-gp inhibitor PSC 883 than control mice. In addition, the fetal accumulation was 2.5-fold higher in presence of PSC 883 suggesting that P-gp function might be more prominent in the blood–brain barrier than in the placenta. The above results indicated that increased accumulation and therefore transplacental exposure of fetuses to xenobiotics in the presence of placental P-gp blockade or reduced placental P-gp activity is expected to have toxicological consequences to the fetus.
Lee et al studied the transplacental transfer of paclitaxel, a known P-gp substrate, in pregnant rats and observed that the paclitaxel uptake in the fetus was lower compared to the uptake in the placenta suggesting that P-gp would affect the fetal transfer of paclitaxel and thus make clinical use of paclitaxel possible in pregnancy (66). Bhuiyan et al showed that the selective serotonin reuptake inhibitor sertraline could influence the placental transfer of the P-gp substrate digoxin after 4 h of sertraline administration in mice. Sertraline caused an increase in the P-gp mediated substrate efflux at the placenta and enhanced the ability of the placenta to prevent xenobiotics from entering the fetus (67). The authors note that the enhancement in the substrate efflux could be positive cooperativity, or P-gp induction, requiring further studies. However, sertraline also caused an increased transfer of digoxin to the maternal and fetal brain indicating a decrease in P-gp activity at maternal and fetal blood–brain barrier (BBB) (67). Bloise et al studied the effects of exposure of sublethal doses of lipopolysaccharides (LPS) on the expression and activity of placental drug transporters in mouse. Abcb1 a/b and Abcg2 mRNA expression levels were unaffected after acute and chronic LPS treatments. However, the acute sub-lethal infection caused a decrease in placental P-gp activity in a time- and gestational-age dependent manner. In addition, it was also observed that fetuses with smaller placentae accumulated the P-gp substrate digoxin to a greater extent as compared to the larger placentae, suggesting that the smaller placentae are less efficient at efflux (68). Complementing the above study, Hodyl et al studied the placental P-gp expression in preterm small for gestational age (SGA) infants on antenatal glucocorticoid therapy. The studies showed that P-gp both at the mRNA and protein level displayed a decrease in expression in placenta from SGA infants compared to appropriately grown infants. This implied that the fetus might be exposed to higher levels of exogenous glucocorticoids due to lower levels of placental P-gp. This also suggested that P-gp might have a role in the pathogenesis of SGA. Nonetheless, another short study in the same publication also indicated that the placental P-gp mRNA and protein expression was unchanged by the timing of the prenatal exogenous glucocorticoids or the fetal sex (69).
Further, using verapamil as the protypical P-gp substrate and cyclosporine as the inhibitor in the non-human primate Macaca nemestrina, Eyal et al observed a 3.9-fold change in brain to plasma AUC ratio in presence of cyclosporine and a 2.2-fold change at the placenta (70). However, the same group in a subsequent publication indicated that these results could be confounded by tissue blood flow and the blood flow should be accounted for while comparing P-gp activity at brain and placenta (71).
In humans, in vivo placental P-gp activity has been inferred by measurement of fetal (cord) and maternal plasma concentrations at the time of C-section in women receiving P-gp substrates such as the anti-HIV protease inhibitors (72). The cord-to-maternal plasma concentration ratios of nelfinavir, ritonavir, saquinavir or lopinavir were very small (<0.3). These results indicate that the placenta excludes protease inhibitors from the fetal compartment, most likely due to P-gp efflux activity. Consequently, the fetal antiviral concentrations and activity of these anti-HIV protease inhibitors would be less than expected. However, caution should be used in interpreting single cord-to-maternal plasma concentration ratio as this ratio is highly dependent on the interval between time of sampling and drug administration. If the baby is delivered soon after drug consumption, the cord-to-maternal plasma concentration may be <1 even in the absence of any efflux, if the drug does not exhibit rapid distribution into body tissues, including the fetal compartment. Thus, it is important that physiologically based pharmacokinetic (PBPK) models be used to interpret such data. Duarte et al reported distribution of fentanyl in the placenta and fetal tissues. The study indicated 86% placental transfer of fentanyl and it primarily deposited in the placental intervillous space suggesting a possible role of efflux by P-gp. Further, the umbilical artery and vein concentrations were equal suggesting that there was no uptake or metabolism of fentanyl by fetal tissues (73).
Polymorphism of P-gp
Hutson et al has reviewed the effect of polymorphism on the placental P-gp expression. Specific P-gp polymorphisms which have been the focus of the studies reviewed included C3435T, G2677T/A, C1236T and T-129C. While most studies report a decrease in protein expression of P-gp due to polymorphism, some suggest an increase (74). Studies by Hitzl et al, indicated that the 3435 T and 2677 T variants decrease placental P-gp expression (25). Hemauer et al investigated the relationship between MDR1 polymorphism and human placental P-gp expression and functionality using inside out vesicles from the placental brush border membrane. The C3435T and G2677T/A alleles were associated with significant decrease in P-gp protein expression while the C1236T variant was related with a trend towards decreased placental P-gp expression. Ironically, in homozygous variants 1236 T/T and 3435 T/T the P-gp activity was apparently increased as shown by the transport of the prototypical P-gp substrate paclitaxel in the human term placenta compared to the homozygous wild type (C/C). The G2677T/A alleles also showed a trend toward increased P-gp activity than the homozygous wild type (C/C) (75). Further, a study by Rahi et al involving immunoblotting and genotyping by PCR in human placental samples indicated that the variant allele 3435 T of P-gp caused significantly higher placental P-gp expression levels. However in a related study by the same group, this increased expression did not translate into higher P-gp activity (76). In a separate study in the human perfused placenta model, Rahi et al had shown that the 3435 T polymorphism may have caused an increased transfer of the antipsychotic agent quetiapine, although no association was found between P-gp expression and placental transfer (77). Bliek et al studied the association between ABCB1 3435C > T polymorphism, periconceptional medication exposure of the mother and risk of a child with cleft lip and or cleft palate. The study showed that mothers carrying 3435TT genotype and using a medication during the periconceptional period displayed a 6.2-fold increased risk of child having cleft palate compared to subjects carrying 3435CC genotype and not using a medication. Hence these data suggest that mothers carrying the 3435C > T polymorphism display a significantly higher risk for a child with cleft palate especially in mothers that use medications during periconceptional period (78). However, since the 3435C > T mutation is synonymous, the possibility of a spurious correlation cannot be discounted. Bogacz et al studied the frequency of G2677T/A and C3435T polymorphism of MDR1 in preeclamptic pregnancy. However, no significant association between the genotypes and the clinical parameters of women with preeclamptic pregnancies was observed. However, an increased prevalence of mutated 2677A allele of G2677T/A MDR1 polymorphism in preeclamptic women was observed (79). The effect of polymorphism on the expression of ABC transporters has also been reviewed elsewhere as well (74,80).
Placental Localization and Expression of P-gp
In situ hybridization studies have shown that P-gp is expressed in the syncytiotrophoblast and cytotrophoblast cells of the placental villi (81). Immunohistochemical studies have shown that P-gp is expressed in the maternal-facing apical membrane of the syncytiotrophoblasts (23,24). In addition to the syncytiotrophoblasts, villous fetal and maternal endothelium is also positive for P-gp expression (25). In this study the endothelial P-gp was detected through double staining with P-gp and endothelial markers (anti-human von Willebrand factor and the anti-human CD31). This localization of P-gp is consistent with its role in protecting the fetus from xenobiotics ingested by the mother. Ushigome et al (82) showed that in the human placenta, P-gp protein is expressed in the brush-border membrane but not in basolateral membrane of the syncytiotrophoblasts. Functionally, the transport of the radiolabeled P-gp substrates, digoxin and vinblastine, into the human placental brush border membrane vesicles was inhibited by the P-gp inhibitors verapamil or the P-gp antibody, C219.
The presence of P-gp in the placenta raises the obvious question: Does placental P-gp expression and activity change with increasing gestational age? Such gestational age dependent expression would have significant consequences for fetal drug toxicity or drug therapy of the fetus with drugs that are P-gp substrates.
Using quantitative PCR, P-gp mRNA levels in first and second trimester human placentae (gestational age range 60–120 days of pregnancy) were approximately 5-6 fold higher than in term C-section placentae (38–41 weeks gestation) (26). Consistent with this observation, another study also found increased levels of P-gp mRNA in human placentae of 24–35 weeks of gestation compared with term placentae (27). In both studies placental P-gp protein expression, quantified using Western or ELISA analysis demonstrated a similar decrease with advancing gestation.
In rats, placental P-gp expression demonstrates different pattern of expression during pregnancy (83). P-gp (in rodents P-gp is coded by Mdr1a and Mdr1b) mRNA expression was detected by polymerase chain reaction (PCR) on 11th day of pregnancy and increases with gestational age. P-gp protein expression was measured by two different antibodies. Antibody C219, which detects an intracellular epitope of P-gp (84), was used to detect P-gp on day 13 of gestation. P-gp was detected on day 15 onwards by another antibody, F4, which recognize an extracellular epitope (85). This discrepancy in P-gp detection time may be due to differential affinities of the antibodies. Another explanation is that the C219 antibody recognized multidrug resistance protein 2 (Mdr2) or sister of P-gp (sP-gp). F4 antibody does not react with any of these P-gp related proteins. Therefore, reports of early detection of P-gp using C219 antibody may be confounded by cross-reactivity with Mdr2 and sP-gp.
While the rat and human placenta are used extensively for studying P-gp and its xenobiotic substrates, it is important to note some structural differences in human and rat placenta with respect to P-gp localization (86). The human placental barrier comprises one syncytiotrophoblast layer which expresses P-gp on the apical membrane. The rat placental labyrinth has three trophoblastic layers. The first layer which faces maternal blood does not represent a barrier to small compounds. The remaining two layers (layer II and III) are thought to be the syncytium. Pavec et al (87) found highest expression of P-gp in the apical membranes of the second or third trophoblast layer but no expression in the first layer. The IInd and IIIrd layers are likely to represent the actual P-gp barriers in the rat placenta. Unlike the human placenta, no P-gp was found in the rat placental endothelial cells.
Zhang et al showed that in the mouse placenta the P-gp protein expression is highest at early gestational ages and decreases with progression of pregnancy. However, expression of placental P-gp transcripts Mdr1a and Mdr1b remained unchanged regardless of gestational age. Further the expression level of Mdr1a was approximately 7-fold greater than that of Mdr1b (88).
Soo et al studied the effect of maternal undernutrition on the placental growth in guinea pigs. It was shown that maternal undernutrition reduced the placental and fetal growth and also altered the placental and fetal brain morphometry at 60–62 days gestation. Maternal undernutrition caused a decrease in the P-gp protein expression in the placenta and fetal brain suggesting that the “small for gestational age” fetus are more susceptible to drug exposure in late gestation. However, there were no significant changes in the mRNA expression of P-gp (89), suggesting post-transcriptional regulation of P-gp expression.
Chung et al investigated the P-gp activity in placenta during pregnancy using verapamil as the prototypical P-gp substrate and cyclosporine as the P-gp inhibitor in a non-human primate (Macaca nemestrina) and it was shown that the activity increases from mid- to late gestational age (90). However, in a subsequent report it was shown that these results are confounded by tissue blood flow (71).
Finally, to translate the above studies in vivo, one must also take into consideration the size of the placenta. Though P-gp expression per mg of protein may decrease but the increase in size with gestational age may, in part, compensate for the decrease. Of course this gets more complicated as the fraction of P-gp transport relative to diffusion may also change with gestational age due to the thinning of the placenta.
P-gp Activity in the Perfused Placenta
Since placentae can be obtained non-invasively, a suitable ex vivo model for studying placental drug transport is the isolated human placental perfusion model which has been discussed elsewhere ((91,92) and references within). This approach allows for study of transport of substances across the maternal and fetal interface. However, availability of donated placentae, technical difficulty, and the additional caution of dealing with potentially biohazardous material limit the widespread use of this technique. Moreover, Bapat et al. reported a 7.1% overall success rate in the dually perfused human placental cotyledon model (93), which emphasizes the challenging nature of these methods.
Experiments performed using dually perfused rat placentae have been used to investigate MDR1 functional activity. Pavek et al demonstrated that in the rat placenta, Mdr1a/1b limits the entry of some xenobiotics into the fetus as well as accelerates some xenobiotic movement in the fetal to maternal direction (87,94). Inhibitors of P-gp, such as PSC833, cyclosporine, quinidine, or chlorpromazine, were able to significantly increase the materno-fetal transplacental passage of rhodamine 123. However, quinidine, an inhibitor of P-gp and rat organic cation transport, was most efficient in inhibiting feto-maternal passage of rhodamine 123. This observation implies that rhodamine 123 has significant component of rat organic cation transport and therefore should be used with caution to evaluate placental P-gp function.
The perfused human term placenta was used to evaluate drug transport in fetal-maternal and maternal-fetal directions (95). As expected, maternal to fetal transfer of saquinavir was low and increased 6- to 8-fold after preperfusion with P-gp inhibitors PSC833 or GG918. The fetal-to-maternal clearance of saquinavir is 108-fold higher than the maternal-fetal clearance. Preperfusion with P-gp inhibitors PSC833 did not increase the placental fetal-to-maternal clearance of saquinavir. These results correlate well with clinical studies in HIV-infected pregnant women and demonstrate low fetal concentrations of saquinavir, a P-gp substrate, indicating poor permeability of the drug across the human placenta (72) due to efflux by P-gp.
Sudhakaran et al investigated the effect of P-gp inhibition on the placental transfer of indinavir in the dually perfused isolated human placenta model. The mean maternal to fetal clearance index of indinavir and vinblastine increased significantly in the presence of PSC833, a known P-gp substrate indicating that P-gp restricts the fetal transfer of indinavir and vinblastine. However, the transfer of indinavir was unaffected in the presence of ritonavir, most likely because of low P-gp inhibitory potential of ritonavir at clinically relevant concentrations (86). Like indinavir (86) and saquinavir (95) (see above), methadone (96) also showed increased fetal drug transfer after inhibition of P-gp in the perfused human placenta. Nekhayeva et al also reported that MDR1 has been demonstrated to regulate the disposition of methadone across the placental barrier in the perfused human placental model (97). Malek et al reported the effects of methadone or methadone in conjunction with cocaine or heroin on the expression of placental P-gp in the ex vivo model of human perfused placenta. It was shown that the P-gp expression significantly increased after treatment with methadone or methadone in conjunction with cocaine or heroin (98). Rahi et al studied the influence of ATP on P-gp dependent transfer of saquinavir in dually perfused human placenta. The results indicated that addition of exogenous ATP does not alter the P-gp dependent transfer of saquinavir. The results also suggested in an indirect way that the placental continues to produce ATP during the perfusion (76).
Other In Vitro Models
Several in vitro models developed from primary tissue and cultures exist to facilitate study of placental MDR1 transport. Each model has its advantages and limitations and it is up to the investigator to determine which model is most suitable. Both Sastry and Bode have thoroughly reviewed the in vitro techniques available for studying transport across the placenta (99,100). It should be noted that although placental perfusion, primary trophoblast and membrane vesicle studies have their limitations, these cell systems express MDR1 as well and may be utilized to understand the complexity of the efflux mechanism.
Primary cultures of human cytotrophoblasts isolated from donated placenta provide a good in vitro tool. When placed in culture conditions however, these cells spontaneously differentiate to syncytiotrophoblasts (99). Furthermore, these cells do not form a confluent monolayer but rather form cell aggregates with large intercellular spaces (99). As a result, primary cytotrophoblasts can be used for uptake and metabolism studies, but cannot be used for trans-trophoblast monolayer transport studies. The presence of functional MDR1 in primary cultures has been established for anticancer agents and makes them a useful model for studying terminally differentiated cytotrophoblasts (101). Beghin et al studied the effects of saquinavir and nelfinavir on P-gp expression and functionality in human cytotrophoblast cells. While saquinavir significantly increased both the expression and functionality of P-gp, nelfinavir exclusively increased the functionality by causing dissociation of P-gp from caveolin-1. These results also suggested that although both the drugs increased P-gp activity, they might act through different mechanisms (102). Beghin et al also studied the effect of selected drugs on the expression and activity of P-gp using rat and human primary trophoblast cultures. In this study while aspirin caused an induction of P-gp activity, it did not have an effect on the P-gp expression in both the cultures. Both methadone and a cardiovascular proprietary compound caused an induction of P-gp activity and expression in both the cultures. Nonetheless these effects on the P-gp activity by both the drugs were substrate specific, suggesting different effects on P-gp activity with use of different known substrates. Thalidomide had an inductive effect in the rat primary trophoblasts, while it had a P-gp inhibitive effect on human primary trophoblast cells. In addition, a more marked effect of these drugs on P-gp was observed after 48 h as compared to 24 h in both the cultures (103), suggesting effects on regulation of P-gp expression.
Human placental membrane vesicles can also be obtained from placental tissue (82,104). The advantage of this technique is that based on the type of vesicle generated, one can investigate the permeability properties of the brush border and basal membranes separately. Like primary cultures, the vesicle preparations have been used to demonstrate functional MDR1 in brush border membranes of trophoblasts but not the basolateral membrane, and can be used to characterize the efflux mechanism (104). Hemauer et al showed that the inside out vesicles obtained from term placenta brush border membranes provided an effective method for identification of drugs that are substrates for P-gp and for studying their interaction with transport of its prototypical substrate paclitaxel. No correlation was observed between P-gp expression and transport activity in the inside out vesicles. Among the opioids tested, methadone and morphine were found to be P-gp substrates that compete with paclitaxel transport while buprenorphine was found to be a P-gp inhibitor. Further all the three opioids were found to be more potent inhibitors of P-gp compared to the known P-gp inhibitor verapamil (105).
Lee et al identified the transport mechanism of paclitaxel in conditionally immortalized rat synctiotrophoblast cell lines. The uptake of paclitaxel was time and temperature dependent and saturable in the cell lines. The uptake of paclitaxel was inhibited by known P-gp substrates such as verapamil and cyclosporine, but the MRP substrates displayed no effect. The studies showed that mRNA of P-gp was expressed in these cell lines and that P-gp was involved in the transport of paclitaxel (106).
Vaidya et al characterized the human term placental villous tissue explant model as a tool for studying the ABC transporter mediated efflux of a model substrate 2,4-dinitrophenyl-S-glutathione (DNP-SG) and showed that while BCRP expression remained unchanged, P-gp and MRP-1 expression decreased with culture time. Further, the studies showed that this model could be used to study coordinated function of glutathione-S-transferase isoform-P1-1 and apically oriented ABC transporters in the formation and export (respectively) of model substrate DNP-SG (107). Additionally, transport of digoxin (another MDR1 substrate) in microvillous membrane vesicles derived from the apical syncytiotrophoblast membrane of human placenta was ATP-dependent, saturable (Kt 2.6 ± 1.8 μM; Jmax 1.2 ± 0.5 nmol/20 min/mg protein), inhibited by the C219 antibody (which binds MDR1), and inhibited by classic MDR1 inhibitors such as verapamil and cyclosporine (82). These data indicate that MDR1 plays a functional role in transporting its substrates away from the fetus.
Recently, Song et al established the placenta slice in Ussing chamber model for studying the placental transport in vitro and showed the P-gp mRNA expression and functional activity in this model (108).
Immortalized Cell Lines
Immortalized cell lines from both normal and malignant tissues appear to be a convenient model for studying placental transport. Trophoblast cell lines have also been developed from human embryonic stem cells (109). Unfortunately, all necessary growth factors have not yet been identified, so these cells propagate quite poorly, limiting their usefulness. The existence of MDR1 in trophoblasts developing from stem cells remains to be shown. Apati et al recently, discussed the low level of expression of the ABC transporters in human pluripotent stem cells. In addition, the analysis of transporter expression and function is challenging due to the constant change of form between pluripotent and partially differentiated cells (110). Considering the variety of other models available, the use of stem cells appears to be less preferred for studying placental transport of xenobiotics.
One immortalized placental cell line showing the ability to propagate readily and form confluent monolayers is the HRP-1 cell line, a trophoblast cell line derived from normal rat placenta. This cell line has been shown to demonstrate a variety of properties similar to those of human trophoblasts. Shi et al demonstrated that the HRP-1 cell line could be very useful in studying permeability and metabolic properties of trophoblasts since it retains functional expression of CYP1A1, the predominant P450 isozyme of the placenta (111). Furthermore, HRP-1 retains activity of common peptidases of the placenta such as angiotensin I converting enzyme and carboxypeptidase N-like enzyme (111). HRP-1’s ability to retain functional asymmetry was also demonstrated by monitoring linoleic acid uptake (previously shown by Lafond et al to be saturable, polarized and dependent on extracellular levels of albumin (112)). Unfortunately, an MDR1-like transporter does not appear to be present in this cell line (111).
Another immortalized cell line and perhaps one of the most widely used is the BeWo cell line derived from a human choriocarcinoma (113). The attractiveness of the BeWo cell line stems from the striking morphological and biochemical similarities to normal trophoblasts as well as their relatively easy maintenance. A clone of BeWo cells isolated by Dr. Alan Schwartz at Washington University in St. Louis can be grown to form confluent monolayers with reasonable transepithelial resistance making them ideal for a wide range of in vitro assays (114). One feature of the BeWo cell line that makes it especially attractive is the expression of functional MDR1 (101). Utoguchi et al showed both biochemical and functional evidence of MDR1 in BeWo cells by Western blotting and uptake studies with known MDR1 inhibitors respectively (101). Utoguchi also showed that uptake of MDR1 substrates such as calcein-AM by BeWo cells could be significantly increased in the presence of MDR1 inhibitors such as quinidine and verapamil (101). Western blots using monoclonal antibody C219, selective for MDR1, further confirmed the presence of P-gp in BeWo cells. However, since the C219 antibody also reacts with MDR3 (115), so at least a part of the blot density attributed to P-gp expression could be due to MDR3.
Since the HRP-1 and BeWo cell lines form confluent monolayers, transport studies can be performed using Side-bi-Side™ diffusion chambers as well as Transwell® inserts. Both methods have been described thoroughly by Bode et al (99). Lastly it is worth noting that all experiments undertaken using immortalized cells should first demonstrate their investigated clone express mRNA and protein of the ABC transport of interest when functional/pharmacological studies are undertaken.
ABCB4
MDR3 which is encoded by ABCB4 is known as a phospholipid flippase (116) and has been detected in the placenta. Although Patel et al did not detect MDR3 transcripts in human placenta (117), Serrano et al detected low levels of mRNA expression in primary human trophoblasts (36), and Evseenko et al found higher expression of MDR3 than MDR1 (P-gp) in trophoblast cells, JAR, and BeWo cells (32). Furthermore, in primary term trophoblasts, mRNA and protein expression of MDR3 was increased by interleukin 6 (IL-6) (29). Expression of MDR1 and MDR3 decreases with differentiation (32), consistent with declining levels of MDR1 with gestational age. The functional relevance of MDR3 in human placenta needs further study.
ABCB5
Volpicelli et al investigated the localization and expression of ABCB5 transporter in the human placenta. Immunohistochemistry and double labeling immunofluorescence staining showed that ABCB5 is expressed in the trophoblast cells in the first trimester of pregnancy with progressive decrease till term. In addition, this transporter was expressed in the partial and complete molar pregnancies and also in 20% of the choriocarcinomas and 40% of the placental site trophoblastic tumors that were examined. This transporter was mostly localized in the cytotrophoblast layer of placental villi (118).
ABCB6
ABCB6 apparently transports heme and heme-like molecules such as coproporphyrin III and protoporphyrin from the cytoplasm across the outer mitochondrial membrane. This transport activity is involved in the synthesis of heme, and may serve to protect the cells from protoporphyrin-induced degradation of cellular DNA and protein (119). While its expression and functional impact in the human placenta are not known, it may have a role in protecting the placenta from metabolic perturbations.
BSEP/ABCB11
In the liver, Na+-taurocholate cotransporting polypeptide (NTCP) accounts for approximately 80% of bile salt uptake, while organic anion transporting polypeptides (OATPs) transport much of the remainder from blood into the hepatocyte on the sinusoidal aspect (120). On the canalicular side, bile salt export pump (BSEP) is the predominant bile salt transporter (121). Since accumulation of bile salts in the fetal compartment would be detrimental to fetal health, maternally-directed transport systems may exist in the placenta. Using real-time PCR, Patel et al did not detect ABCB4 (MDR3) or ABCB11 (BSEP) mRNA transcripts in human 3rd trimester placentaeplacentae, while they detected low levels in 1st trimester placentaeplacentae. Additionally, they did not detect transcripts of the hepatic sinusoidal bile acid transporter NTCP in human placentaeplacentae (117). Serrano et al. found very low mRNA expression of BSEP in human term trophoblasts and cultured choriocarcinoma cells, and detected protein expression by Western blotting, but expression was too low for immunohistochemistry (36). These data suggest that the fetal to maternal excretion of bile salts may utilize a different pathway. Furthermore, mRNA levels of Bsep in rat placentaeplacentae exceeded the expression in fetal rat liver; (122) which may suggest a species difference in placental bile salt transport between humans and rodents. Marin et al suggested a role for ABCCs and ABCG2 in the fetal to maternal transport of bile salts and biliary pigments such as bilirubin, rather than BSEP (123).
ABCC Subfamily
ABCC1
Since the first report documenting the expression of MRP1 (gene symbol ABCC1) in the human placenta at the mRNA and proteins levels (13), the physiological role of this ABC efflux pump for the developing fetus has elicited much interest. Microscopic examination of sections of term placenta showed that MRP1 is most abundant within terminal villi at the abluminal surface of fetal endothelial cells, with lower levels at the basal (fetal-facing) membrane of the syncytiotrophoblast layer (23,24). Consistent with this localization, the MRP1 protein can be detected in primary cultures of cytotrophoblast cells (23,32) and in purified basal membranes isolated from the syncytiotrophoblast of term placenta (23,24). MRP1 was also detected on the apical syncytiotrophoblast membrane (13,34). The list of endogenous and exogenous solutes that are pumped out of cells by MRP1 in an energy-dependent manner is exhaustive and has been the subject of several reviews (124–126). Anionic glutathione, glucuronide and sulfate conjugates of natural substances comprise one important category of substrates. These include bilirubin mono- and diglucuronides (127), 17β-estradiol glucuronide (128), the dianionic bile salt, hyodeoxycholate-6-O-glucuronide (127), cysteinyl leukotriene C4, the glutathione S-conjugates of prostaglandins A and J2 (129), the glutathione S-conjugate of 4-hydroxynonenal (130), as well as dehydroepiandrosterone sulfate and estrone-3-sulfate (131). MRP1 substrates also include unconjugated natural solutes, many of which are highly relevant to fetal well-being, including reduced and oxidized glutathione (127,132), unconjugated bilirubin (133) and folic acid (134,135). A host of unmodified, hydrophobic drugs and toxins also count among the substrates of MRP1: the mycotoxin aflatoxin B1 (136,137), the antiviral protease inhibitors saquinavir and ritonavir (138), the metalloid salt forms of arsenic (139,140) and antimony (140), the folic acid derivative leucovorin and folic acid antagonist methotrexate (141) as well as many antineoplastic drugs classified as anthracyclines and plant alkaloid (132,142). To this list can be added the conjugated metabolites of many of these as well as other xenobiotics (4,126) as well as several substrates transported (or co-transported) in the presence of reduced glutathione (143,144).
With this MRP1 stalwart transporter expressed in the placenta, it is logical to assume that its physiological role is to reinforce the barrier between the fetal and maternal circulations. However, such a protective role for MRP1 in the human placenta is difficult to rationalize based on its subcellular localizations. The basal orientation at the syncytiotrophoblast exports substrates towards the fetal circulation (23,24) whereas the abluminal expression within fetal endothelial cells is directed away from the fetal circulation toward the syncytiotrophoblast (24). Although these two localizations appear to be at cross purposes, their relative abundances suggest that the clearance of solutes from the fetal blood takes precedence over export from the syncytiotrophoblast towards the fetus (13). Unconjugated bilirubin is one candidate for this clearance pathway since there is strong evidence that MRP1 mediates its efflux from various cell types (133,145). Moreover, fetal bilirubin concentrations increase throughout the course of gestation (146), which may be linked to the small increase in MRP1 mRNA levels in term compared to first trimester placenta reported by Pascolo et al. (147). This is especially relevant in light of the fact that increased bilirubin production in rats has been correlated with an upregulation of Mrp1 at the mRNA and protein levels in the liver and spleen (148). The MRP1-mediated export of bilirubin from fetal endothelial cells would logically precede the subsequent steps of bilirubin elimination proposed by Briz et al., that is OATP1B3 mediated uptake at the basal surface of the syncytiotrophoblast followed by ATP-dependent efflux into maternal blood at the apical side (149). The extent to which the fetal-facing MRP1 in the syncytiotrophoblast (23,24) would hinder this vectorial transfer across the placenta is not known. The identity of the apical (maternal-facing) bilirubin-transporting pump is not currently known, although there is indirect evidence that P-gp may be involved (150).
The high expression of MRP1 in fetal endothelial cells may also constitute a local cellular defense against oxidative stress by exporting glutathione disulfide (GSSG), as recently shown for human aortic endothelial cells (151). Endothelial cell dysfunction related to oxidative stress is purported to play a role in the etiopathogenesis of preeclampsia (152) and the major lipid peroxidation product, 4-hydroxy-2-nonenal, could be detected within villous endothelium in an experimental model of oxidative stress (153). The fact that both GSSG and the glutathione-S conjugate of 4-hydroxy-2-nonenal are substrates of MRP1, which in turn is upregulated in cell culture under conditions that provoke oxidative stress (154), supports the notion that MRP1 protects fetal endothelial cells by exporting potentially noxious endothelium-derived products. The trophoblast layer is also a source of 4-hydroxy-2-nonenal, both under normal and pre-eclamptic conditions (155). While export of 4-hydroxy-2-nonenal-S-conjugate at the basal side would seem to be of dubious benefit to the fetus, this effect would be tempered by the actions of MRP2 at the apical side, which also transports this metabolite (156). Furthermore, 4-hydroxy-2-nonenal-S-conjugate is subject to downstream reduction by cytosolic NADPH-dependent aldo-keto reductase and oxidation by aldehyde dehydrogenase (157), enzymes that are present in macrophages of several tissues (158). It is not known whether placental macrophages also express these enzymes but it is tempting to speculate that disposition of these toxic metabolites within macrophages is one possible avenue that would prevent recirculation back to the fetal blood.
Notwithstanding these potentially protective mechanisms afforded by MRP1, an essential role in fetal development has been discounted because Mrp1(-/-) knockout mice are viable and fertile (159). The pharmacological importance of MRP1 in the placenta may be of a greater concern. For those drugs in the maternal circulation that are substrates for MRP1 as well as apically oriented pumps such as MRP2 and P-gp, the net maternal-fetal transfer will be determined by the relative protein abundances, their affinities, as well as the presence of modulatory agents (4,160–163). This is well illustrated by the poor maternal to fetal transfer of saquinavir, a substrate for two apical placental pumps, P-gp and MRP2, as well as MRP1 (72,138,161,164). Conversely, maternal ingestion of drugs or toxins such as aflatoxin B, which is transported by MRP1 and not MRP2 or P-gp, results in considerable fetal exposure (136,165). Experiments designed to test Mrp1(-/-) embryos for toxicity after challenging pregnant mice with xenobiotics have not yet been reported but will no doubt be helpful in assessing the specific role of Mrp1. However, the interspecies difference is cause for concern when extrapolating data that describes the maternal-fetal exchange of solutes in the rodent model to humans. In particular, the endoplacental yolk sac is the major site of Mrp1 expression in the rat placenta, a structure having no human equivalent after the first trimester (166), whereas the fetal capillaries of the labyrinth zone are negative for Mrp1 (122).
Mitra and Audus investigated the ability of the BCRP and MRP transporters to efflux sulfate conjugates from the placenta. In studies with the BeWo cells, the model substrates 4-nitrophenyl sulfate and acetaminophen sulfate displayed minimal interaction with BCRP. However, the studies indicated that one or more of the MRP isoforms namely MRP1 and/or 5 but not limited to these, play a major role in the elimination of acetaminophen sulfate across the apical side (maternal facing) and 4-nitrophenyl sulfate across the basolateral side (fetal facing) of the trophoblast cells (167). Biondi et al showed that the MRP1 was responsible for the efflux of c-AMP from the forskolin and prostaglandin E2 (PGE2) stimulated trophoblast cells. To obtain these results MK-571 (MRP1 inhibitor) was used. It was also indicated that estrone and IL-1β stimulated the MRP1 expression and increased the efflux of c-AMP from the trophoblast cells. These studies were performed in the HTR-8/SVneo cells, JAR cells and the placental villous tissue explants (168). However, in these experiments the extracellular c-AMP levels are modified while the intracellular levels of c-AMP were unaltered (168) as opposed to that observed elsewhere (169). Vaidya et al investigated the involvement of the placental ABC transporters in the efflux of DNP-SG in human placental villous tissue and observed the placental efflux was caused by ABC transporters. To evaluate the contribtions of the individual transporters, further studies were performed in Sf9 (Spodoptera frugiperda insect cells) membrane vesicles overexpressing the transporters. The studies suggested that MRP1 was responsible for the efflux of DNP-SG and it was localized apically in the human placental synctiotrophoblasts (34). Further, the same group also characterized the human term placental villous tissue explant model as a tool for studying the ABC transporter mediated efflux of a model substrate DNP-SG and showed that MRP1 expression decreases with culture time in this model (107).
Rahi et al ruled out the possibility of MRP1 transporters being involved in the placental efflux of saquinavir by conducting experiments in the presence of known MRP1 inhibitors MK-571 and probenecid in dually perfused model of human placenta (170). Gedeon et al investigated the potential role of the involvement of MRPs in the fetal to maternal efflux of glyburide in the dually perfused model of human placental cotyledon. Studies were done in the presence of indomethacin, an MRP inhibitor, which suggested that at therapeutic concentrations there is minimal involvement of MRPs in the transport of glyburide across placenta (171).
ABCC2
MRP2 is expressed on the apical syncytiotrophoblast membrane (13) and has an MDR1-like core but containing an additional 5 N-terminal cytoplasmic domains playing a role in membrane targeting (172). In contrast to MDR1/P-gp, MRP2 prefers anionic substrates, which are generally made somewhat more hydrophilic through formation of glucuronide, glutathione, and sulfate conjugates; thus its substrate selectivity is quite similar to MRP1. It is most highly expressed in the canalicular (apical) membrane of hepatocytes, but is also expressed at lower levels in the kidney, intestine, and placenta. Its physiologic role in the liver has been debated but it is important for excreting reduced and oxidized glutathione, glucuronide and sulfated conjugates of steroids and bile acids, as well as other endogenous compounds like bilirubin and leukotrienes. Furthermore, it is known to excrete numerous drugs, toxins, and their conjugates into bile (172). In the intestine, MRP2 may inhibit absorption of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) (173).
Little direct functional data are published to substantiate its impact in human placenta. First, MRP2 expression increases nearly two-fold from early preterm (<32 weeks of gestation) to term (>37 weeks of gestation) in human placenta (35). This suggests a physiologic role in pregnancy, although its exact role is unknown. Notably, steroid hormones such as progesterone and estrogens, as well as their glucuronide conjugates, increase with gestational age; thus MRP2 may be involved in their excretion into maternal blood. St-Pierre et al. reported ATP-dependent uptake of 14C-DNP-SG and 3H-E217G in human term placental apical membrane vesicles (13). May et al. reported that the materno-fetal transfer of talinolol in the human placental perfusion model was sensitive to probenecid, consistent with MRP2 involvement in the transplacental transfer of this substrate (174). Bridges et al examined the disposition of the mercuric ions in the placental and fetal tissues. It was observed the Mrp2 knock out dams displayed greater amounts of fetal and placental mercury compared to the Wistar dams suggesting that Mrp2 may be responsible for the efflux of mercury from the placenta and fetus (175).
Further, in the absence of placental expression of the bile salt export pump (ABCB11) (13), MRP2 may be involved in the excretion of bile salt conjugates synthesized by the maturing fetal liver. Interestingly, Mrp2 expression in rat placenta increases during obstructive cholestasis and furthermore upon treatment with ursodiol (176). Furthermore, Azzaroli et al showed that in pregnant women with intrahepatic cholestasis of pregnancy treatment with ursodeoxycholate MRP2 mRNA and protein expression were increased, while cord blood concentrations of bilirubin and bile salts were decreased, while expression of MRP3 and MRP4 protein did not change (37). Bilirubin and conjugated bile acids are substrates for MRP1 and MRP2, and these transporters may be involved in the transfer of such endogenous compounds synthesized by the fetal liver, across the placental syncytiotrophoblast (123). These data support the role of MRP2 in placental excretion of bile salt conjugates. The mechanisms through which elevated bile salts regulate the placental expression has not been elucidated. However, the nuclear receptors pregnane X receptor (PXR), constitutive androstane receptor (CAR), and farnesoid X receptor regulate the expression of MRP2 in the liver (172).
The expression and activity of UDP-glucuronosyltransferases isoforms 2B4 and 2B7 have been shown (177); these enzymes may protect the fetus by producing glucuronide conjugates, which could be efficiently excreted by MRP2. Zidovudine-glucuronide transport clearance was 12.5-fold greater in the fetal to maternal direction compared to the maternal to fetal direction (178). These data are consistent with the activity of an active transport process at the fetal-maternal blood barrier. Although it is not known which transporter(s) may be involved, MRP2 is positioned in the apical syncytiotrophoblast, while MRP1 and 3 are located in the fetal capillary to transport zidovudine-glucuronide towards maternal blood. In fact, MRP1/3 and MRP2 may work in series to accomplish the secretion of zidovudine-glucuronide into maternal blood.
ABCC3
In the human placenta, MRP3 (gene symbol ABCC3), a 170 kDa protein is predominantly expressed in the blood vessel endothelia and shows minor expression in the apical syncytiotrophoblast as demonstrated by immunofluorescence and immunoblotting studies (13). MRP3 mRNA expression is similar in the human liver and trophoblasts, however the expression in the BeWo, JAR and JEG-3 choriocarcinoma cell lines is very poor (36). Its expression in BeWo cells was up-regulated marginally upon polarization, however no significant difference was observed in mRNA expression in first and third trimester placentae (147).
MRP3 is highly expressed in the large intestine and moderately in the liver in humans, rats and mice. Mrp3 is barely detectable in the mouse placenta (179). In the rat placenta, Mrp3 was predominantly immunolocalized on the epithelium of the endoplacental yolk sac and to a lesser extent in the endothelium of the maternal arteries and the syncytiotrophoblast layers of the labyrinth zone, although localization to the apical side of the syncytiotrophoblast was not confirmed (122). As in the case of MRP1, the preferential subcellular localization of this transporter may suggest a protective role in the efflux of toxic substances that reach the fetus, initially out of the fetal circulation and then out of the syncytiotrophoblast in the feto-maternal direction. Strong expression displayed by the epithelial cells surrounding the yolk sac diverticula may suggest that Mrp3-mediated efflux into the yolk sac is an excretory pathway in the rat placenta. Furthermore, Mrp3 expression in maternal endothelial arteries may be associated with an additional role for Mrp3 in either limiting or enhancing the distribution of xenobiotics from the fetal compartment to the maternal circulation. Also, the fetal expression of Mrp3 mRNA in the rat placenta exceeded that in the fetal liver till 20 days of gestation reflecting that Mrp3-mediated placental efflux of substrates may be important in early stages of gestation when the fetal liver shows poor expression (122). In the rat liver, Mrp3 is expressed on the sinusoidal side of the hepatocytes and plays a functional role in the efflux of substrates such as organic anions. It is up-regulated along with Mrp1 when Mrp2 is down-regulated (180). There is some evidence indicating that Mrp3 expression in the rat liver may be dependent on CAR-mediated pathway (181). Mrp3 is involved in the efflux of several organic anions including sulfates and glucuronide conjugates such as 17β estradiol 17-β-D-glucuronide (Km = 33.4 μM) as well as methotrexate. It also transports bile salts such as glycocholate and taurocholate (Km = 15.9 μM) as well as their sulfate conjugates such as taurochenodeoxycholate 3-sulfate and taurolithocholate 3-sulfate (Km = 3.1 μM) (182–184). MRP3 substrate specificity resembles that of Mrp3; however, taurocholate is not a substrate for MRP3 (185). As opposed to MRP1 or MRP2, glutathione conjugates are poor substrates of MRP3 (184). Like Mrp1 and Mrp2, Mrp3 expression is up-regulated during maternal cholestasis and upon treatment with ursodeoxycholic acid (UDCA), suggesting a defensive role for Mrp3 in limiting fetal exposure to elevated levels of maternal bile acids (176). In another study, in placentae from patients with intrahepatic cholestasis of pregnancy, MRP3 mRNA expression was increased by treatment with ursodeoxycholate, but protein expression was unchanged (37). Additionally, MRP3 may also be involved in limiting exposure to anti-cancer agents such as etoposide, teniposide and methotrexate (186) as well as anti-diabetic agents such as glyburide (187). In summary, MRP3 appears to have a protective role in the human placenta and might be involved in the feto-maternal efflux of substrates from the fetal endothelial capillaries in addition to some efflux activity at the apical syncytiotrophoblast.
ABCC4
MRP4 (gene product ABCC4) was detected at the mRNA level in the placenta of mice (179) and rats (188) but at low levels compared to the kidney, where it has its highest expression and localizes to the apical membrane of proximal tubule cells (189). In human liver, it resides at the basolateral surface of hepatocytes where it mediates the efflux of glutathione into sinusoidal blood with co-transport of monoanionic bile salts (190). In contrast, MRP4 is localized to the apical membrane of the proximal tubule in the kidney (189). The expanded role for MRP4 now includes cyclic nucleotides, urate (191), steroid conjugates (192), prostaglandins (193), and nucleoside analogs such as adefovir and tenofovir (194).
MRP4 has been detected in apical (but not basolateral) membranes of human term placental syncytiotrophoblasts (37). In this study, the expression of MRP4 protein was not different in placentae from women with or without intrahepatic cholestasis of pregnancy (ICP), or in ICP patients treated with ursodiol (ursodeoxycholic acid) (37). MRP4 mRNA levels in BeWo, JAR, and JEG-3 cells tended to be higher than in primary human placental trophoblasts (36). Overall, the impact of MRP4 in the human placenta needs further investigation.
ABCC5
MRP5 (gene symbol ABCC5) is a 190 kDa protein and its expression at the mRNA and protein levels has been reported in the mouse (195) and human placenta (147,196). Immunofluorescence and immunoblotting studies indicated predominant localization at the basal side of the syncytiotrophoblast, though minor expression on the apical side was also reported (38). In addition to the syncytiotrophoblast, it is also localized in and around the fetal capillary vessels (38). MRP5 expression decreases with increase in gestational age (38). MRP5 mRNA expression was six fold higher in preterm placentae as compared to term placentae, and showed a good correlation with the protein expression in pre-term and term placentae. Nonetheless, confounding factors such as the cause of preterm delivery should be taken into account while interpreting such data. Its expression in isolated trophoblast cells increased in parallel with human chorionic gonadotropin production indicating that MRP5 expression is influenced by the cellular differentiation process (38). Similarly, MRP5 mRNA expression in BeWo cells was 1500-fold higher in polarized cells as compared to non-polarized cells (147). MRP5 mRNA has also been detected in mouse placenta (179). Recently, Shuster et al studied the gestational age-dependent changes in the gene expression of ABC transporters in pregnant mice and found that Abcc5 gene expression in the placenta decreased during pregnancy (197).
MRP5 is involved in the ATP-dependent transport of cyclic nucleotide analogs such as 3′,5′-cyclic nucleotides, cAMP (Km = 379 μM) and cGMP (Km = 2.1 μM) (198,199). ATP-dependent transport of 3H-cGMP was detected in isolated human placental basolateral membrane vesicles, indicating a functional role for MRP5 as an export pump for cyclic nucleotides, particularly cGMP (38). cGMP plays an important role in cytotrophoblast differentiation (200), nitric oxide mediated placental angiogenesis (201) and maintenance of fetoplacental vascular tone by reducing fetal blood pressure (202). MRP5 may thus be important for regulating intracellular levels of cyclic nucleotides that act as secondary messengers or for providing extracellular nucleotides for paracrine signaling. Besides cGMP-mediated signal transduction, MRP5 may also play a role in the export of organic anions, and nucleoside monophosphates of nucleoside analogs used in anticancer and antiviral therapy (203).
ABCC6 (MRP6)
Mrp6 (gene ABCC6) is a novel member of Mrp family. ABCC6 is highly homologous to several other representatives of the multidrug resistance protein family able to transport various organic anionic conjugates in vitro, however, no physiological substrate or function could be initially assigned to it (204). Using identified Mrp6 transporter substrates, anionic cyclopentapeptides and endothelin receptor antagonist BQ-123 (cyclic pentapeptide), Mrp6 was found to be expressed in rat hepatocytes (205). In a study to assess mRNA levels of various transporters in rat placenta and comparing the levels with maternal liver and kidney, it was found that Mrp6 mRNA levels were highest in liver and kidney and lowest in placenta (188). In a separate study, Mrp6 mRNA was barely detectable in the placenta (i.e., less than 0.1% of the adult levels in the liver) (122). Similarly, mRNA expression of Mrp6 was found to be barely detectable in mice (179,195). Mrp6 protein expression in the human placenta has not been reported.
ABCC10, ABCC11 and ABCC12
ABCC10 has been reported to be expressed in the human placenta in earlier reports (31,206,207). However, not much is known about its functional significance.
MRP8 and MRP9 (gene products ABCC11 and ABCC12, respectively) are the most recent members of the MRP family to be cloned (208,209). In both of these early investigations, a panel of tissues was examined for expression of MRP8 and MRP9 mRNAs, which showed that the placenta does express MRP8 but not MRP9 (208,209). Bera et al, has also identified MRP8 by EST database mining and gene prediction program and showed that it is expressed at very low levels in the human placenta (210). Like MRP4, MRP8 mRNA levels in BeWo, JAR, and JEG-3 cells tended to be higher than in primary human placental trophoblasts (36). The substrate list of MRP8 is extensive and as diverse as that of MRP1, MRP2, MRP3 and MRP5. Energy-dependent efflux of cyclic nucleotides (cAMP and cGMP) is mediated by MRP8 (211). MRP8 shares several endogenous glutathione-, glucuronide- and sulfate-conjugated substrates with MRP1 and MRP2: cysteinyl leukotriene C4, estradiol-17β-glucuronide and estrone-3-sulfate and dehydroepiandrosterone sulfate (212). Folic acid and the antifolate methotrexate are additional shared substrates. The monoanionic bile salts glycocholate and taurocholate have also been identified as substrates of MRP8 (212).
The assignment of a role to MRP8 in placental physiology remains highly speculative, without information that compares the level of MRP8 mRNA transcripts with that of other MRPs in the placenta and without confirmation that the protein is detectable. Nevertheless, it is interesting to note that MRP8 sorted to the apical membrane in transfected, polarized Madin-Darby canine kidney (MDCK) cells and to the axonal domain of neurons (213). Assuming that MRP8 is expressed in the placenta it would likewise be localized to the apical domain of the syncytiotrophoblast, then this transporter could contribute to the ATP-dependent bile acid transport system previously identified in isolated membrane vesicles (214). The identity of the transporter(s) responsible for the export of fetal bile acids to the maternal circulation role has remained elusive until now. The liver BSEP has always been an unlikely candidate because its affinity for monoionic bile salts is considerably higher than that reported in placental vesicles (176,215,216), and because expression of BSEP has only been detected at the mRNA level in human (117) and rat placenta (122) but not at the protein level. Recently, Vinot et al showed an inverse correlation between placental transfer of maraviroc, and the placental expression levels of ABCC10 and ABCC11 suggesting that these transporters could be involved in its limited fetal exposure (217).
ABCG Subfamily
ABCG2
Introduction
A third protein sub-family of the ABC superfamily which has been identified in placenta is breast cancer resistant protein (BCRP). Western blotting showed expression of BCRP in the BeWo cell line and indirect immunofluorescence microscopy indicates BCRP is localized at the apical membrane (218). Additional cellular uptake studies in the presence of known BCRP inhibitors such as GF120918, Ko143 and PSC833 suggest functional expression (218). Due to the very high levels of BCRP expression seen by Ceckova et al, it was suggested that BCRP may play a predominant role in the efflux of xenobiotics and may even be the predominant ABC transporter of the placenta. Hormones can play a role in the modulation of BCRP much in the same way as seen with MDR1. For example, 17 β-estradiol has been shown to reverse BCRP mediated drug resistance (219). This transporter is highly expressed in the placenta and the liver, but is also found in the intestine, lung, and kidney. Like MRP2, it can also transport some glucuronide conjugates as well as some unconjugated anionic and cationic compounds, but it appears to prefer sulfate conjugates (220). Unlike MDR1 and MRP2, its monomeric structure has only six transmembrane domains and one ATP-binding cassette, but it functions as a homotetramer (221). Its normal role may be to transport sulfated steroid precursors such as dehydroepiandrosterone-3-sulfate out of cells where they are produced, as in the placenta (220,222).
Although ABCG2 is highly expressed in the human placenta, there is a relative paucity of functional data. Inhibition of this homotetrameric transporter by GF120918 has been shown to double the fetus/maternal plasma ratio of topotecan in mice, indicating its functional impact in the mouse placenta (223). Furthermore, ABCG2 functional activity has been determined in microvillous membrane vesicles from human placenta. In this model, ATP-dependent transport of mitoxantrone was demonstrated, and the activity was inhibited by the ABCG2 inhibitor fumitremorgin C (224). Additionally, the high level of sulfotransferase (SULT) 2B1 mRNA in human placenta (222) suggests a role for ABCG2 in the excretion of sulfate conjugates formed by this enzyme.
Structure and Homology
The mouse Bcrp1 and BCRP amino-acid sequences are 81% identical and 86% similar, with very high homology in the ATP-binding region and a superimposable hydrophobicity profile. Both human and mouse analogs of the protein have six transmembrane domains (TMD) and one nucleotide binding domain (NBD). Two cytosolic and two extracellular (between the fifth and sixth TMD) N-glycosylation sites have been reported for Bcrp1. Only one of the extracellular N-glycosylation sites is conserved in the human BCRP sequence (225). The general structure of ABC transporters comprises 12 TMD regions split in two similar halves, each with a NBD and 6 TMDs regions. Since BCRP has six TMD regions and a single NBD it is often termed as a half transporter. Some studies suggest that human BCRP may function as a homodimer (226,227) or a homotetramer (221). Whether or not it can function as a heterodimer with other ABC transporters is not known.
Expression
BCRP (gene symbol ABCG2), a 70 kDa protein is very highly expressed on the apical (maternal facing) side of the human placental syncytiotrophoblast (Fig. 1), and is expressed at lower levels in the liver canalicular membrane and the apical membrane of the epithelium in the small intestine (39,40,228). In the human placenta, its expression is highly variable (26) and does demonstrate gestational age dependency where protein expression increases with advancing pregnancy with unaltered mRNA expression (229). Vaidya et al characterized the human term placental villous tissue explant model as a tool for studying the ABC transporter mediated efflux of a model substrate DNP-SG and showed that BCRP expression remains unchanged with time in culture in this model (107). The murine homolog of BCRP (Bcrp1) is highly expressed in the kidney, moderately expressed in the liver and shows low expression in the placenta at the mRNA level (223,230). Cygalova et al showed that in rat placenta the Bcrp mRNA expression decreases from mid-stage to the end of gestation (231) which was consistent with that reported by others (232). Conversely the expression of Bcrp in rat fetal body tissue increased as pregnancy progresses (231). Although Bcrp mRNA levels were low towards the end of pregnancy, pronounced efflux activity of Bcrp in the ex vivo model of perfused term rat placenta was observed (233), suggesting that the protective function of the placental Bcrp was further strengthened by fetal Bcrp as the pregnancy progressed (231). This finding is also supported by another set of experiments by the same group using cimetidine as a model Bcrp substrate in rats (231). Memon et al studied the regional localization of ABCG2/BCRP transporter in human term placenta and found that the BCRP mRNA and protein levels are similar across the placenta and the sampling location does not cause variations in its expression (234).
Ridano et al showed that the organophosphate pesticide chlorpyrifos increased the expression of ABCG2 without affecting the cell viability and morphology in the human placental JEG-3 cells (235). Kummu et al studied the effect of selected heavy metals on the expression and function of ABCG2 in the BeWo cells. While cadmium did not inhibit the mRNA or protein expression of ABCG2, it inhibited the function of ABCG2. This was evident from the finding that the efflux of mitoxantrone (a known BCRP substrate) and PhIP were inhibited in presence of cadmium (236). Feinshtein et al investigated the effect of short-term exposure (1–2 h) of cannabidiol on the function of BCRP. Cannabidiol inhibited BCRP hence reducing the efflux of mitoxantrone in a concentration dependent manner in BeWo, JAR, and MCF7/P-gp cells. Short-term exposure of cannabidiol also led to an increased fetal to maternal ratio of glyburide (another BCRP substrate) in the ex vivo placental perfusion model (237).
Myllynen et al showed that the placental BCRP can restrict the fetal exposure to harmful foreign compounds including food borne chemical carcinogen PhIP in the perfused human placental model. These studies were done in presence of BCRP inhibitor KO143 and the placental transfer correlated well with the BCRP expression levels in the placenta. While the fetal transfer of PhIP increased in presence of ABCG2 inhibitor it had no effect in the presence of ABCC1/ABCC2 inhibitor probenecid. In addition, in vitro studies with the BeWo cells suggested that PhIP also affects the expression levels of some ABC transporters at the mRNA level. A higher concentration (10 μM) of PhIP decreased expression of BCRP while the mRNA levels were upregulated both at lower (1 μM) and higher concentrations (10 μM) (238). Ursodiol (ursodeoxycholic acid, the drug of choice for intrahepatic cholestasis in pregnancy) was shown to cause an increase in the expression of BCRP at the mRNA and protein level thus causing an efflux of bile acids from the fetal side (239). Recently, Jebbink et al studied the relation between placental transport of bile acids and ABCG2 expression in preeclamptic pregnancies complicated by hemolysis elevated liver enzymes and low platelets and found that the lower levels of bile acids in the fetal compartment compared to maternal levels is not related with the placental ABCG2 expression levels (240). Saito et al reported that there is an association between methylation levels of microRNA 328, a non-coding small RNA known to influence BCRP expression in the cancer cells, and BCRP expression levels and the methylation levels of microRNA 328 are involved in the inter-individual differences in the BCRP expression levels in the placenta (241).
Substrate Specificity, Biochemical Function and Polymorphism
BCRP functions as a high-capacity drug transporter with wide substrate specificity, with some overlap with both P-gp and MRP substrates. It can transport hydrophobic cationic or anionic compounds including cytotoxic compounds (mitoxantrone, topotecan, flavopiridol, methotrexate), fluorescent dyes (rhodamine 123, BODIPY-prazosin) (40,242) and dipyridamole (243) as well as hydrophilic sulfate, glucuronide or glutathione conjugates such as estrone-3-sulfate (E1S) and E217G (242). Among the sulfate conjugates, BCRP affinity for 4-methylumbelliferone sulfate > E1S > 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole (E3040) sulfate (Km range: 12.9–26.9 μM), in BCRP-transfected mouse lymphoma P388 cell membrane vesicles (220). ATP-dependent transport of glucuronide and glutathione conjugates, including DNP-SG, was also detected, but was much less compared to that of the sulfate conjugates (220). Substrate specificity characterization for the mouse Bcrp1 has not been reported, but is expected to be very similar to that of human BCRP due to the structural similarities between BCRP and Bcrp1 as described above.
As established for BCRP-expressing transfected cell lines, Bcrp1 expressing cell lines also exhibit cross-resistance to topotecan, mitoxantrone and doxorubicin (40,228,244) and show reduced cellular accumulation and increased ATP-dependent efflux of mitoxantrone (225). Both BCRP and Bcrp1 show major differences in substrate specificities due to a R482G mutation (245,246). Presence of arginine at the 482 position leads to acquisition of resistance against methotrexate.
Zhou et al. showed that Bcrp1 significantly limits fetal distribution of glyburide in pregnant mice and has a minimal effect on the systemic clearance of the drug (247). Moreno-Sanz et al showed that URB937, a fatty acid amide hydrolase inhibitor, behaves as a substrate for the placental Bcrp efflux transporter in both mice and rats. In addition, the higher efflux in mice is correlated well with the higher expression of Abcg2 mRNA levels in mice compared to rats (248). In pregnant P-gp-deficient mice treated with the Bcrp and P-gp inhibitor GF120918, the fetal concentration of Bcrp substrate topotecan was found to be 2-fold higher than that in the control group, indicating that Bcrp1 has a role in the apical efflux from the placenta, and may protect the fetus from xenobiotic exposure (223). The disposition of the Bcrp substrate genistein was studied in wild-type and Bcrp1(-/-) pregnant mice. Bcrp1 was found to limit fetal distribution genistein in the pregnant mouse (249). Blazquez et al studied Abcg2-mediated bile acid transport in the rat placenta. In vivo studies in rats showed that glycocholic acid when given with fumitremorgin C, the Abcg2 inhibitor, caused a marked reduction in the placental transfer of glycocholic acid. Furthermore, in obstructive cholestasis (while the maternal serum bile levels increased several fold), there was a modest increase in the fetal bile levels suggesting a possible role of placental Abcg2. In addition, following intravenous administration in bile duct ligated Abcg2 knock out mice, the fetal serum, liver and placental bile acid levels were markedly higher suggesting a role of Abcg2 in maternally-directed placental transport of bile acids (250).
In dually perfused rat placental studies, the feto-maternal clearance of cimetidine was 25 times higher than the materno-fetal clearance, and was partly inhibited by Bcrp inhibitors fumitremorgin C and GF120918, suggesting a defensive role for Bcrp in the rat placenta (233). Hofman et al investigated the effect of purine cyclic dependent kinase inhibitors on placental Abcg2 in the dually perfused rat term placenta. Olomoucine II and purvalanol A caused significant inhibition of placental Abcg2. The fetal to maternal ratio in presence of the inhibitors was 3-fold higher compared to the control (no inhibitors). While roscovitine and bohemine were able to inhibit Abcg2 more efficiently than the model inhibitor furmitremorgin C, olomoucine showed negligible inhibition of rat placental Abcg2 (251).
Kolwankar et al. reported ATP-dependent uptake of mitoxantrone in human term placental apical plasma membrane vesicles that was inhibited by 65% in the presence of the BCRP inhibitor fumitremorgin C (224). GF120918 sensitive mitoxantrone uptake has also been reported in BeWo cell lines and transfected MDCKII-BCRP cells (218). Zhou et al showed that glyburide, a drug intended for gestational diabetes, is a substrate for mouse Bcrp1 in in vitro uptake and transport experiments using transfected MDCK cells (247). Feinshtein et al investigated the involvement of ABC placental transporters in the transport of nitrofurantoin, an antibacterial agent, in the placental choriocarcinoma JAR cells. While BCRP was responsible for the efflux of nitrofurantoin, P-gp and the MRPs were not involved (252). Similar results regarding involvement of Bcrp were shown in wild type and Bcrp1(-/-) pregnant mice elsewhere (253). Gedeon et al utilized 70% right side out vesicles obtained from the brush border membrane (maternal facing membrane) of the human placentae and showed that there was a significant increase in the intra-vesicular concentration of glyburide in the presence of the BCRP inhibitor novobiocin compared to the control vesicles. This suggested that glyburide was a substrate for BCRP and is responsible for the efflux in the fetal to maternal direction (254). In a separate set of in vitro experiments, Hofman et al studied the combination of either olomoucine II or puvalanol A with mitoxantrone (ABCG2/Abcg2 substrate) for antiproliferative activity in different placental cell lines. In the BeWo cells the combination of either purine inhibitor with methotrexate (MTX) fell into the additive category, while in the HRP-1 cells and rat trophoblast cells, the combination of olomoucine II with MTX was additive but that of purvalanol with MTX was synergistic against proliferation (251).
The protective role of BCRP in the placenta has been a topic of intense research investigation in recent years, however, there is limited evidence of functional activity of BCRP in the human placenta (12). Pollex et al investigated the potential role of BCRP in the placental efflux of glyburide using dual placental perfusion model. In the presence of the BCRP inhibitor nicardipine, the fetal to maternal transfer of glyburide showed a significant increase as opposed to that in the absence of the inhibitor. While this finding suggested that BCRP was responsible for the efflux of glyburide, the efflux was not completely inhibited by nicardipine suggesting that there could be other efflux transporters (255).
Recently, Kumar et al developed a bioluminescent pregnant mouse model to demonstrate the Abcg2 transporter function at the blood placental barrier. It was shown that following co-administration of the Abcg2 inhibitors KO143 and gefitinib with the Abcg2 substrate D-luciferin, the bioluminescent signal from the placenta and fetus increased. However, the mice treated with the Pgp inhibitor DCPQ showed unchanged bioluminescence. Hence, this study demonstrated the efflux activity of Abcg2 at the blood placental barrier in mice by bioluminescent imaging (256).
Polymorphism in BCRP has been reviewed earlier (74,80). Polymorphisms that occur at a higher frequency in BCRPs are G34A and C421A. Kobayashi et al. conducted a study in a Japanese cohort to investigate the effect of polymorphism on BCRP expression. Twenty polymorphisms were detected and with some polymorphisms there was an evidence of alteration of BCRP expression (257). Pollex et al also investigated the effect of single nucleotide polymorphism (SNP) on BCRP mediated efflux of glyburide in transfected human embryonic kidney cells. The apparent Kt and Vmax values of these transfected cells expressing the polymorphic variant Q141K of the BCRP transporter were significantly higher compared to the wild type. This suggested that the Q141K SNP variant could alter the placental pharmacokinetics of glyburide and hence could have clinical significance in individuals carrying this variant (258). Wang et al studied the association between BCRP polymorphism in children and isolated septal defects in a Han Chinese population. The results suggested that the ABCG2 mRNA and protein expression levels did not alter among the different genotypes of 421C > A polymorphism. The ABCG2 mRNA and protein expression were also significantly different for the GG genotype as compared to the AA phenotype of the 34G > A polymorphism. It was concluded that 34G > A polymorphism in children causes septal defects in the Han Chinese population probably through regulation of BCRP levels in placenta (259).
BCRP is certainly one of the most important ABC transporter in the placenta. It is expressed in a variety of vital organs in body and transports a variety of structurally diverse xenobiotics. BCRP also exhibits polymorphisms which could alter its expression and the pharmacokinetics of the BCRP substrates. While BCRP is known to restrict the entry of several xenobiotics into the placenta, various xenobiotics can alter the BCRP expression in the placenta. BCRP appears to have a protective function in the placenta and several studies are currently underway.
ABCG5/8
ABCG5 and ABCG8 (termed sterolin-1 and sterolin-2, respectively) are indispensable for the regulation of dietary-sterol (plant sterols) absorption and selective sterol excretion by the liver into bile. Mutations of the ABCG5 and ABCG8, cause sitosterolemia (260,261). In a study to assess the mRNA levels of various transporters in rat placenta, placenta sterolin transporter mRNA levels were assessed and compared with maternal liver and kidney levels. Abcg5 mRNA levels were highest in the liver. Placental levels were approximately two times higher than those in kidney and liver. Higher Abcg8 expression levels were found in placenta, comparable with that in both liver and kidney. This presence suggests a role for the sterolin transporters in the elimination of sterols, particularly plant sterols, within the placenta (188).
Hormones, Cytokines and Placental ABC Transporters
Hormones, Prostaglandins and Nuclear Receptors
Due to the fluctuating hormone levels experienced during pregnancy, steroidal modification of MDR1 expression at the placenta is an area of interest. For example, it has been shown that the hormone progesterone, which has varying levels during pregnancy, can have both inhibitory effects (above 10 μM) and stimulatory effects (below 1 μM) on MDR1 (262). The method of progesterone’s modulation is believed to be through an allosteric binding site (262). Studies in other cell lines also showed that progesterone’s interaction with MDR1 may be sensitive to stereochemistry which may also be the case in placental cell lines. Namely, metabolites in the 5β-metabolic pathway of progesterone were determined to be more potent inhibitors than metabolites of the 5α-metabolic pathway of progesterone (263). Coles et al investigated the effect of progesterone and estradiol on P-gp protein levels and function in estrogen receptor (ER) α positive JAR cells. While progesterone and β-estradiol caused an induction in the levels of P-gp in NCI-ADR-RES cells, induction caused by estradiol in JAR cells (ERα expressing) was at very low concentrations (more sensitive) as opposed to the NCI-ADR-RES cells (ERα non-expressing). In addition, both cell lines also showed a decrease in the uptake of P-gp substrate. As the induction was abolished in the presence of a transcriptional inhibitor, it was proposed that transcriptional regulation could be a cause for the induction of P-gp by β-estradiol or progesterone (264). Additionally, tamoxifen (an ER antagonist) reverses MDR1 mediated drug resistance in vitro (265) and it is possible that environmental estrogens could have similar effects. Jin and Audus investigated the effects of bisphenol A (BPA), a plastic component known to have estrogenic effects, on MDR1 expression in BeWo cells (266). They found that acute treatment with BPA had a direct stimulatory effect on MDR1 as evidenced by decreased calcein accumulation in BeWo cells. Chronic exposure however had no effect on calcein accumulation, suggesting that BPA does not alter MDR1 expression; they confirmed this with immunoblots (266). The role of estrogen and other hormones in MDR1 modulation however remains unclear and is still an area of active research.
Wang et al showed that the BCRP expression is induced at the transcriptional level in BeWo cells by progesterone through the progesterone receptor (PR) isoform PRB and not PRA (41). Further it was also shown that PRA represses the activity of PRB (41). Oda et al examined the induction of Abcg2 by its substrate mitoxantrone in the TRT-TBT 18d-1 rat placental trophoblast cells. In this study it was shown that the expression of Abcg2 was induced by exposure to mitoxantrone (more than 1 μM) through increased expression of ER α in the TR-TBT 18d-1 cells. In other words, ERα induction by mitoxantrone increased the Abcg2 expression in these cells (43). Sieppi et al studied the effects of xenoestrogens on ABCG2 expression in human placental explants (267). BPA caused a decrease in the ABCG2 mRNA expression after 24 h in term placenta explants. However, it did not have an effect at 48 h and neither in the first trimester explants. Similarly, E2 did not have an effect on mRNA expression regardless of the time or the stage of pregnancy. Furthermore, while E2 caused a decrease in the ABCG2 protein expression in term placental explants at 24 and 48 h, BPA caused a decrease only at lower concentrations (1 nM) and not at (100 nM), 48 h after exposure. No effects were observed on protein expression for either compound in first trimester explants. Para-nonylphenol caused a decrease in ABCG2 protein expression at 48 h in term placenta. Studies with the ER antagonist fulvestrant suggested the involvement of ER in the actions of BPA, E2 and para-nonylphenol (267).
Manceau et al investigated the influence of spontaneous syncytialization and induction by glucocorticoids on the ABC drug transporter expression in primary human cytotrophoblast cells. While there are conflicting evidences elsewhere (32,35,38) synctialization caused a spontaneous increase in the expression of ABCB1, ABCC2 and ABCC5 between 24 and 48 h incubation times. Dexamethasone and betamethasone caused an induction of ABCB1 gene expression in human cytotrophoblast cells whereas prednisone did not. It was also suggested that structural similarity between dexamethasone and betamethasone may be responsible for these effects (31). Petropoulos et al performed studies to investigate the effect of glucocorticoids on regulation of placental Abcg2 mRNA and Bcrp1 protein expression and function in mouse. Following treatments with high doses of dexamethasone (1 mg/kg), the Abcg2 mRNA expression was significantly down regulated and Bcrp1 function was significantly inhibited in the placentae derived from female fetuses. However, in the placentae derived from male fetuses, only the Bcrp function was significantly inhibited. Hence, these studies showed that there is a dose dependent regulatory effect of synthetic glucocorticoids on the mouse placental Abcg2 mRNA and Bcrp1 activity. Furthermore, at the gene level the synthetic glucocorticoids had a greater effect on female-derived placentae rather than male-derived. Nonetheless it is worthy to note that the levels of Bcrp1 protein expression remain unchanged (44). Petropoulos et al also studied the effect of glucocorticoids on regulation of P-gp in mice. Following treatment of dexamethasone from embryonic day (E) 12.5 to 18.5, the Abcb1a mRNA expression (at 1 mg/kg dose) and the P-gp protein expression (at 0.1 and 1 mg/kg and only on E 18.5) was significantly up regulated. Further high doses of dexamethasone from E 9.5 to 15.5 caused significantly increased levels of placental Abcb1a mRNA. However, it is worth noting that these changes do not appear to translate into alteration of P-gp activity and P-gp mediated drug transfer (268). These findings are in contrast with those of Mark et al where Abcb1a mRNA expression was down regulated following treatment of dexamethasone in rats (269), although the functional significance of this finding is unclear. Kalabis et al found that pregnant guinea pigs treated with betamethasone at 40/41 and 50/51 days of gestation have decreased expression of P-gp at the mRNA and protein levels (270). Salje et al observed that St. John’s wort and thyroxine can significantly induce the placental expression of Abcb1 in Wistar pregnant rats (271). In addition, dexamethasone induced Abcb1 expression in the fetal brain and the liver whereas thyroxine induced expression of Abcb1 in the fetal brain. Rifampin also induced Abcb1 expression in the placenta and the fetal organs however it did not translate into higher P-gp activity because of a concomitant inhibitory effect by rifampin (271).
Wang et al showed that estriol, human placental lactogen and human prolactin increased the expression of BCRP at the mRNA and protein level in BeWo cells. While testosterone and human chorionic gonadotrophin by itself did not affect BCRP expression, testosterone in conjugation with 17-β estradiol increased BCRP mRNA and protein expression through ERβ mediated induction of testosterone receptor (45). Yasuda et al studied the response of ABCG2 promoter to the treatment of sex hormones in BeWo cells (42). While earlier it has been reported that the expression level of ABCG2 in BeWo cells is regulated by treatment with sex hormones (272), later it was reported that estradiol exposure for 72 h had no effect on the response of the ABCG2 promoter in the BeWo cells. This discrepancy was attributed to insufficient function of ABCG2 promoter in the batch used for regulation of ABCG2. On the contrary, consistent with earlier findings, treatment with progesterone for 72 h significantly suppressed the response of ABCG2 promoter. Furthermore it was shown that this effect was achieved by progesterone by changing expression levels of PR-A and PR-B (42). Ietta et al showed that 17-β estradiol modulates the macrophage migration inhibitory factor in a dose dependent manner by acting on the placental tissue and down regulating the mRNA and protein expression of ABCA1 in the human first trimester chorionic villous explants (273).
Evseenko et al, showed that, on treatment with growth factors and steroid hormones, BCRP and MDR3 mRNA (or protein) expression increased. Although growth factors and steroids had no observable effect on MDR1 and MRP1 expressions after 12-h exposure, interestingly, MDR1 expression levels in estradiol were increased after 48-h of exposure (29). Hence, it is quite evident that steroids and the steroid receptors could affect the expression (and function in most cases) of placental ABC transporters mostly P-gp and BCRP. In addition, hormones such as placental lactogen, prolactin and certain proinflammatory agents also alter the expression of various ABC transporters.
Anger et al studied the placental ABC transporter expression in insulin managed diabetes patients. No significant changes were observed in the protein expression of MDR1, MRP2 and BCRP between the insulin managed gestational and type 1 diabetes mellitus patients and the controls. However, there was a significant positive correlation between the HbA1c and the BCRP mRNA and protein levels in both the insulin managed diabetics which suggested that the placental ABC transporter expression could be altered in the case of poorly managed diabetes (274). Mason et al studied the expression of ABC transporters in different conditions of pregnancy. While the placental expression of women in labor and women with preeclampsia was unaltered, MDR1 and BCRP protein and mRNA expression in placentae of women with labor and preterm inflammation was increased (275).
Mason et al studied the effect of prostaglandin E2 (PGE2) on the expression of multidrug resistant transporters in vitro in the human placental cells. The treatment of placental cells with PGE2 caused an upregulation in BCRP expression whereas blocking of the E-prostanoid (EP) 1 and 3 receptors caused an attenuation of the increase in BCRP expression. While PGE2 decreased P-gp mRNA, it did not cause a significant alteration in the P-gp expression and function. Overall the results suggested that PGE2 caused an upregulation of BCRP expression through the EP1 and 3 receptors (276).
Gahir et al examined the effect of PXR on the expression of the ABC transporters in placenta. It was observed that the levels of the major ABC transporters (namely Mdr1a, Bcrp, Mrp1, 2 and 3) in PXR knock out mice were higher as compared to the wild type mice with fully functional PXR. This increase of expression was observed from mid-gestation to term in the case of pregnant rats. These finding suggest a repressive role of PXR in the expression of the placental transporters. Following PXR activation by PCN, no significant change was observed in the expression of ABC transporters in the placenta unlike liver where a 30-fold increase was observed. This suggested a tissue specific role of PXR with respect to regulation of the ABC transporters (28).
It is worthy to note that the expression of PXR and CAR was not detected at the mRNA level or was found to be very low in human primary cytotrophoblast cells and in the human placental tissue (277). In other in vitro models such as the Bewo and the JEG cells, mRNA expression of PXR and CAR was not observed. However, transcripts of Pxr and Car have been reported in placenta of other species such as the mouse. Nonetheless activation of Pxr does not produce induction of placental ABC transporters (277).
Cytokines and Wnt/β-catenin Pathway
Studies on the effects of cytokines on the transcription regulation of placental ABC transporters (using primary trophoblast cells in monolayer culture) have been shown (29). Treatment with cytokines showed a reduction and/or inhibition of mRNA and protein expression of MDR1 and BCRP by tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β. Although interleukin (IL)-6 had little effect on the expression of MDR1 and BCRP, it was found to increase expression levels of MDR3 mRNA and protein under the same conditions. Tumor necrosis factor-α and IL-1β had little effect on the expression of MDR3 mRNA or protein. MRP1 mRNA was shown to have increased expression levels by TNF-α, IL-1β, and IL-6. It was also suggested by Mason et al that the pro-inflammatory cytokines particularly interlukin-8, may have a possible role in the increase of the transporter expression (275). In primary trophoblasts, the expression of MRP1 is induced by inflammatory cytokines TNFα, IL1β, and IL6 (29). The induction of MRP1 as well as its substrate selectivity are consistent with its putative role in mitigating toxicity and oxidative stress in the trophoblast under adverse conditions (278). Petrovic et al. studied the impact of inflammation induced by polyinosinic/polycytidylic acid (poly(I:C)) on the placental drug transporters in pregnant rats. Inflammation caused a significant down regulation of placental Abcb1a/b, Abcc1, Abcc3 and Abcg2 mRNA. It was suggested that the poly(I:C) mediated induction of TNF-α, IFN-α and IL-6 might be involved in the decreasing levels of these placental transporters. Further, significant changes in the protein expression of P-gp and Bcrp were not observed in these rats. Hence, the biological significance of the changes in the levels of mRNA expression remains to be understood (30).
In addition, Mikheev et al. through microarray studies demonstrated that there is a significant downregulation of the canonical Wnt pathway and inhibition of β-catenin protein expression depending on the gestational age (279). These changes in the signal transduction pathways might regulate placental transporter activity through transcriptional effects as well as through indirect effects on cell proliferation, polarization and differentiation. Other related studies in cancer cells have demonstrated downregulation of P-gp expression through inhibition or interference with the Wnt/b-catenin pathway (280,281).
The regulation of expression of ABCA1 is under investigation. Reported transcription factors that might regulate expression of ABCA1 are liver X receptor (LXR), peroxisome proliferator-activator receptor γ (PPARγ), sterol regulatory binding protein, inflammatory mediators (endotoxin and cytokines), SCAN-domain containing zinc finger protein, and coregulators (48,282–285).
Recent Findings from Studies Involving Multiple ABC Transporters
Hemauer et al (286) investigated the role of placental ABC transporters in the efflux of drugs used for gestational diabetes namely glyburide, rosiglitazone and metformin in inside-out brush border placental membrane vesicles. While P-gp contributed to a small fraction of glyburide efflux, BCRP and MRP1 were responsible for a greater fraction. P-gp and BCRP were involved in the placental efflux of metformin. Rosiglitazone, metformin, and the protypical P-gp substrate paclitaxel were also effluxed by P-gp. While the rate of rosiglitazone transport by P-gp was significantly lower than that of metformin, both the drugs had similar affinity for P-gp (286). Hemauer et al. (287) also investigated the placental disposition of buproprion, an antidepressant used for smoking cessation and its hydroxyl metabolite, in inside out placental vesicles. It was indicated that both P-gp and BCRP work in parallel to eliminate buproprion from the placenta with P-gp acting at lower buproprion concentrations due to its high affinity for buproprion while BCRP working at high concentration due to its higher total transport capacity for buproprion. On the contrary, ABC transporters were not involved in the placental elimination of its hydroxyl metabolite (287).
Prouillac et al investigated the effect of zearalenone, a non-steroidal estrogenic mycotoxin and a food contaminant, on the ABC transporter expression in the BeWo cells. Zearalenone caused a significant increase in MRP1 protein expression after treatment with 10 μM of zearalenone. A non-significant increase in the expression of BCRP and MRP2 was also observed after treatment with 10 μM zearalenone for 24 or 48 h. Further, expression of mRNA of MRP1, MRP2 and BCRP was induced after 24 h exposure to zearalenone (288).
Stefulj et al investigated the placental transport of cholesterol from the maternal to fetal compartment in the human placental endothelial cells and observed that ABCA1 and ABCG1 and not ABCG4 are involved in the efflux of cholesterol into the fetal side. Activation of LXR by endogenous or synthetic agonists induces ABCA1 and ABCG1 expression consequently causing efflux of cholesterol (289). Blazquez et al showed that incubation with acetaminophen caused an increase in MRP2 expression and a reduction in the mRNA levels of BCRP in the JEG-3 cells. Further, following treatment with acetaminophen there was a marked decrease in the BCRP mediated efflux of the BCRP selective substrate BODIPY-prazosin indicating a reduction in its transport activity in the JEG-3 and BeWo cells. Also, while the efflux of BODIPY-prazosin was sensitive to the known BCRP inhibitor fumitremorgin, it was not sensitive to known inhibitors of the MDR1 substrate rhodamine123 and the MRP 1-4 substrate calcein. In the rat placenta, treatment with acetaminophen in the last 3 days of pregnancy caused a moderate increase in Mrp1 mRNA expression but not a simultaneous increase in the Mrp1 protein expression. The Bcrp expression was also increased at the mRNA level post treatment with acetaminophen however the western blot analysis displayed a decrease in the expression of Bcrp protein. Further acetaminophen treatment caused a decrease in the placental transfer of glycocholic acid possibly due to the decrease in levels of placental Bcrp in in situ perfused rat placenta. Further maternal treatment with acetaminophen in rats caused significantly higher levels of bile acids in the fetal compartment during maternal cholestasis. In the same paper it was also suggested that decrease in the expression of BCRP/Bcrp at the protein level is potentially due to oxidative stress and there could be nuclear factor erythroid 2–related factor 2- (a protein that regulates expression of antioxidant proteins) dependent expression of the ABC transporters in the placenta (290). Lye et al investigated the effect of bacterial and viral challenges on the expression of placental ABC transporters in human placental explants from first and third trimesters. LPS exposure decreased the expression of ABCB1 and ABCG2 at the mRNA and protein level in the first trimester explants while poly(I:C) altered the ABCB1 expression at the mRNA level in the explants from the third trimester. These results suggested that bacterial infections could alter the fetal exposure to toxins in the first trimester whereas the viral toxins could alter exposure in the third trimester (291).
Cygalova et al (292) investigated the effect of P-gp and Bcrp over a broad range of concentrations of their substrates namely glyburide and rhodamine 123, respectively, by using the dually perfused rat placental perfusion model. It was shown that glyburide and rhodamine 123 interact with rat placental Bcrp and P-gp, respectively, and both the transporters are able to transport the compounds from the fetal to the maternal compartments against a concentration gradient. A study involving a dual substrate such as BODIPY FL prazosin was included to investigate whether the number of drug transporters (ie, 1 vs. 2) involved is reflected in the transplacental pharmacokinetics. However, this correlation did not hold true, indicating that lipophilicity predominated in the placental transport of this compound (292). Maraviroc, an anti-retroviral compound, displayed a higher drug transfer in the fetal to maternal direction than in the maternal to fetal direction in the ex vivo model of placental perfusion strongly suggesting the involvement of the efflux drug transporters localized on the apical side of the placental barrier. It was suggested that maraviroc might be a substrate for ABCC2, ABCC10 and ABCC11 (217). Neumanova et al (293) studied the transplacental passage of the antiretroviral drug tenofovir and its prodrug tenofovir disoproxil fumarate. The studies showed that in the in situ model of dually perfused rat term placenta, tenofovir diisoproxil fumarate was a dual substrate for placental ABCB1 and ABCG2, and that its passage was restricted from mother to fetus while tenofovir transport was not affected by any of these transporters (293). Cerveny et al investigated the effect of long term application of tenofovir and emtricitabine on the expression of P-gp and Bcrp in pregnant Wistar rats. Both the drugs did not significantly affect the mRNA expression of Mdr1a, Mdr1b or Bcrp in the placenta, maternal or fetal organs. In addition, the expression levels of transporters were also monitored in maternal and fetal organs. While Mdr1a expression was higher in maternal vs. fetal organs, the expression of Bcrp was comparable in both the organs. The expression of Mdr1b was higher in some maternal organs than the fetal organs (294). Petrovic et al, observed a time dependent decrease in the levels of mRNA of Bcrp 24 h post-treatment with endotoxin in rats. A dose and time dependent down regulation of mRNA levels of Mdr1a and Mdr1b was observed 18–24 h post endotoxin treatment in rats. A similar down regulation of mRNA levels of Mrp1, 2 and 3 was also observed. However the down regulation with respect to time differed with the Mrp transporter subtype (295).
Bervellier et al (296) studied the expression of the placental transporters by quantitative PCR in cytotrophoblast cells isolated from first trimester and term human placentae after 24 and 72 h in culture. Certain transporter genes (namely ABCA9 and ABCA12/MRP9) were significantly transcribed at higher levels in the first trimester than the term placental trophoblast cells. Further, certain transporter genes such as ABCA3/ABC3 were transcribed at significantly higher levels in the synctiotrophoblast as compared to cytotrophoblasts (296). Myllynen et al (297) compared the ABCB1 and ABCG2 transporter expression in the placenta of other species. While in human, the expression of ABCB1 mRNA and protein decreases with advancing gestation, there are some reports that the same pattern for protein expression is also observed in mice. These changes in the protein expression appear to translate into their functional activity. In guinea pigs the mRNA expression follows the same pattern as in the case of humans and mice. However functional data is not available for the guinea pigs. There is conflicting information about the mRNA levels of Abcb1 in the case of rats and functional data is not available. While there are conflicting data about the mRNA and protein expression levels of ABCG2 in the case of humans, in the case of mice and rats the protein and mRNA levels of Abcg2 decrease from mid to late gestation. Nonetheless in the study with the largest study population, the BCRP expression profile was similar to the rodents. Comparing humans and rodents, the spatial orientation of both the transporters was similar and they were expressed in the trophoblast cells at the primary interface between mother and fetus (297). Coles et al showed the P-gp levels in placenta at mid-gestation are elevated as compared to late gestation in mice (298). This was earlier shown by Kalabis et al as well (299). The MRP1 protein levels on the other hand were significantly lower at mid-gestation compared to late gestation. However, the mRNA levels of both the transporters remain unaltered. Demonstration with P-gp substrates like saquinavir showed greater placental and fetal uptake at late-gestation compared to mid-gestation. While saquinavir is also a substrate for MRP1, despite the fact that MRP1 levels are higher at late gestation, saquinavir showed increased placental transport probably because of the basolateral localization (298). Cressman et al (300) studied the impact of malaria infection on the expression of placental ABC transporters in pregnant mice. It was observed that the placental mRNA expression of Abcb1a, Abcb1b, Abcc1, Abcc2, Abcc3, and Abcg2 were significantly downregulated in the mice group with malaria infection as compared to the control group. In addition, the Mdr1 protein expression was also decreased in the malaria infected group compared to control (300).
Kozlowska-Rup et al studied the localization of various placental ABC transporters and compared their expression in the normal placenta with that in the placenta with gestational diabetes by performing immunohistochemistry. While P-gp, MDR3 and BCRP were found in all parts of the placenta, MRP1 was observed only in the cytotrophoblasts and synctiotrophoblasts. P-gp and BCRP were localized on the apical side of synctiotrophoblasts, MRP1 on the apical and basolateral side and diffuse distribution of MDR3. In placentae from gestational diabetic patients, these placentae displayed weaker MRP1 and MDR3 positive reactions in the synctiotrophoblasts and also a slightly lower expression of P-gp in the amniotic epithelium and decidual cells and MDR3 in the amniotic epithelium (301). Yoshino et al, investigated the effects of arsenic (AsIII) on primary cultured chorion and amnion cells prepared from fetal membranes. The protein expression levels of MRP2 were down regulated in the chorionic cells but not in the amnionic cells. The P-gp expression was minimal its mRNA expression was detectable. The expression of MRP1 was comparable in both the cell lines. Further, cyclosporine and MK571 strongly sensitized both the cells to toxicity by arsenic. These results suggested that MRP2 was involved in the arsenic accumulation and subsequent differential toxicity between the two cell lines (302).
Lye et al investigated the effect of oxygen on the expression of placental ABC transporters in the first trimester placental villous explants. While ABCG2 mRNA was induced by hyperoxic conditions, BCRP protein expression was higher in hypoxic conditions indicating a disconnect between the effect of oxygen on the mRNA and protein expression of BCRP. In addition, the mRNA expression of P-gp remained unchanged in different oxygen tensions but the P-gp expression was higher in the cytotrophoblast or syncytium of villous explants under hypoxic conditions (303). Recently, Javam et al investigated the effect of oxygen tension on the expression of P-gp and BCRP in human term placenta using term placental explants subjected to different tensions of oxygen. While P-gp mRNA and protein expression levels increased under hypoxic (3% O2) conditions at both 24 and 48 h, the BCRP protein and mRNA levels remained unchanged (304). In addition, Javam et al also observed that glycosylated P-gp in fresh placental tissue was deglycosylated after culturing for 6 days. Total P-gp expression decreased, but the localization of P-gp was unaltered. This observation was important in understanding the post-translation regulation of placental P-gp expression and localization (304).
Conclusions
As discussed it is evident that several transporters of the ABC family are expressed in the placenta. Of the transporters discussed in this review, P-gp and BCRP are the most studied and likely have the greatest impact on transplacental fetal exposure. These transporters are important in the transport of several nutrients, endogenous substances and xenobiotics across the placenta and also in protecting the fetus from the harmful xenobiotic substances.
Several in vitro and ex vivo models have been developed to study the expression and function of ABC transporters. Several animal models have also been used in studying various aspects of these transporters. In addition, while several models have been developed to study placental transport, further characterization is needed. While animal models are widely used to study placental transport of xenobiotics, human studies remain to be limited due to ethical concerns. Wide utilization of modern methods like laser capture microdissection followed by RNA sequencing, single cell sequencing, application of bioinformatics might help to overcome existing problems.
Various hormones, cytokines and other components of recognized signal transduction pathways that govern placental development play a vital role in regulation of the expression and function of these transporters. Further modulation of the expression and activity with gestational age and polymorphism make placental transport studies complicated. Placental transport involves transport across the fetal capillaries along with the trophoblast and more studies are required at this barrier to investigate its impact on the overall placental transport. Placental ABC transporters may have a high degree of biological impact and pharmaceutical importance in pregnancy outcomes. More research is needed on their regulatory mechanisms and the influence of various pathologies (especially inflammation) on their expression and function. Also, the role of the MRPs in oxidative stress and the intracellular regulation of reduced and oxidized glutathione in the placenta needs to be more clearly elucidated. In general, more information is needed on the influence of placental ABC transporters in homeostasis of endogenous compounds (such as lipids, bile acids, and hormones) and in fetal development. A better understanding of the functional and regulatory mechanisms of placental ABC transporters will aid in developing optimal medications for use in pregnancy.
Abbreviations
- ABC:
-
ATP binding cassette
- APS:
-
Antiphospholipid syndrome
- BBB:
-
Blood–brain barrier
- BCRP:
-
Breast cancer resistance protein
- BPA:
-
Bisphenol A
- BSEP:
-
Bile salt export pump
- CAR:
-
Constitutive androstane receptor
- DNP-SG:
-
2,4-dinitrophenyl-S-glutathione
- EP:
-
E-prostanoid
- ER:
-
Estrogen receptor
- FDA:
-
Food and Drug Administration
- GSSG:
-
Glutathione disulfide
- HDL-C:
-
High density lipoprotein cholesterol
- ICP:
-
Intrahepatic cholestasis of pregnancy
- IL:
-
Interleukin
- IV:
-
Intravenous
- LDL:
-
Low density lipoprotein
- LOX-1:
-
Oxidized LDL receptor-1
- LPS:
-
Lipopolysaccharides
- LXR:
-
Liver X receptor
- MDCK:
-
Madin-Darby canine kidney
- MDR:
-
Multidrug resistance protein
- MRP:
-
Multidrug resistance associated proteins
- MTX:
-
Methotrexate
- NBD:
-
Nucleotide binding domain
- NTCP:
-
Na+-taurocholate cotransporting polypeptide
- OATP:
-
Organic anion transporting polypeptides
- Ox-LDL:
-
Oxidized low density lipoprotein
- PCR:
-
Polymerase chain reaction
- PET:
-
Positron emission tomography
- PGE2:
-
Prostaglandin E2
- P-gp:
-
P-glycoprotein
- PhIP:
-
2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine
- poly(I:C):
-
Polyinosinic/Polycytidylic
- PPARγ:
-
Peroxisome proliferator-activator receptor γ
- PR:
-
Progesterone receptor
- PXR:
-
Pregnane X receptor
- SGA:
-
Small for gestational age
- TMD:
-
Transmembrane domains
- TNF:
-
Tumor necrosis factor-α
References
Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188(4):1039–45.
Moe AJ. Placental amino acid transport. Am J Physiol. 1995;268(6 Pt 1):C1321–31.
Smith CH, Moe AJ, Ganapathy V. Nutrient transport pathways across the epithelium of the placenta. Annu Rev Nutr. 1992;12:183–206.
Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–37.
Kaufmann P, Scheffen I. Placental development. In: Polin RA, Fox WW, editors. Fetal and neonatal physiology. Philadelphia: WB Saunders; 1992. p. 47-56.
Fant ME, Yeakley J, Harrison RW. Evidence for carrier-mediated transport of glucocorticoids by human placental membrane vesicles. Biochim Biophys Acta. 1983;731(3):415–20.
Kudo Y, Urabe T, Fujiwara A, Yamada K, Kawasaki T. Carrier-mediated transport system for cephalexin in human placental brush-border membrane vesicles. Biochim Biophys Acta. 1989;978(2):313–8.
Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28(3):235–69.
Nau H. Physicochemical and structural properties regulating placental drug transfer. In: Polin RA, Fox WW, editors. Philadelphia: WB Saunders; 1992. p. 130–141.
Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514.
Ganapathy V, Prasad PD, Ganapathy ME, Leibach FH. Placental transporters relevant to drug distribution across the maternal-fetal interface. J Pharmacol Exp Ther. 2000;294(2):413–20.
Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res. 2008;25(6):1244–55.
St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–503.
Ceckova-Novotna M, Pavek P, Staud F. P-glycoprotein in the placenta: expression, localization, regulation and function. Reprod Toxicol. 2006;22(3):400–10.
Aye IL, Paxton JW, Evseenko DA, Keelan JA. Expression, localisation and activity of ATP binding cassette (ABC) family of drug transporters in human amnion membranes. Placenta. 2007;28(8-9):868–77.
Kalabis GM, Petropoulos S, Gibb W, Matthews SG. Breast cancer resistance protein (Bcrp1/Abcg2) in mouse placenta and yolk sac: ontogeny and its regulation by progesterone. Placenta. 2007;28(10):1073–81.
Yeboah D, Kalabis GM, Sun M, Ou RC, Matthews SG, Gibb W. Expression and localisation of breast cancer resistance protein (BCRP) in human fetal membranes and decidua and the influence of labour at term. Reprod Fertil Dev. 2008;20(2):328–34.
Nikitina L, Wenger F, Baumann M, Surbek D, Korner M, Albrecht C. Expression and localization pattern of ABCA1 in diverse human placental primary cells and tissues. Placenta. 2011;32(6):420–30.
Bhattacharjee J, Ietta F, Giacomello E, Bechi N, Romagnoli R, Fava A, et al. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta. 2010;31(5):423–30.
Albrecht C, Nikitina L, Wenger F, Baumann M, Surbek D, Korner M. Localisation of ABCA1 in first trimester and term placental tissues. Placenta. 2010;31(8):741–2.
Baumann M, Korner M, Huang X, Wenger F, Surbek D, Albrecht C. Placental ABCA1 and ABCG1 expression in gestational disease: pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta. 2013;34(11):1079–86.
Plosch T, Gellhaus A, van Straten EM, Wolf N, Huijkman NC, Schmidt M, et al. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia? Placenta. 2010;31(10):910–8.
Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285(3):C584–91.
Nagashige M, Ushigome F, Koyabu N, Hirata K, Kawabuchi M, Hirakawa T, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta. 2003;24(10):951–8.
Hitzl M, Schaeffeler E, Hocher B, Slowinski T, Halle H, Eichelbaum M, et al. Variable expression of P-glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics. 2004;14(5):309–18.
Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R963–9.
Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27(6-7):602–9.
Gahir SS, Piquette-Miller M. Gestational and pregnane X receptor-mediated regulation of placental ATP-binding cassette drug transporters in mice. Drug Metab Dispos. 2011;39(3):465–71.
Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35(4):595–601.
Petrovic V, Piquette-Miller M. Impact of polyinosinic/polycytidylic acid on placental and hepatobiliary drug transporters in pregnant rats. Drug Metab Dispos. 2010;38(10):1760–6.
Manceau S, Giraud C, Decleves X, Scherrmann JM, Artiguebieille F, Goffinet F, et al. ABC drug transporter and nuclear receptor expression in human cytotrophoblasts: influence of spontaneous syncytialization and induction by glucocorticoids. Placenta. 2012;33(11):927–32.
Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1357–65.
Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75(3):451–62.
Vaidya SS, Walsh SW, Gerk PM. Formation and efflux of ATP-binding cassette transporter substrate 2,4-dinitrophenyl-S-glutathione from cultured human term placental villous tissue fragments. Mol Pharm. 2009;6(6):1689–702.
Meyer Zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, et al. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33(7):896–904.
Serrano MA, Macias RI, Briz O, Monte MJ, Blazquez AG, Williamson C, et al. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta. 2007;28(2-3):107–17.
Azzaroli F, Mennone A, Feletti V, Simoni P, Baglivo E, Montagnani M, et al. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007;26(8):1139–46.
Meyer Zu Schwabedissen HE, Grube M, Heydrich B, Linnemann K, Fusch C, Kroemer HK, et al. Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol. 2005;166(1):39–48.
Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–64.
Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22(47):7340–58.
Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD, et al. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol. 2008;73(3):845–54.
Yasuda S, Kobayashi M, Itagaki S, Hirano T, Iseki K. Response of the ABCG2 promoter in T47D cells and BeWo cells to sex hormone treatment. Mol Biol Rep. 2009;36(7):1889–96.
Oda K, Nishimura T, Higuchi K, Ishido N, Ochi K, Iizasa H, et al. Estrogen receptor alpha induction by mitoxantrone increases Abcg2 expression in placental trophoblast cells. J Pharm Sci. 2013;102(9):3364–72.
Petropoulos S, Gibb W, Matthews SG. Glucocorticoid regulation of placental breast cancer resistance protein (Bcrp1) in the mouse. Reprod Sci. 2011;18(7):631–9.
Wang H, Unadkat JD, Mao Q. Hormonal regulation of BCRP expression in human placental BeWo cells. Pharm Res. 2008;25(2):444–52.
Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29.
Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, et al. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257(1):29–33.
Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng JF, et al. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci U S A. 2000;97(14):7987–92.
Schmid KE, Davidson WS, Myatt L, Woollett LA. Transport of cholesterol across a BeWo cell monolayer: implications for net transport of sterol from maternal to fetal circulation. J Lipid Res. 2003;44(10):1909–18.
Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, et al. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104(8):R25–31.
Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang L-H, Yu L, et al. Mutations in the ABC 1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet. 1999;354:1341.
Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4):336–45.
Christiansen-Weber TA, Voland JR, Wu Y, Ngo K, Roland BL, Nguyen S, et al. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol. 2000;157(3):1017–29.
Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem. 2013;288(48):34414–26.
Albrecht C, Soumian S, Tetlow N, Patel P, Sullivan MHF, Lakasing L, et al. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta. 2007;28(7):701–8.
Schubert C. World of reproductive biology. Biol Reprod. 2013;88(1):1.
Chigusa Y, Tatsumi K, Kondoh E, Fujita K, Nishimura F, Mogami H, et al. Decreased lectin-like oxidized LDL receptor 1 (LOX-1) and low Nrf2 activation in placenta are involved in preeclampsia. J Clin Endocrinol Metab. 2012;97(10):E1862–70.
Chigusa Y, Kondoh E, Mogami H, Nishimura F, Ujita M, Kawasaki K, et al. ATP-binding cassette transporter A1 expression is decreased in preeclamptic placentas. Reprod Sci. 2013;20(8):891–8.
Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L. ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics. 2012;7(5):464–72.
Houde AA, Guay SP, Desgagne V, Hivert MF, Baillargeon JP, St-Pierre J, et al. Adaptations of placental and cord blood ABCA1 DNA methylation profile to maternal metabolic status. Epigenetics. 2013;8(12):1289–302.
Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502.
Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94(8):4028–33.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97(11):2517–24.
Lankas GR, Wise LD, Cartwright ME, Pippert T, Umbenhauer DR. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod Toxicol. 1998;12(4):457–63.
Smit JW, Huisman MT, van Tellingen O, Wiltshire HR, Schinkel AH. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J Clin Invest. 1999;104(10):1441–7.
Lee NY, Lee KB, Kang YS. Pharmacokinetics, placenta, and brain uptake of paclitaxel in pregnant rats. Cancer Chemother Pharmacol. 2014;73(5):1041–5.
Bhuiyan M, Petropoulos S, Gibb W, Matthews SG. Sertraline alters multidrug resistance phosphoglycoprotein activity in the mouse placenta and fetal blood-brain barrier. Reprod Sci. 2012;19(4):407–15.
Bloise E, Bhuiyan M, Audette MC, Petropoulos S, Javam M, Gibb W, et al. Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PLoS ONE. 2013;8(6):e65728.
Hodyl NA, Stark MJ, Butler M, Clifton VL. Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta. 2013;34(4):325–30.
Eyal S, Chung FS, Muzi M, Link JM, Mankoff DA, Kaddoumi A, et al. Simultaneous PET imaging of P-glycoprotein inhibition in multiple tissues in the pregnant nonhuman primate. J Nucl Med. 2009;50(5):798–806.
Ke AB, Eyal S, Chung FS, Link JM, Mankoff DA, Muzi M, et al. Modeling cyclosporine A inhibition of the distribution of a P-glycoprotein PET ligand, 11C-verapamil, into the maternal brain and fetal liver of the pregnant nonhuman primate: impact of tissue blood flow and site of inhibition. J Nucl Med. 2013;54(3):437–46.
Marzolini C, Rudin C, Decosterd LA, Telenti A, Schreyer A, Biollaz J, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16(6):889–93.
de Barros DL, Moises EC, Carvalho Cavalli R, Lanchote VL, Duarte G, da Cunha SP. Distribution of fentanyl in the placental intervillous space and in the different maternal and fetal compartments in term pregnant women. Eur J Clin Pharmacol. 2009;65(8):803–8.
Hutson JR, Koren G, Matthews SG. Placental P-glycoprotein and breast cancer resistance protein: influence of polymorphisms on fetal drug exposure and physiology. Placenta. 2010;31(5):351–7.
Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79(6):921–5.
Rahi M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. Influence of adenosine triphosphate and ABCB1 (MDR1) genotype on the P-glycoprotein-dependent transfer of saquinavir in the dually perfused human placenta. Hum Exp Toxicol. 2008;27(1):65–71.
Rahi M, Heikkinen T, Hartter S, Hakkola J, Hakala K, Wallerman O, et al. Placental transfer of quetiapine in relation to P-glycoprotein activity. J Psychopharmacol. 2007;21(7):751–6.
Bliek BJ, van Schaik RH, van der Heiden IP, Sayed-Tabatabaei FA, van Duijn CM, Steegers EA, et al. Maternal medication use, carriership of the ABCB1 3435C > T polymorphism and the risk of a child with cleft lip with or without cleft palate. Am J Med Genet A. 2009;149A(10):2088–92.
Bogacz A, Mrozikiewicz PM, Deka-Pawlik D, Seremak-Mrozikiewicz A, Bartkowiak-Wieczorek J, Barlik M, et al. Frequency of G2677T/A and C3435T polymorphisms of MDR1 gene in preeclamptic women. Ginekol Pol. 2013;84(9):781–7.
Pollex EK, Hutson JR. Genetic polymorphisms in placental transporters: implications for fetal drug exposure to oral antidiabetic agents. Expert Opin Drug Metab Toxicol. 2011;7(3):325–39.
Mylona P, Hoyland JA, Sibley CP. Sites of mRNA expression of the cystic fibrosis (CF) and multidrug resistance (MDR1) genes in the human placenta of early pregnancy: no evidence for complementary expression. Placenta. 1999;20(5-6):493–6.
Ushigome F, Koyabu N, Satoh S, Tsukimori K, Nakano H, Nakamura T, et al. Kinetic analysis of P-glycoprotein-mediated transport by using normal human placental brush-border membrane vesicles. Pharm Res. 2003;20(1):38–44.
Novotna M, Libra A, Kopecky M, Pavek P, Fendrich Z, Semecky V, et al. P-glycoprotein expression and distribution in the rat placenta during pregnancy. Reprod Toxicol. 2004;18(6):785–92.
Georges E, Bradley G, Gariepy J, Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990;87(1):152–6.
Chu TM, Kawinski E, Lin TH. Characterization of a new monoclonal antibody F4 detecting cell surface epitope and P-glycoprotein in drug-resistant human tumor cell lines. Hybridoma. 1993;12(4):417–29.
Sudhakaran S, Rayner CR, Li J, Kong DC, Gude NM, Nation RL. Inhibition of placental P-glycoprotein: impact on indinavir transfer to the foetus. Br J Clin Pharmacol. 2008;65(5):667–73.
Pavek P, Staud F, Fendrich Z, Sklenarova H, Libra A, Novotna M, et al. Examination of the functional activity of P-glycoprotein in the rat placental barrier using rhodamine 123. J Pharmacol Exp Ther. 2003;305(3):1239–50.
Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol. 2008;74(3):714–23.
Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK, Morrison JL. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod Toxicol. 2012;33(3):374–81.
Chung FS, Eyal S, Muzi M, Link JM, Mankoff DA, Kaddoumi A, et al. Positron emission tomography imaging of tissue P-glycoprotein activity during pregnancy in the non-human primate. Br J Pharmacol. 2010;159(2):394–404.
Myllynen P, Vahakangas K. Placental transfer and metabolism: an overview of the experimental models utilizing human placental tissue. Toxicol In Vitro. 2013;27(1):507–12.
Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90(1):67–76.
Bapat P, Hutson J, Kedar R, Berger H, Koren G. Evaluating the success rate of the dual perfusion of the human placenta ex vivo. Placenta. 2014;35:A96.
Pavek P, Fendrich Z, Staud F, Malakova J, Brozmanova H, Laznicek M, et al. Influence of P-glycoprotein on the transplacental passage of cyclosporine. J Pharm Sci. 2001;90(10):1583–92.
Molsa M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. Functional role of P-glycoprotein in the human blood-placental barrier. Clin Pharmacol Ther. 2005;78(2):123–31.
Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol. 2005;69(12):1869–78.
Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GD, Ahmed MS. Bidirectional transfer of methadone across human placenta. Biochem Pharmacol. 2005;69(1):187–97.
Malek A, Obrist C, Wenzinger S, von Mandach U. The impact of cocaine and heroin on the placental transfer of methadone. Reprod Biol Endocrinol. 2009;7:61.
Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol Med. 2006;122:225–39.
Sastry BV. Techniques to study human placental transport. Adv Drug Deliv Rev. 1999;38(1):17–39.
Utoguchi N, Chandorkar GA, Avery M, Audus KL. Functional expression of P-glycoprotein in primary cultures of human cytotrophoblasts and BeWo cells. Reprod Toxicol. 2000;14(3):217–24.
Beghin D, Forestier F, Noel-Hudson MS, Gavard L, Guibourdenche J, Farinotti R, et al. Modulation of endocrine and transport functions in human trophoblasts by saquinavir and nelfinavir. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):55–9.
Beghin D, Delongeas JL, Claude N, Farinotti R, Forestier F, Gil S. Comparative effects of drugs on P-glycoprotein expression and activity using rat and human trophoblast models. Toxicol In Vitro. 2010;24(2):630–7.
Ushigome F, Takanaga H, Matsuo H, Yanai S, Tsukimori K, Nakano H, et al. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur J Pharmacol. 2000;408(1):1–10.
Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem Pharmacol. 2009;78(9):1272–8.
Lee NY, Lee HE, Kang YS. Identification of p-glycoprotein and transport mechanism of Paclitaxel in syncytiotrophoblast cells. Biomol Ther (Seoul). 2014;22(1):68–72.
Vaidya SS, Walsh SW, Gerk PM. Application of human placental villous tissue explants to study ABC transporter mediated efflux of 2,4-dinitrophenyl-S-glutathione. Curr Pharm Biotechnol. 2011;12(5):814–23.
Song D, Guo J, Han F, Zhang W, Wang Y. Establishment of an in vitro model of the human placental barrier by placenta slice culture and ussing chamber. Biosci Biotechnol Biochem. 2013;77(5):1030–4.
Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20(12):1261–4.
Apati A, Szebenyi K, Erdei Z, Varady G, Orban TI, Sarkadi B. The importance of drug transporters in human pluripotent stem cells and in early tissue differentiation. Expert Opin Drug Metab Toxicol. 2016;12(1):77–92.
Shi F, Soares MJ, Avery M, Liu F, Zhang X, Audus KL. Permeability and metabolic properties of a trophoblast cell line (HRP-1) derived from normal rat placenta. Exp Cell Res. 1997;234(1):147–55.
Lafond J, Simoneau L, Savard R, Gagnon MC. Linoleic acid transport by human placental syncytiotrophoblast membranes. Eur J Biochem. 1994;226(2):707–13.
Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28(7):1231–6.
Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273(5 Pt 1):C1596–604.
Schinkel AH, Roelofs MEM, Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by p-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991;51(10):2628–35.
Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, et al. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994;354(3):263–6.
Patel P, Weerasekera N, Hitchins M, Boyd CA, Johnston DG, Williamson C. Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C, OATP-D, OATP-E and NTCP gene transcripts in 1st and 3rd trimester human placenta. Placenta. 2003;24(1):39–44.
Volpicelli ER, Lezcano C, Zhan Q, Girouard SD, Kindelberger DW, Frank MH, et al. The multidrug-resistance transporter ABCB5 is expressed in human placenta. Int J Gynecol Pathol. 2014;33(1):45–51.
Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, et al. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443(7111):586–9.
Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20(3):273–92.
Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–71.
St-Pierre MV, Stallmach T, Freimoser Grundschober A, Dufour JF, Serrano MA, Marin JJ, et al. Temporal expression profiles of organic anion transport proteins in placenta and fetal liver of the rat. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1505–16.
Marin JJ, Macias RI, Briz O, Perez MJ, Serrano MA. Molecular bases of the excretion of fetal bile acids and pigments through the fetal liver-placenta-maternal liver pathway. Ann Hepatol. 2005;4(2):70–6.
Keppler D. Export pumps for glutathione S-conjugates. Free Radic Biol Med. 1999;27(9-10):985–91.
Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167(1):3–23.
Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006;580(4):1103–11.
Jedlitschky G, Leier I, Buchholz U, Hummel-Eisenbeiss J, Burchell B, Keppler D. ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocyte canalicular isoform MRP2. Biochem J. 1997;327(Pt 1):305–10.
Loe DW, Almquist KC, Cole SP, Deeley RG. ATP-dependent 17 beta-estradiol 17-(beta-D-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J Biol Chem. 1996;271(16):9683–9.
Evers R, Cnubben NH, Wijnholds J, van Deemter L, van Bladeren PJ, Borst P. Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett. 1997;419(1):112–6.
Renes J, de Vries EE, Hooiveld GJ, Krikken I, Jansen PL, Muller M. Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J. 2000;350(Pt 2):555–61.
Qian YM, Song WC, Cui H, Cole SP, Deeley RG. Glutathione stimulates sulfated estrogen transport by multidrug resistance protein 1. J Biol Chem. 2001;276(9):6404–11.
Mao Q, Deeley RG, Cole SP. Functional reconstitution of substrate transport by purified multidrug resistance protein MRP1 (ABCC1) in phospholipid vesicles. J Biol Chem. 2000;275(44):34166–72.
Rigato I, Pascolo L, Fernetti C, Ostrow JD, Tiribelli C. The human multidrug-resistance-associated protein MRP1 mediates ATP-dependent transport of unconjugated bilirubin. Biochem J. 2004;383(Pt 2):335–41.
Stark M, Rothem L, Jansen G, Scheffer GL, Goldman ID, Assaraf YG. Antifolate resistance associated with loss of MRP1 expression and function in Chinese hamster ovary cells with markedly impaired export of folate and cholate. Mol Pharmacol. 2003;64(2):220–7.
Zeng H, Chen ZS, Belinsky MG, Rea PA, Kruh GD. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res. 2001;61(19):7225–32.
Loe DW, Stewart RK, Massey TE, Deeley RG, Cole SP. ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistance protein (MRP) gene. Mol Pharmacol. 1997;51(6):1034–41.
Lorico A, Nesland J, Emilsen E, Fodstad O, Rappa G. Role of the multidrug resistance protein 1 gene in the carcinogenicity of aflatoxin B1: investigations using mrp1-null mice. Toxicology. 2002;171(2-3):201–5.
Jones K, Bray PG, Khoo SH, Davey RA, Meaden ER, Ward SA, et al. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS. 2001;15(11):1353–8.
Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279(31):32700–8.
Salerno M, Petroutsa M, Garnier-Suillerot A. The MRP1-mediated effluxes of arsenic and antimony do not require arsenic-glutathione and antimony-glutathione complex formation. J Bioenerg Biomembr. 2002;34(2):135–45.
Koike K, Conseil G, Leslie EM, Deeley RG, Cole SP. Identification of proline residues in the core cytoplasmic and transmembrane regions of multidrug resistance protein 1 (MRP1/ABCC1) important for transport function, substrate specificity, and nucleotide interactions. J Biol Chem. 2004;279(13):12325–36.
Renes J, de Vries EG, Nienhuis EF, Jansen PL, Muller M. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126(3):681–8.
Leslie EM, Ito K, Upadhyaya P, Hecht SS, Deeley RG, Cole SP. Transport of the beta -O-glucuronide conjugate of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by the multidrug resistance protein 1 (MRP1). Requirement for glutathione or a non-sulfur-containing analog. J Biol Chem. 2001;276(30):27846–54.
Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996;271(16):9675–82.
Gennuso F, Fernetti C, Tirolo C, Testa N, L’Episcopo F, Caniglia S, et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1). Proc Natl Acad Sci U S A. 2004;101(8):2470–5.
Sikkel E, Pasman SA, Oepkes D, Kanhai HH, Vandenbussche FP. On the origin of amniotic fluid bilirubin. Placenta. 2004;25(5):463–8.
Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun. 2003;303(1):259–65.
Cekic D, Bellarosa C, Garcia-Mediavilla MV, Rigato I, Pascolo L, Ostrow JD, et al. Upregulation in the expression of multidrug resistance protein Mrp1 mRNA and protein by increased bilirubin production in rat. Biochem Biophys Res Commun. 2003;311(4):891–6.
Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371(Pt 3):897–905.
Hanko E, Tommarello S, Watchko JF, Hansen TW. Administration of drugs known to inhibit P-glycoprotein increases brain bilirubin and alters the regional distribution of bilirubin in rat brain. Pediatr Res. 2003;54(4):441–5.
Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res. 2005;97(7):637–44.
Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv. 2005;60(12):807–16.
Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159(3):1031–43.
Tatebe S, Sinicrope FA, Kuo MT. Induction of multidrug resistance proteins MRP1 and MRP3 and gamma-glutamylcysteine synthetase gene expression by nonsteroidal anti-inflammatory drugs in human colon cancer cells. Biochem Biophys Res Commun. 2002;290(5):1427–33.
Santoso DI, Rogers P, Wallace EM, Manuelpillai U, Walker D, Subakir SB. Localization of indoleamine 2,3-dioxygenase and 4-hydroxynonenal in normal and pre-eclamptic placentae. Placenta. 2002;23(5):373–9.
Reichard JF, Doorn JA, Simon F, Taylor MS, Petersen DR. Characterization of multidrug resistance-associated protein 2 in the hepatocellular disposition of 4-hydroxynonenal. Arch Biochem Biophys. 2003;411(2):243–50.
Alary J, Fernandez Y, Debrauwer L, Perdu E, Gueraud F. Identification of intermediate pathways of 4-hydroxynonenal metabolism in the rat. Chem Res Toxicol. 2003;16(3):320–7.
Brown KE, Broadhurst KA, Mathahs MM, Kladney RD, Fimmel CJ, Srivastava SK, et al. Immunodetection of aldose reductase in normal and diseased human liver. Histol Histopathol. 2005;20(2):429–36.
Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57(23):5238–42.
Huisman MT, Smit JW, Crommentuyn KM, Zelcer N, Wiltshire HR, Beijnen JH, et al. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS. 2002;16(17):2295–301.
Williams GC, Liu A, Knipp G, Sinko PJ. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2). Antimicrob Agents Chemother. 2002;46(11):3456–62.
Park S, Sinko PJ. P-glycoprotein and mutlidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J Pharmacol Exp Ther. 2005;312(3):1249–56.
Bakos E, Evers R, Sinko E, Varadi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57(4):760–8.
Forestier F, de Renty P, Peytavin G, Dohin E, Farinotti R, Mandelbrot L. Maternal-fetal transfer of saquinavir studied in the ex vivo placental perfusion model. Am J Obstet Gynecol. 2001;185(1):178–81.
Abdulrazzaq YM, Osman N, Yousif ZM, Trad O. Morbidity in neonates of mothers who have ingested aflatoxins. Ann Trop Paediatr. 2004;24(2):145–51.
Gonzalez-Crussi F. The human yolk sac and yolk sac (endodermal sinus) tumors. A review. Perspect Pediatr Pathol. 1979;5:179–215.
Mitra P, Audus KL. MRP isoforms and BCRP mediate sulfate conjugate efflux out of BeWo cells. Int J Pharm. 2010;384(1-2):15–23.
Biondi C, Ferretti ME, Lunghi L, Medici S, Cervellati F, Pavan B, et al. cAMP efflux from human trophoblast cell lines: a role for multidrug resistance protein (MRP)1 transporter. Mol Hum Reprod. 2010;16(7):481–91.
Chiavegatti T, Costa Jr VL, Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol. 2008;153(6):1331–40.
Rahi MM, Heikkinen TM, Hakala KE, Laine KP. The effect of probenecid and MK-571 on the feto-maternal transfer of saquinavir in dually perfused human term placenta. Eur J Pharm Sci. 2009;37(5):588–92.
Gedeon C, Anger G, Lubetsky A, Miller MP, Koren G. Investigating the potential role of multi-drug resistance protein (MRP) transporters in fetal to maternal glyburide efflux in the human placenta. J Obstet Gynaecol. 2008;28(5):485–9.
Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302(2):407–15.
Dietrich CG, de Waart DR, Ottenhoff R, Schoots IG, Elferink RP. Increased bioavailability of the food-derived carcinogen 2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol Pharmacol. 2001;59(5):974–80.
May K, Minarikova V, Linnemann K, Zygmunt M, Kroemer HK, Fusch C, et al. Role of the multidrug transporter proteins ABCB1 and ABCC2 in the diaplacental transport of talinolol in the term human placenta. Drug Metab Dispos. 2008;36(4):740–4.
Bridges CC, Joshee L, Zalups RK. Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: role of Mrp2. Reprod Toxicol. 2012;34(4):628–34.
Serrano MA, Macias RI, Vallejo M, Briz O, Bravo A, Pascual MJ, et al. Effect of ursodeoxycholic acid on the impairment induced by maternal cholestasis in the rat placenta-maternal liver tandem excretory pathway. J Pharmacol Exp Ther. 2003;305(2):515–24.
Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, et al. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol. 2002;63(3):409–19.
Collier AC, Keelan JA, Van Zijl PE, Paxton JW, Mitchell MD, Tingle MD. Human placental glucuronidation and transport of 3′azido-3′-deoxythymidine and uridine diphosphate glucuronic acid. Drug Metab Dispos. 2004;32(8):813–20.
Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33(7):947–55.
Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, et al. Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278(3):G438–46.
Xiong H, Yoshinari K, Brouwer KL, Negishi M. Role of constitutive androstane receptor in the in vivo induction of Mrp3 and CYP2B1/2 by phenobarbital. Drug Metab Dispos. 2002;30(8):918–23.
Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem. 2000;275(4):2905–10.
Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B. The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett. 2003;140–141:133–43.
Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem. 1999;274(21):15181–5.
Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res. 2002;19(1):34–41.
Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96(12):6914–9.
Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27(11-12):1096–102.
Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31(2):153–67.
van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13(3):595–603.
Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38(2):374–84.
Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288(2):F327–33.
Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, et al. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J. 2003;371(Pt 2):361–7.
Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100(16):9244–9.
Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2):619–27.
Aleksunes LM, Cui Y, Klaassen CD. Prominent expression of xenobiotic efflux transporters in mouse extraembryonic fetal membranes compared with placenta. Drug Metab Dispos. 2008;36(9):1960–70.
Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst. 1998;90(22):1735–41.
Shuster DL, Bammler TK, Beyer RP, Macdonald JW, Tsai JM, Farin FM, et al. Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metab Dispos. 2013;41(2):332–42.
Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275(39):30069–74.
Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278(20):17664–71.
Sawai K, Azuma C, Koyama M, Hashimoto K, Kimura T, Samejima Y, et al. The novel role of 3′,5′-guanosine monophosphate (cGMP) on the differentiation of trophoblasts: comparison with the effects of 3′,5′-adenosine monophosphate (cAMP). Early Pregnancy. 1996;2(4):244–52.
Yamahara K, Itoh H, Chun TH, Ogawa Y, Yamashita J, Sawada N, et al. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc Natl Acad Sci U S A. 2003;100(6):3404–9.
Clifton VL, Read MA, Leitch IM, Giles WB, Boura AL, Robinson PJ, et al. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J Clin Endocrinol Metab. 1995;80(10):2888–93.
Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97(13):7476–81.
Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem. 2002;277(19):16860–7.
Madon J, Hagenbuch B, Landmann L, Meier PJ, Stieger B. Transport function and hepatocellular localization of mrp6 in rat liver. Mol Pharmacol. 2000;57(3):634–41.
Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49(2):230–8.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6):452–77.
Yabuuchi H, Shimizu H, Takayanagi S, Ishikawa T. Multiple splicing variants of two new human ATP-binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun. 2001;288(4):933–9.
Tammur J, Prades C, Arnould I, Rzhetsky A, Hutchinson A, Adachi M, et al. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene. 2001;273(1):89–96.
Bera TK, Lee S, Salvatore G, Lee B, Pastan I. MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med. 2001;7(8):509–16.
Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278(32):29509–14.
Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol. 2005;67(2):545–57.
Bortfeld M, Rius M, Konig J, Herold-Mende C, Nies AT, Keppler D. Human multidrug resistance protein 8 (MRP8/ABCC11), an apical efflux pump for steroid sulfates, is an axonal protein of the CNS and peripheral nervous system. Neuroscience. 2006;137(4):1247–57.
Marin JJ, Bravo P, el-Mir MY, Serrano MA. ATP-dependent bile acid transport across microvillous membrane of human term trophoblast. Am J Physiol. 1995;268(4 Pt 1):G685–94.
Noe J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123(5):1659–66.
Serrano MA, Brites D, Larena MG, Monte MJ, Bravo MP, Oliveira N, et al. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta. J Hepatol. 1998;28(5):829–39.
Vinot C, Gavard L, Treluyer JM, Manceau S, Courbon E, Scherrmann JM, et al. Placental transfer of maraviroc in an ex vivo human cotyledon perfusion model and influence of ABC transporter expression. Antimicrob Agents Chemother. 2013;57(3):1415–20.
Ceckova M, Libra A, Pavek P, Nachtigal P, Brabec M, Fuchs R, et al. Expression and functional activity of breast cancer resistance protein (BCRP, ABCG2) transporter in the human choriocarcinoma cell line BeWo. Clin Exp Pharmacol Physiol. 2006;33(1-2):58–65.
Imai Y, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance. Jpn J Cancer Res. 2002;93(3):231–5.
Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278(25):22644–9.
Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279(19):19781–9.
Her C, Wood TC, Eichler EE, Mohrenweiser HW, Ramagli LS, Siciliano MJ, et al. Human hydroxysteroid sulfotransferase SULT2B1: two enzymes encoded by a single chromosome 19 gene. Genomics. 1998;53(3):284–95.
Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92(20):1651–6.
Kolwankar D, Glover DD, Ware JA, Tracy TS. Expression and function of ABCB1 and ABCG2 in human placental tissue. Drug Metab Dispos. 2005;33(4):524–9.
Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59(17):4237–41.
Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, et al. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565(1):6–16.
Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97(5):626–30.
Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–9.
Bloise E, Ortiga-Carvalho TM, Reis FM, Lye SJ, Gibb W, Matthews SG. ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Update. 2016;22(2):164–81.
Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326(1):181–7.
Cygalova L, Ceckova M, Pavek P, Staud F. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol Lett. 2008;178(3):176–80.
Yasuda S, Itagaki S, Hirano T, Iseki K. Expression level of ABCG2 in the placenta decreases from the mid stage to the end of gestation. Biosci Biotechnol Biochem. 2005;69(10):1871–6.
Staud F, Vackova Z, Pospechova K, Pavek P, Ceckova M, Libra A, et al. Expression and transport activity of breast cancer resistance protein (Bcrp/Abcg2) in dually perfused rat placenta and HRP-1 cell line. J Pharmacol Exp Ther. 2006;319(1):53–62.
Memon N, Bircsak KM, Archer F, Gibson CJ, Ohman-Strickland P, Weinberger BI, et al. Regional expression of the BCRP/ABCG2 transporter in term human placentas. Reprod Toxicol. 2014;43:72–7.
Ridano ME, Racca AC, Flores-Martin J, Camolotto SA, de Potas GM, Genti-Raimondi S, et al. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod Toxicol. 2012;33(3):331–8.
Kummu M, Sieppi E, Wallin K, Rautio A, Vahakangas K, Myllynen P. Cadmium inhibits ABCG2 transporter function in BeWo choriocarcinoma cells and MCF-7 cells overexpressing ABCG2. Placenta. 2012;33(10):859–65.
Feinshtein V, Erez O, Ben-Zvi Z, Eshkoli T, Sheizaf B, Sheiner E, et al. Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: an ex vivo study. Am J Obstet Gynecol. 2013;209(6):573 e571-573 e515.
Myllynen P, Kummu M, Kangas T, Ilves M, Immonen E, Rysa J, et al. ABCG2/BCRP decreases the transfer of a food-born chemical carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in perfused term human placenta. Toxicol Appl Pharmacol. 2008;232(2):210–7.
Azzaroli F, Raspanti ME, Simoni P, Montagnani M, Lisotti A, Cecinato P, et al. High doses of ursodeoxycholic acid up-regulate the expression of placental breast cancer resistance protein in patients affected by intrahepatic cholestasis of pregnancy. PLoS ONE. 2013;8(5):e64101.
Jebbink J, Veenboer G, Boussata S, Keijser R, Kremer AE, Elferink RO, et al. Total bile acids in the maternal and fetal compartment in relation to placental ABCG2 expression in preeclamptic pregnancies complicated by HELLP syndrome. Biochim Biophys Acta. 2015;1852(1):131–6.
Saito J, Hirota T, Furuta S, Kobayashi D, Takane H, Ieiri I. Association between DNA methylation in the miR-328 5′-flanking region and inter-individual differences in miR-328 and BCRP expression in human placenta. PLoS ONE. 2013;8(8):e72906.
Mao Q, Unadkat J. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;1(1):E118–33.
Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, et al. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res. 2005;22(12):2023–34.
Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59(1):8–13.
Allen JD, Jackson SC, Schinkel AH. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Res. 2002;62(8):2294–9.
Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62(17):5035–40.
Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an obstetric-fetal pharmacology research unit network and University of Washington Specialized Center of Research Study. Mol Pharmacol. 2008;73(3):949–59.
Moreno-Sanz G, Sasso O, Guijarro A, Oluyemi O, Bertorelli R, Reggiani A, et al. Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: interaction with the Abcg2 transporter in the blood-placenta barrier. Br J Pharmacol. 2012;167(8):1620–8.
Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72(4):967–75.
Blazquez AG, Briz O, Romero MR, Rosales R, Monte MJ, Vaquero J, et al. Characterization of the role of ABCG2 as a bile acid transporter in liver and placenta. Mol Pharmacol. 2012;81(2):273–83.
Hofman J, Ahmadimoghaddam D, Hahnova L, Pavek P, Ceckova M, Staud F. Olomoucine II and purvalanol A inhibit ABCG2 transporter in vitro and in situ and synergistically potentiate cytostatic effect of mitoxantrone. Pharmacol Res. 2012;65(3):312–9.
Feinshtein V, Holcberg G, Amash A, Erez N, Rubin M, Sheiner E, et al. Nitrofurantoin transport by placental choriocarcinoma JAr cells: involvement of BCRP, OATP2B1 and other MDR transporters. Arch Gynecol Obstet. 2010;281(6):1037–44.
Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos. 2007;35(12):2154–8.
Gedeon C, Anger G, Piquette-Miller M, Koren G. Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta. 2008;29(1):39–43.
Pollex E, Lubetsky A, Koren G. The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta. Placenta. 2008;29(8):743–7.
Kumar JS, Wei BR, Madigan JP, Simpson RM, Hall MD, Gottesman MM. Bioluminescent imaging of ABCG2 efflux activity at the blood-placenta barrier. Sci Rep. 2016;6:20418.
Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101.
Pollex EK, Anger G, Hutson J, Koren G, Piquette-Miller M. Breast cancer resistance protein (BCRP)-mediated glyburide transport: effect of the C421A/Q141K BCRP single-nucleotide polymorphism. Drug Metab Dispos. 2010;38(5):740–4.
Wang C, Xie L, Li H, Li Y, Mu D, Zhou R, et al. Associations between ABCG2 gene polymorphisms and isolated septal defects in a Han Chinese population. DNA Cell Biol. 2014;33(10):689–98.
Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69(2):278–90.
Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27(1):79–83.
Shapiro AB, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur J Biochem. 1999;259(3):841–50.
Ichikawa-Haraguchi M, Sumizawa T, Yoshimura A, Furukawa T, Hiramoto S, Sugita M, et al. Progesterone and its metabolites: the potent inhibitors of the transporting activity of P-glycoprotein in the adrenal gland. Biochim Biophys Acta. 1993;1158(3):201–8.
Coles LD, Lee IJ, Voulalas PJ, Eddington ND. Estradiol and progesterone-mediated regulation of P-gp in P-gp overexpressing cells (NCI-ADR-RES) and placental cells (JAR). Mol Pharm. 2009;6(6):1816–25.
Ramu A, Glaubiger D, Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984;44(10):4392–5.
Jin H, Audus KL. Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta. 2005;26(Suppl A):S96–103.
Sieppi E, Vahakangas K, Rautio A, Ietta F, Paulesu L, Myllynen P. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol Cell Endocrinol. 2016;429:41–9.
Petropoulos S, Gibb W, Matthews SG. Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta. 2010;31(9):803–10.
Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. Changes in the placental glucocorticoid barrier during rat pregnancy: impact on placental corticosterone levels and regulation by progesterone. Biol Reprod. 2009;80(6):1209–15.
Kalabis GM, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can J Physiol Pharmacol. 2009;87(11):973–8.
Salje K, Lederer K, Oswald S, Dazert E, Warzok R, Siegmund W. Effects of rifampicin, dexamethasone, St. John’s Wort and Thyroxine on maternal and foetal expression of Abcb1 and organ distribution of talinolol in pregnant rats. Basic Clin Pharmacol Toxicol. 2012;111(2):99–105.
Yasuda S, Itagaki S, Hirano T, Iseki K. Effects of sex hormones on regulation of ABCG2 expression in the placental cell line BeWo. J Pharm Pharm Sci. 2006;9(1):133–9.
Ietta F, Bechi N, Romagnoli R, Bhattacharjee J, Realacci M, Di Vito M, et al. 17{beta}-Estradiol modulates the macrophage migration inhibitory factor secretory pathway by regulating ABCA1 expression in human first-trimester placenta. Am J Physiol Endocrinol Metab. 2010;298(3):E411–8.
Anger GJ, Cressman AM, Piquette-Miller M. Expression of ABC Efflux transporters in placenta from women with insulin-managed diabetes. PLoS ONE. 2012;7(4):e35027.
Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW. ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos. 2011;39(6):1000–7.
Mason CW, Lee GT, Dong Y, Zhou H, He L, Weiner CP. Effect of prostaglandin E2 on multidrug resistance transporters in human placental cells. Drug Metab Dispos. 2014;42(12):2077–86.
Pavek P, Smutny T. Nuclear receptors in regulation of biotransformation enzymes and drug transporters in the placental barrier. Drug Metab Rev. 2014;46(1):19–32.
Petrovic V, Teng S, Piquette-Miller M. Regulation of drug transporters during infection and inflammation. Mol Interv. 2007;7(2):99–111.
Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, et al. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15(9):866–77.
Riganti C, Salaroglio IC, Caldera V, Campia I, Kopecka J, Mellai M, et al. Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/beta-catenin pathway. Neuro-Oncology. 2013;15(11):1502–17.
Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/beta-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104(10):1303–8.
Plosch T, van Straten EM, Kuipers F. Cholesterol transport by the placenta: placental liver X receptor activity as a modulator of fetal cholesterol metabolism? Placenta. 2007;28(7):604–10.
Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22(8):2607–19.
Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim Biophys Acta. 2005;1735(1):1–19.
Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun. 2000;274(3):794–802.
Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010;202(4):383 e381-387.
Hemauer SJ, Patrikeeva SL, Wang X, Abdelrahman DR, Hankins GD, Ahmed MS, et al. Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion. Biochem Pharmacol. 2010;80(7):1080–6.
Prouillac C, Videmann B, Mazallon M, Lecoeur S. Induction of cells differentiation and ABC transporters expression by a myco-estrogen, zearalenone, in human choriocarcinoma cell line (BeWo). Toxicology. 2009;263(2–3):100–7.
Stefulj J, Panzenboeck U, Becker T, Hirschmugl B, Schweinzer C, Lang I, et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ Res. 2009;104(5):600–8.
Blazquez AG, Briz O, Gonzalez-Sanchez E, Perez MJ, Ghanem CI, Marin JJ. The effect of acetaminophen on the expression of BCRP in trophoblast cells impairs the placental barrier to bile acids during maternal cholestasis. Toxicol Appl Pharmacol. 2014;277(1):77–85.
Lye P, Bloise E, Javam M, Gibb W, Lye SJ, Matthews SG. Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. Am J Pathol. 2015;185(6):1666–75.
Cygalova LH, Hofman J, Ceckova M, Staud F. Transplacental pharmacokinetics of glyburide, rhodamine 123, and BODIPY FL prazosin: effect of drug efflux transporters and lipid solubility. J Pharmacol Exp Ther. 2009;331(3):1118–25.
Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28(1):9–17.
Cerveny L, Neumanova Z, Karbanova S, Havlova I, Staud F. Long-term administration of tenofovir or emtricitabine to pregnant rats; effect on Abcb1a, Abcb1b and Abcg2 expression in the placenta and in maternal and fetal organs. J Pharm Pharmacol. 2016;68(1):84–92.
Petrovic V, Wang JH, Piquette-Miller M. Effect of endotoxin on the expression of placental drug transporters and glyburide disposition in pregnant rats. Drug Metab Dispos. 2008;36(9):1944–50.
Berveiller P, Degrelle SA, Segond N, Cohen H, Evain-Brion D, Gil S. Drug transporter expression during in vitro differentiation of first-trimester and term human villous trophoblasts. Placenta. 2015;36(1):93–6.
Myllynen P, Kummu M, Sieppi E. ABCB1 and ABCG2 expression in the placenta and fetus: an interspecies comparison. Expert Opin Drug Metab Toxicol. 2010;6(11):1385–98.
Coles LD, Lee IJ, Hassan HE, Eddington ND. Distribution of saquinavir, methadone, and buprenorphine in maternal brain, placenta, and fetus during two different gestational stages of pregnancy in mice. J Pharm Sci. 2009;98(8):2832–46.
Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod. 2005;73(4):591–7.
Cressman AM, McDonald CR, Silver K, Kain KC, Piquette-Miller M. Malaria infection alters the expression of hepatobiliary and placental drug transporters in pregnant mice. Drug Metab Dispos. 2014;42(4):603–10.
Kozlowska-Rup D, Czekaj P, Plewka D, Sikora J. Immunolocalization of ABC drug transporters in human placenta from normal and gestational diabetic pregnancies. Ginekol Pol. 2014;85(6):410–9.
Yoshino Y, Yuan B, Kaise T, Takeichi M, Tanaka S, Hirano T, et al. Contribution of aquaporin 9 and multidrug resistance-associated protein 2 to differential sensitivity to arsenite between primary cultured chorion and amnion cells prepared from human fetal membranes. Toxicol Appl Pharmacol. 2011;257(2):198–208.
Lye P, Bloise E, Dunk C, Javam M, Gibb W, Lye SJ, et al. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34(9):817–23.
Javam M, Audette MC, Iqbal M, Bloise E, Gibb W, Matthews SG. Effect of oxygen on multidrug resistance in term human placenta. Placenta. 2014;35(5):324–30.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors gratefully acknowledge the following sources of support: The Thomas F. and Kate Miller Jeffress Memorial Trust, the VCU School of Pharmacy, the National Institutes of Health (MD002254), VCU Presidential Research Incentive Program and grant P01DA032507 from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, A.A., Vaidya, S.S., St-Pierre, M.V. et al. Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm Res 33, 2847–2878 (2016). https://doi.org/10.1007/s11095-016-2028-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2028-8