Abstract
Purpose
We investigated whether the pregnancy-related hormones, estriol (E3), testosterone, human placental lactogen (hPL), human prolactin (hPRL), and human chorionic gonadotropin (hCG) affect BCRP expression in human placental BeWo cells.
Materials and Methods
The effects of these hormones on BCRP protein and mRNA expression in BeWo cells were determined by immunoblotting and quantitative real-time RT-PCR, respectively. The effects of these hormones on membrane localization of BCRP in BeWo cells were examined by immunofluorescent confocal microscopy.
Results
E3, hPL, and hPRL significantly increased BCRP protein and mRNA approximately two to threefold at physiological concentrations. Induction of BCRP by E3 was abrogated by the estrogen receptor (ER) antagonist ICI-182,780. However, knock-down of ERα by RNA interference did not abolish the inductive effect of E3. Testosterone by itself did not affect BCRP expression at physiological concentrations. However, testosterone together with 17β-estradiol (E2) increased BCRP protein and mRNA approximately twofold, and this induction was abolished by ICI-182,780 or the testosterone receptor (TR) antagonist flutamide or knock-down of ERα expression. Further analysis revealed that E2 increased TR mRNA approximately 5.9-fold, suggesting that testosterone in combination with E2 increases BCRP expression, possibly through E2-mediated up-regulation of TR. hCG at physiological concentrations had no effect on BCRP expression.
Conclusions
E3, hPL, hPRL, and testosterone in combination with E2 may up-regulate BCRP expression in the placenta during pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The human breast cancer resistance protein (BCRP, gene symbol ABCG2) is the second member of the subfamily G of the large ATP-binding cassette (ABC) transporter superfamily (1–3), with a broad range of substrates from highly lipophilic substances (e.g., mitoxantrone and dipyridamole) to hydrophilic organic anions [e.g., estrone-3-sulfate, 17β-estradiol 17-(β-D-glucuronide), and methotrexate] (4–6). BCRP is expressed in normal human tissues (7), and has been shown to play an important role in the absorption, distribution, and elimination of drugs that are BCRP substrates (8–13).
Since BCRP is most abundantly expressed in the apical membrane of the placental syncytiotrophoblasts (7), we are particularly interested in the functional role of the transporter in the placenta. Currently available data suggest that BCRP protects the fetus from toxicity of xenobiotics and their metabolites by limiting placental penetration of these compounds. For example, Bcrp1, the murine homolog of BCRP, has been shown to increase the fetus/plasma ratio of topotecan twofold in pregnant mice treated with the BCRP inhibitor GF120918 compared with vehicle-treatment controls (9). Most recently, an ex vivo study (14) using dually perfused rat placenta also clearly demonstrated that rat Bcrp1 can actively transport cimetidine from the fetal to the maternal compartment against a concentration gradient. In humans, a recent placental perfusion study showed that glyburide was actively transported from the fetus to the maternal circulation by a transporter other than P-glycoprotein, because this glyburide transport activity did not appear to be inhibited by the potent P-glycoprotein inhibitor verapamil (15). Since glyburide is a BCRP substrate (16) and verapamil is not a BCRP inhibitor (6), and given the fact that BCRP is abundantly expressed in the placenta, it is highly likely that BCRP plays a significant role in limiting placental penetration of glyburide.
We have recently demonstrated that Bcrp1 expression in placenta, kidney, and liver of pregnant mice is significantly increased at mid-gestational ages compared with early or term pregnancy (17). These results suggest that not only fetal distribution but systemic exposure to drugs that are BCRP substrates could be influenced by pregnancy, particularly at mid-gestational stages. Likewise, BCRP expression in human placenta at preterm (28 ± 1 week) is also approximately two times greater than that at term (39 ± 2 week) (18). To date, little is known about the molecular mechanisms by which BCRP expression is regulated during pregnancy. We have hypothesized that the pregnancy-related hormones are involved in the up-regulation of BCRP in human placenta (19). We have indeed shown that progesterone (P4) and E2, respectively, increase and decrease BCRP expression in the human placental BeWo cells, and P4 in combination with E2 further increases BCRP expression compared with P4-treatment alone (19). Similar to P4 and E2, the levels of other pregnancy-related hormones, such as E3, testosterone, hPL or hPRL, also continuously increase throughout the course of pregnancy. The effects of these hormones on BCRP expression have not yet been investigated.
Therefore, in the present study, we analyzed the effects of E3, testosterone, hPL, hPRL, and hCG on BCRP expression in the model human placental BeWo cells. We found that E3, hPL, and hPRL increased BCRP expression. Testosterone by itself did not alter BCRP expression; however, the combined testosterone plus E2 treatment increased BCRP expression. BCRP expression was not altered by hCG. These results provide new insight into the regulation of BCRP expression in human placenta by pregnancy-related hormones.
MATERIALS AND METHODS
Materials
Estriol (E-1253), 17β-estradiol (E-2758), testosterone (T-5411), flutamide (F-9397), and hCG (C-2047) were purchased from Sigma (St. Louis, MO). hPL (P-2984) was from Spring Bioscience (Fremont, CA). Recombinant hPRL was purchased from the National Hormone and Peptide Program of the University of California at Los Angeles. 7a,17b-[9-[(4,4,5,5,5-pentafluoropentyl)-sulfinyl]-nonyl]-estra-1,3,5(10)-triene-3,17-diol (ICI-182,780) was from Tocris Cookson (Ellisville, MO). HPLC grade DMSO was from Fisher Scientific (Pittsburgh, PA) and used as the solvent to dissolve the steroid hormones, ICI-182,780 and flutamine. The peptide hormones hCG, hPL, and hPRL were dissolved in phosphate-buffered saline (PBS). The Complete™ protease inhibitor cocktail was from Roche Molecular Biochemicals (Mannheim, Germany). The Laemmli sample buffer and 2-mercaptoethanol were from Bio-Rad (Hercules, CA). BeWo cells were from ATCC (Manassas, VA). RPMI 1640 phenol-red free medium was from Gibco (Grand Island, NY). PBS and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, CA). Charcoal/dextran-stripped FBS was purchased from HyClone (Logan, UT).
Cell Culture and Whole Cell Lysate Preparation
The BeWo cells were maintained in RPMI 1640 phenol-red free medium supplemented with 10% FBS as previously described (19). To examine BCRP expression in the BeWo cells treated with various hormones, the cells were first cultured in dishes in RPMI 1640 phenol-red free medium supplemented with 5% charcoal/dextran-stripped FBS for at least 48 h to achieve 60–70% confluence. The medium was then replaced with fresh medium, and the hormones at various concentrations were then added into the medium. Cell culture was continued for an additional 48 h. For studies in which cells were treated with a combination of E2 and testosterone, the cells were first primed with 5 × 10−9 M E2 for 24 h. The medium was then replaced with fresh medium, and the cells were incubated with E2 at the same concentration in the presence of testosterone for 48 h. The cells were then harvested for immunoblotting or mRNA isolation or immunofluorescent confocal microscopy. Only cells within eight passages after purchase were used in these experiments. The concentration of DMSO used was 0.1% (v/v). No effects of the vehicle on cell viability, BCRP protein and mRNA expression, and the plasma membrane localization of the transporter were observed at this concentration.
Whole cell lysates were prepared as previously described (19). Protein concentrations were determined by the Bio-Rad Dc protein assay kit (Bio-Rad, Hercules, CA) using bovine serum albumin as standard.
SDS-Polyacrylamide Gel Electrophorsis and Immunoblotting
The whole cell lysates (20 μg each lane) were subjected to immunoblotting using a BCRP-specific monoclonal antibody BXP-21 (Kamiya Biomedical, Seattle, WA) and a monoclonal antibody against β-actin (Sigma) for detection of BCRP and β-actin, respectively, as previously described (19). Relative BCRP protein levels were determined by densitometric analysis of the immunoblots using the NIH Scion Image software (Scion Corp., Frederick, MD). β-actin was used as an internal control. For detection of ERα, the monoclonal antibody NCL-ER-6F11 against human ERα (Novocastra Laboratories Ltd., Newcastle, UK) was used as the primary antibody at 1:50 dilution, and the goat anti-mouse HRP-conjugated antibody (Bio-Rad) was used as the second antibody at 1:5,000 dilution.
Confocal Microscopy
The effect of hormone treatment on plasma membrane localization of BCRP was examined by immunofluorescent confocal microscopy using BXP-21 as previously described (19).
Total RNA Isolation and Quantitative Real-Time TaqMan RT-PCR Analysis
The effect of hormone treatment on mRNA expression of BCRP and testosterone receptor (TR) was quantified by TaqMan real-time reverse transcription-polymerase chain reaction (RT-PCR) as follows. After treatment of the BeWo cells with various hormones, total cellular RNA was isolated using the Trizol® reagent (Invitrogen) according to the manufacturer’s instructions. To eliminate contamination of genomic DNA, all RNA samples were treated with DNase I (Promega, Madison, WI) and purified by ethanol precipitation prior to RT-PCR. The concentration of RNA was determined by measuring optical density at 260 nm. The OD260 nm/OD280 nm ratios of all RNA samples were determined to be between 1.7 and 2.0, to ensure that all RNA samples are highly pure. RNA integrity was examined by agarose gel electrophoresis. Single-strand cDNA used for analysis of BCRP was then synthesized from 0.25 μg of purified total RNA using a TaqMan® reverse transcription kit (Applied Biosystems, Branchberg, NJ) and single-strand cDNA used for analysis of TR was synthesized from 2.5 μg of purified total RNA, using a high capacity cDNA archive kit (Applied Biosystems, Foster City, CA), all in a volume of 25 μl. The synthesized cDNA was further purified by ethanol precipitation and dissolved in 25 μl of pure H2O. Real-time PCR reactions were then performed using a TaqMan® universal PCR master mix on the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Reactions were carried out in triplicates in a MicroAmp optical 96-well plate in a total volume of 20 μl. Each reaction mixture contained 10 μl of 2× TaqMan® universal PCR master mix, 6.1 μl of sterile Millipore water, 0.47 μl of forward primer (235 nM), 0.47 μl of reverse primer (235 nM), 0.47 μl of probe (118 nM) and 2.5 μl of reverse-transcription products. PCR conditions were as follows: 50°C for 2 min; 95°C for 10 min; 95°C for 15 s, 60°C for 1 min (40 cycles). Quantification of relative mRNA levels was carried out by determining the threshold cycle (C T), which is defined as the cycle at which the 6-carboxyfluorescein reporter fluorescence exceeds ten times the standard deviation of the mean baseline emission for cycles 3 to 10. β-actin was used as an internal control. The mRNA levels of BCRP or TR were normalized to those of β-actin according to the following formula: C T(BCRP or TR) − C T(β-actin) = ΔC T. Thereafter, the relative mRNA levels of BCRP or TR after hormone treatment were calculated using the ΔΔC T method: ΔC T(test hormone) − ΔC T(vehicle) = ΔΔC T(test hormone). The fold-changes of mRNA levels of BCRP or TR in BeWo cells upon treatment with a respective hormone were expressed as \( 2^{{ - \Delta \Delta {\text{C}}}}_{{\text{T}}} \). The primer pairs and probes for BCRP were the same as previously described (19). The primer pairs and probe for β-actin and TR (Hs00171172_ml AR) were purchased from Applied Biosystems (Foster City, CA). The concentration of DMSO used in all experiments was 0.1% (v/v).
Knock-down of ERα expression using siRNAs
Two small interfering RNA (siRNA) preparations of 25-bp double-stranded oligoribonucleotide targeting ERα were purchased from Invitrogen (Carlsbad, CA). The siRNA sequences (sense) were 5′-UAGCCAGGCACAUUCUAGAAGGUGG-3′ and 5′-CCACCUUCUAGAAUGUGCCUGGCUA-3′. The BeWo cells were maintained in RPMI 1640 phenol-red free medium as described above. To knock-down ERα expression, the cells were transfected with a combination of two siRNAs (each at 75 nM) or the control without siRNAs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Six hours after transfection, the medium was replaced with fresh medium containing 5% charcoal-stripped FBS. After 48 h, the cells were harvested and assessed for ERα expression by immunoblotting. To investigate the effect of knockdown of ERα expression on BCRP expression in the presence of hormones, 48 h after transfection, the medium was replaced with fresh medium containing 10−6 M E3 and the cell culture continued for another 48 h, or the medium was replaced with fresh medium containing 5 × 10−9 M E2 and the cells were primed for 24 h, followed by incubation with E2 at the same concentration and testosterone at 3 × 10−9 M for another 48 h. The cells were then harvested, and BCRP protein and mRNA expression determined by immunoblotting and real-time PCR, respectively.
Statistical Analysis
Data were analyzed for statistical significance using one-way ANOVA analysis or Student’s t test. Differences with p values of <0.05 were considered statistically significant.
RESULTS
Hormone treatment did not affect the plasma membrane localization of BCRP
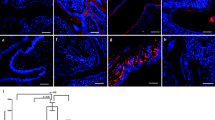
We first examined whether the treatment with E3, testosterone, hPL or hPRL can affect membrane localization of BCRP in BeWo cells using immunofluorescent confocal microscopy. BCRP was found to be predominantly expressed on the plasma membrane of BeWo cells with some intracellular expression (Fig. 1a). Treatment with 10−6 M E3 (Fig. 3b), 3 × 10−9 M testosterone (Fig. 3c), 3 × 10−9 M testosterone plus 5 × 10−9 M E2 (Fig. 3d), 1,000 ng/ml hPL (Fig. 3e) or 200 ng/ml hPRL (Fig. 3f) for 48 h had no qualitative effect on the plasma membrane localization of the transporter. We previously showed that treatment with E2 alone had no significant effect on BCRP localization (19). Thus, the levels of BCRP protein determined in whole cell lysates should reflect the levels of BCRP protein expression on the plasma membrane. We therefore determined BCRP protein expression using whole cell lysates in all of the subsequent immunoblotting experiments.
Confocal microscopy of BeWo cells. BeWo cells were treated with 0.1% (v/v) DMSO (a), 10−6 M E3 (b), 3 × 10−9 M testosterone (c), 3 × 10−9 M testosterone plus 5 × 10−9 M E2 (d), 1,000 ng/ml hPL (e), or 200 ng/ml hPRL (f) for 48 h. BCRP was then detected using the BCRP-specific monoclonal antibody BXP-21 as described in “Materials and Methods”. Selected areas of BeWo cells are shown, and BCRP protein expression is indicated in green. Images have been enhanced for maximal contrast between the black background and green fluorescence and were not intended for quantitative determination of BCRP expression. Arrows indicate the plasma membrane localization of BCRP.
E3 stimulated BCRP expression
To examine the effect of E3 on BCRP protein and mRNA, the BeWo cells were treated with E3 at various concentrations (10−8–10−5 M) for 48 h. E3 at 10−6 and 10−5 M significantly increased BCRP protein expression approximately twofold; however, β-actin expression was not affected under the same experimental conditions (Fig. 2a). E3 did not significantly affect BCRP protein expression at concentrations below 10−7 M. The inductive effect of 10−6 M E3 was abolished by the addition of ten times molar excess of the ER antagonist ICI-182,780 (Fig. 2b). ICI-182,780 itself at 10−5 M had no significant effect on BCRP protein expression (data not shown). Similarly, E3 at 10−6 M significantly increased BCRP mRNA expression approximately 2.5-fold, and this induction was completely abrogated by the addition of 10−5 M ICI-182,780 (Fig. 2c). To test if the inhibition of the inductive effect of E3 by ICI-182,780 was due to blocking ERα, we knocked down ERα expression by RNA interference. Transfection of BeWo cells with two siRNAs markedly down-regulated ERα protein expression by more than 80% (Fig. 2d) and down-regulation of ERα by RNA interference persisted for at least 5 days (data not shown); however, knock-down of ERα had no significant effect on E3-mediated induction of BCRP protein (Fig. 2b), suggesting that induction of BCRP by E3 is unlikely mediated by ERα.
Effect of E3 on BCRP expression in BeWo cells. Relative BCRP protein or BCRP mRNA or ERα protein levels normalized to β-actin were determined as described in “Materials and Methods”. Data shown are means ± S.E. from three independent experiments. Immunoblots shown are the representative results obtained in typical experiments. Difference in BCRP protein or mRNA levels is statistically significant: *p < 0.05 compared with the vehicle controls, $ p < 0.05 compared with the 10−6 M E3 plus 10−5 M ICI-182,780 treatment, and Δ p < 0.01 and # p < 0.05 compared E3 treatment alone, using one-way ANOVA analysis. Difference in ERα protein is statistically significant: **p < 0.01 compared with the vehicle control using Student’s t test. a Effect of E3 at various concentrations (10−8–10−5 M) on BCRP protein after 48 h treatment. b Effect of 10−5 M ICI-182,780 treatment (ICI) or RNA interference targeting ERα (siRNA) on E3-mediated induction of BCRP protein after 48 h treatment. c Effect of E3 and E3 plus ICI-182,780 on BCRP mRNA after 48 h treatment. d Effect of RNA interference (siRNA) on ERα protein expression after 48 h treatment. RNA interference was performed by transfection with a combination of two types of siRNA. Relative BCRP protein or BCRP mRNA or ERα protein levels of the vehicle controls are set as 1.
Testosterone together with E2 increased BCRP expression
To examine the effect of testosterone on BCRP expression, the BeWo cells were treated with testosterone at various concentrations (10−10 to 10−8 M) for 48 h. No effect of the testosterone treatments at these concentrations on BCRP protein expression was observed (data not shown). We then examined the combined effect of testosterone and E2 on BCRP expression. The BeWo cells were first primed with 5 × 10−9 M E2 for 24 h. The cells were then switched to fresh medium and incubated with E2 at the same concentration and testosterone at 3 × 10−9 M for 48 h. As shown in Fig. 3a, testosterone alone did not significantly affect BCRP protein expression. E2 alone decreased BCRP protein by approximately 50%. However, testosterone in combination with E2 significantly increased BCRP protein expression approximately twofold compared to the vehicle control. The expression of β-actin was not significantly influenced by any of these treatments. Furthermore, induction of BCRP protein by the combination of testosterone and E2 was completely abrogated by the addition of either 10−6 M ICI-182,780 or 10−6 M flutamide, a testosterone receptor antagonist (Fig. 3a). ICI-182,780 at 10−5 M and 10−6 M or flutamide at 10−6 M had no significant effect on BCRP protein (data not shown). The combined effect of testosterone and E2 on BCRP mRNA was similar to the effect on BCRP protein (Fig. 3b). Likewise, the addition of ICI-182,780 or flutamide completely reversed the inductive effect of the combined testosterone plus E2 treatment on BCRP mRNA (Fig. 3b). To test if testosterone in combination with E2 increased BCRP expression through ERα, we examined the effect of knock-down of ERα on BCRP expression. We noticed that down-regulation of ERα by RNA interference abrogated the inductive effect of testosterone and E2 on BCRP protein expression (Fig. 3c). Further analysis revealed that E2 treatment increased TR mRNA approximately 5.9-fold, and the addition of ICI-182,780 completely blocked this induction (Fig. 3d). Therefore, testosterone together with E2 increased BCRP expression, possibly through ERα-mediated induction of TR.
Effect of testosterone on BCRP expression in BeWo cells. Relative BCRP protein or BCRP mRNA or TR mRNA levels normalized to β-actin were determined as described in “Materials and Methods”. The concentrations of testosterone (T), E2, ICI-182,780 (ICI) and flutamide (F) used were 3 × 10−9, 5 × 10−9, 10−6, and 10−6 M, respectively. Data shown are means ± S.E. from three independent experiments. Immunoblots shown are the representative results obtained in typical experiments. Difference in BCRP protein or BCRP mRNA levels is statistically significant: *p < 0.05 compared with the vehicle controls, and Δ p < 0.01 and # p < 0.05 compared with the testosterone plus E2 treatment, using one-way ANOVA analysis. Difference in TR mRNA is statistically significant: *p < 0.05 compared with the vehicle control, and # p < 0.05 compared with E2 treatment alone, using one-way ANOVA analysis. a Effect of testosterone and E2 in the presence or absence of ICI-182,780 or flutamine on BCRP protein after 48 h treatment. b Effect of testosterone and E2 in the presence or absence of ICI-182,780 or flutamine on BCRP mRNA after 48 h treatment. c Effect of testosterone and E2 with or without RNA interference targeting ERα (siRNA) on BCRP protein after 48 h treatment. RNA interference was performed by transfection with a combination of two types of siRNA. d Effect of E2 in the presence or absence of ICI-182,780 on TR mRNA after 48 h treatment. Relative BCRP protein or BCRP mRNA or TR mRNA levels of the vehicle controls are set as 1.
Peptide hormones hPL and hPRL induced BCRP expression
To examine the effect of hPL, hPRL, or hCG on BCRP protein and mRNA, the BeWo cells were treated with hPL (10–5,000 ng/ml), hPRL (100–1,000 ng/ml), or hCG (2–200 ng/ml) for 48 h. hPL at 1,000 or 5,000 ng/ml significantly increased BCRP protein expression approximately twofold; however, β-actin expression was not affected (Fig. 4a). hPL did not increase BCRP protein expression at concentrations below 100 ng/ml. hPL at 1,000 ng/ml also significantly increased BCRP mRNA approximately twofold (Fig. 4b). Likewise, hPRL at 200 or 1,000 ng/ml significantly increased BCRP protein approximately twofold, but had no effect on β-actin expression (Fig. 5a). hPRL did not significantly alter BCRP protein expression at concentrations below 50 ng/ml. Likewise, hPRL at 200 ng/ml significantly increased BCRP mRNA approximately threefold (Fig. 5b). No significant effect of hCG on BCRP protein expression was observed at the concentrations tested (data not shown).
Effect of hPL on BCRP expression in BeWo cells. Relative BCRP protein or BCRP mRNA levels normalized to β-actin were determined as described in “Materials and Methods”. Data shown are means ± S.E. from three independent experiments. Immunoblots shown are the representative results obtained in typical experiments. Difference in BCRP protein levels is statistically significant: *p < 0.05 compared with the vehicle controls using one-way ANOVA analysis. Difference in BCRP mRNA is statistically significant: *p < 0.05 compared with the vehicle control using Student’s t test. a Effect of hPL at various concentrations (10–5,000 ng/ml) on BCRP protein after 48 h treatment. b Effect of 1,000 ng/ml hPL on BCRP mRNA after 48 h treatment. Relative BCRP protein or BCRP mRNA levels of the vehicle controls are set as 1.
Effect of hPRL on BCRP expression in BeWo cells. Relative BCRP protein or mRNA levels normalized to β-actin were determined as described in “Materials and Methods”. Data shown are means ± S.E. from three independent experiments. Immunoblots shown are the representative results obtained in typical experiments. Difference in BCRP protein levels is statistically significant: *p < 0.05 and **p < 0.01 compared with the vehicle controls using one-way ANOVA analysis. Difference in BCRP mRNA is statistically significant: **p < 0.01 compared with the vehicle control using Student’s t test. a Effect of hPRL at various concentrations (10–1,000 ng/ml) on BCRP protein after 48 h treatment. b Effect of 200 ng/ml hPRL on BCRP mRNA after 48 h treatment. Relative BCRP protein or mRNA levels of the vehicle controls are set as 1.
DISCUSSION
We have previously shown that P4 and E2, respectively, up-regulate and down-regulate BCRP expression in BeWo cells (19). In the present study, we further examined the effect of several other pregnancy-related hormones (E3, testosterone, hPL, hPRL, and hCG) on BCRP expression in BeWo cells. E3 is a quantitatively predominant endogenous estrogen formed during pregnancy. The plasma E3 concentration during pregnancy increases steadily to around 10−6 M prior to delivery (20). At this concentration, E3 significantly increased both BCRP protein and mRNA levels (Fig. 2a,c). This increase was abolished by ICI-182,780 (Fig. 2b); however, knock-down of ERα by RNA interference showed no effect on E3-mediated induction of BCRP (Fig. 2b), suggesting that E3 up-regulates BCRP expression through mechanisms other than ERα. Among estrogens, while E2 has nearly the highest and equal binding affinity for both ERα and ERβ, E3 has been shown to possess preferential binding affinity for ERβ over ERα (difference up to 18-fold) (21). Therefore, it is highly likely that E3 induces BCRP through transcriptional regulation by ERβ, which has been shown to be expressed in BeWo cells (19). We will test this hypothesis in future studies.
Pregnancy produces a significant increase in the plasma concentration of testosterone. The plasma testosterone concentration at delivery is approximately 3 × 10−9 M (22). At this concentration, testosterone by itself showed little effect on BCRP expression (Fig. 3a,b). The plasma E2 concentration during pregnancy also increases steadily to around 8 × 10−8 M at term (23,24). While consistent with our previous finding that E2 by itself at a subthreshold concentration (5 × 10−9 M) decreased BCRP expression (19), E2 together with testosterone increased BCRP expression (Fig. 3a,b). Further analysis revealed that down-regulation of ERα by RNA interference abolished the combined inductive effect of testosterone and E2 (Fig. 3c), and E2 strongly induced TR mRNA (Fig. 3d). These data suggest that the increase in BCRP expression by a combination of testosterone and E2 is possibly mediated by E2-induced synthesis of TR that is controlled by ERα at a transcriptional level. This notion is further supported by data showing that the addition of either ICI-182,780 or flutamide abolished the increase in BCRP expression by a combined testosterone plus E2 treatment (Fig. 3a,b). Thus, flutamide could directly inhibit the binding of testosterone to TR, which is required for the induction of BCRP by testosterone. On the other hand, ICI-182,780 could reverse the inductive effect of E2 on TR through inhibition of ERα (Fig. 3d), thus indirectly blocking the inductive effect of testosterone on BCRP expression. These data suggest that endogenous TR expression in BeWo cells is low, and hence testosterone exerts its function only after TR is induced by E2 through ERα. Several studies (25–27) also reported up-regulation of TR by E2, in various tissues and cell lines. Thus E2 seems able to alter androgenic signaling in these tissues and cells through interaction with testosterone via ERα and TR.
hPL and hPRL play a pivotal role during pregnancy to regulate and coordinate growth and metabolism in the mother and fetus (28–31). Like other pregnancy-related hormones such as P4, E2 and E3, the plasma concentrations of hPL and hPRL continuously rise throughout pregnancy to around 6,000 (32) and 200 ng/ml (33), respectively, in the third trimester. hPL at both 1,000 and 5,000 ng/ml, and hPRL at 200 ng/ml significantly increased BCRP expression (Figs. 4 and 5), suggesting that hPL could induce BCRP expression throughout the course of pregnancy, and hPRL may up-regulate BCRP only in the later stages of pregnancy. hPL and hPRL belong to a family of hormones believed to have arisen from a common ancestral gene (34). The signal transduction mechanisms involved in hPL and hPRL actions are similar and have been extensively studied. The biological activities of hPL and hPRL are mediated by a specific membrane receptor, the PRL receptor (34). Binding of hPL or hPRL to the PRL receptor leads to receptor dimerization and activation of the intracellular tyrosine kinase, JAK2 (35). The activated JAK2, in turn, phosphorylates the receptor at specific tyrosine residues within the cytoplasmic domain and thus recruits the STAT proteins, including STAT1, 3, and 5. Then, JAK2 phosphorylates the STAT proteins at specific tyrosine residues, leading to their dissociation from the receptor, dimerization, and translocation into the nucleus. In the nucleus, the STAT dimers bind to specific DNA sequences and induce gene transcription (36). The Na+/taurocholate cotransporting polypeptide, a major uptake transporter for bile salts in the liver, has been shown to be up-regulated by mouse PL and ovine PRL through the JAK/STAT5 signal transduction pathway (37). Whether hPL or hPRL can up-regulate BCRP expression through the JAK/STAT pathway remains to be investigated. BCRP has been shown to be strongly induced in the mammary gland of lactating mice and human, and is responsible for the secretion of xenobiotics (38) and vitamin B2 (39) into milk. Since hPL and hPRL are essential lactogenic hormones responsible for regulating mammary differentiation and milk secretion (40), our data that hPL and hPRL can induce BCRP expression in BeWo cells suggest that these hormones may also play a role in the up-regulation of BCRP in the mammary gland.
Recent studies support the notion that BCRP plays a significant role in the placental-to-maternal transport of drugs (9,14). It also becomes clear that BCRP expression in the placenta and other tissues significantly changes with gestational ages (17,18,41). Therefore, elucidating the molecular mechanisms by which BCRP expression is regulated during pregnancy is essential for the understanding of how drugs are distributed across the placental barrier and how pregnancy affects the systemic exposure to BCRP substrate drugs. Currently available data suggest that the pregnancy-related hormones play a crucial role in regulating BCRP expression during pregnancy; however, the mechanisms appear to be quite complex. The data obtained from this study and the study published earlier (19) indicate that multiple hormones, including P4, E2, E3, testosterone, hPL, and hPRL, can all contribute to the up-regulation of BCRP. Moreover, interaction between different hormones appears to play an essential role in regulating BCRP expression as well. We and others have shown that E2 by itself down-regulates BCRP expression (19,42); however, when combined with P4, E2 can further increase P4-induced BCRP expression through up-regulation of the progesterone receptor B (PRB) expression (19). Similarly, as demonstrated in this study, E2 can induce TR expression through ERα, leading to increased BCRP expression by testosterone which by itself has no effect on BCRP expression in BeWo cells. Although not having been investigated in this study, interaction involving other combinations of hormones is also possible. For example, E2 has been shown to interact with ovine PRL, leading to repression of ovine PRL-induced expression of the Na+/taurocholate cotransporting polypeptide in liver cells (43). Whether E2 is capable of suppressing hPRL-mediated up-regulation of BCRP in BeWo cells remains to be determined. Finally, in a recent study using pregnant mice, we demonstrated a significant correlation between mRNA expression of Bcrp1 and the nuclear receptors AhR and HIF1α in mouse placenta (17), further suggesting a complex scenario involving the molecular mechanisms regulating BCRP expression during pregnancy in which both nuclear receptors (e.g., ERα, TR and AhR) and membrane receptors (e.g., the PRL receptor) could be involved, either independently or cooperatively.
In summary, the present data establish that E3, hPL, hPRL, or the combination of testosterone with E2 up-regulates BCRP expression in BeWo cells. Our data demonstrate the significance of cross-talk between ERα and TR in regulating BCRP expression in the placenta. These data support the hypothesis that BCRP expression in the placenta and other tissues can be regulated by multiple hormones, either independently or cooperatively. Further studies will be necessary to elucidate the molecular mechanisms by which BCRP expression is regulated by these hormones. Such studies will help elucidate how pregnancy affects drug disposition in pregnant women.
Abbreviations
- ABC:
-
ATP-binding cassette
- AhR:
-
aryl hydrocarbon receptor
- BCRP:
-
breast cancer resistance protein
- DMSO:
-
dimethyl sulfoxide
- E2 :
-
17β-estradiol
- E3 :
-
estriol
- hCG:
-
human chorionic gonadotropin
- HIF1α:
-
hypoxia-inducible factor 1α
- hPL:
-
human placental lactogen
- hPRL:
-
human prolactin
- JAK2:
-
Janus kinase 2
- P4 :
-
progesterone
- PBS:
-
phosphate-buffered saline
- STAT:
-
the signal transducers and activators of transcription
References
L. A. Doyle, W. Yang, L. V. Abruzzo, T. Krogmann, Y. Gao, A. K. Rishi, and D. D. Ross. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 95:15665–15670 (1998).
R. Allikmets, L. M. Schriml, A. Hutchinson, V. Romano-Spica, and M. Dean. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 58:5337–5339 (1998).
K. Miyake, L. Mickley, T. Litman, Z. Zhan, R. Robey, B. Cristensen, M. Brangi, L. Greenberger, M. Dean, T. Fojo, and S. E. Bates. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 59:8–13 (1999).
Q. Mao, and J. D. Unadkat. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 7:E118–133 (2005).
L. A. Doyle, and D. D. Ross. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 22:7340–7358 (2003).
Y. Zhang, A. Gupta, H. Wang, L. Zhou, R. R. Vethanayagam, J. D. Unadkat, and Q. Mao. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm. Res. 22:2023–2034 (2005).
M. Maliepaard, G. L. Scheffer, I. F. Faneyte, M. A. van Gastelen, A. C. Pijnenborg, A. H. Schinkel, M. J. van De Vijver, R. J. Scheper, and J. H. Schellens. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61:3458–3464 (2001).
S. Cisternino, C. Mercier, F. Bourasset, F. Roux, and J. M. Scherrmann. Expression, up-regulation, and transport activity of the multidrug-resistance protein abcg2 at the mouse blood–brain barrier. Cancer Res. 64:3296–3301 (2004).
J. W. Jonker, J. W. Smit, R. F. Brinkhuis, M. Maliepaard, J. H. Beijnen, J. H. Schellens, and A. H. Schinkel. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Cancer Inst. 92:1651–1656 (2000).
C. M. Kruijtzer, J. H. Beijnen, H. Rosing, W. W. ten Bokkel Huinink, M. Schot, R. C. Jewell, E. M. Paul, and J. H. Schellens. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 20:2943–2950 (2002).
P. Breedveld, N. Zelcer, D. Pluim, O. Sonmezer, M. M. Tibben, J. H. Beijnen, A. H. Schinkel, O. van Tellingen, P. Borst, and J. H. Schellens. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug–drug interactions. Cancer Res. 64:5804–5811 (2004).
M. Hirano, K. Maeda, S. Matsushima, Y. Nozaki, H. Kusuhara, and Y. Sugiyama. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol. Pharmacol. 68:800–807 (2005).
H. Zaher, A. A. Khan, J. Palandra, T. G. Brayman, L. Yu, and J. A. Ware. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol. Pharm. 3:55–61 (2006).
F. Staud, Z. Vackova, K. Pospechova, P. Pavek, M. Ceckova, A. Libra, L. Cygalova, P. Nachtigal, and Z. Fendrich. Expression and transport activity of breast cancer resistance protein (Bcrp/Abcg2) in dually perfused rat placenta and HRP-1 cell line. J. Pharmacol. Exp. Ther. 319:53–62 (2006).
J. Kraemer, J. Klein, A. Lubetsky, and G. Koren. Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety. Am. J. Obstet. Gynecol. 195:270–274 (2006).
C. Gedeon, J. Behravan, G. Koren, and M. Piquette-Miller. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 27:1096–1102 (2006).
H. Wang, X. Wu, K. Hudkins, A. Mikheev, H. Zhang, A. Gupta, J. D. Unadkat, and Q. Mao. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am. J. Physiol. Endocrinol. Metab. 291:E1295–1304 (2006).
H. E. Meyer zu Schwabedissen, M. Grube, A. Dreisbach, G. Jedlitschky, K. Meissner, K. Linnemann, C. Fusch, C. A. Ritter, U. Volker, and H. K. Kroemer. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab. Dispos. 34:524–533 (2006).
H. Wang, L. Zhou, A. Gupta, R. R. Vethanayagam, Y. Zhang, J. D. Unadkat, and Q. Mao. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. 290:E798–807 (2006).
M. Peter, H. G. Dorr, and W. G. Sippell. Changes in the concentrations of dehydroepiandrosterone sulfate and estriol in maternal plasma during pregnancy: a longitudinal study in healthy women throughout gestation and at term. Horm. Res. 42:278–281 (1994).
B. T. Zhu, G. Z. Han, J. Y. Shim, Y. Wen, and X. R. Jiang. Quantitative structure–activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 147:4132–4150 (2006).
M. A. Rivarola, M. G. Forest, and C. J. Migeon. Testosterone, androstenedione and dehydroepiandrosterone in plasma during pregnancy and at delivery: concentration and protein binding. J. Clin. Endocrinol. Metab. 28:34–40 (1968).
S. Batraand, and L. P. Bengtsson. 17 beta-Estradiol and progesterone concentrations in myometrium of pregnancy and their relationships to concentrations in peripheral plasma. J. Clin. Endocrinol. Metab. 46:622–626 (1978).
F. S. Khan-Dawood, and M. Y. Dawood. Estrogen and progesterone receptor and hormone levels in human myometrium and placenta in term pregnancy. Am. J. Obstet. Gynecol. 150:501–505 (1984).
J. Fujimoto, M. Nishigaki, M. Hori, S. Ichigo, T. Itoh, and T. Tamaya. The effect of estrogen and androgen on androgen receptors and mRNA levels in uterine leiomyoma, myometrium and endometrium of human subjects. J. Steroid. Biochem. Mol. Biol. 50:137–143 (1994).
O. O. Adesanya-Famuyiwa, J. Zhou, G. Wu, and C. Bondy. Localization and sex steroid regulation of androgen receptor gene expression in rhesus monkey uterus. Obstet. Gynecol. 93:265–270 (1999).
S. Rodriguez-Cuenca, M. Monjo, A. M. Proenza, and P. Roca. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am. J. Physiol. Endocrinol. Metab. 288:E200–207 (2005).
M. J. Soares. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal–fetal interface. Reprod. Biol. Endocrinol. 2:51 (2004).
S. Handwerger. Clinical counterpoint: the physiology of placental lactogen in human pregnancy. Endocr. Rev. 12:329–336 (1991).
S. Handwerger, and M. Freemark. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatr. Endocrinol. Metab. 13:343–356 (2000).
M. Freemark. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm. Res. 65(Suppl 3):41–49 (2006).
I. Sanchez-Vera, B. Bonet, M. Viana, A. Quintanar, and A. Lopez-Salva. Increased low-density lipoprotein susceptibility to oxidation in pregnancies and fetal growth restriction. Obstet. Gynecol. 106:345–351 (2005).
O. A. Kletzky, F. Rossman, S. I. Bertolli, L. D. Platt, and D. R. Mishell, Jr. Dynamics of human chorionic gonadotropin, prolactin, and growth hormone in serum and amniotic fluid throughout normal human pregnancy. Am. J. Obstet. Gynecol. 151:878–884 (1985).
V. Goffin, K. T. Shiverick, P. A. Kelly, and J. A. Martial. Sequence–function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr. Rev. 17:385–410 (1996).
H. Rui, J. Y. Djeu, G. A. Evans, P. A. Kelly, and W. L. Farrar. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J. Biol. Chem. 267:24076–24081 (1992).
C. Schindler, and J. E. Darnell, Jr. Transcriptional responses to polypeptide ligands: the JAK–STAT pathway. Annu. Rev. Biochem. 64:621–651 (1995).
J. Cao, P. M. Gowri, T. C. Ganguly, M. Wood, J. F. Hyde, F. Talamantes, and M. Vore. PRL, placental lactogen, and GH induce NA(+)/taurocholate-cotransporting polypeptide gene expression by activating signal transducer and activator of transcription-5 in liver cells. Endocrinology. 142:4212–4222 (2001).
J. W. Jonker, G. Merino, S. Musters, A. E. van Herwaarden, E. Bolscher, E. Wagenaar, E. Mesman, T. C. Dale, and A. H. Schinkel. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 11:127–129 (2005).
A. E. van Herwaarden, E. Wagenaar, G. Merino, J. W. Jonker, H. Rosing, J. H. Beijnen, and A. H. Schinkel. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 27(4):1247–1253 (2007).
M. C. Neville, T. B. McFadden, and I. Forsyth. Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia. 7:49–66 (2002).
S. Yasuda, S. Itagaki, T. Hirano, and K. Iseki. Expression level of ABCG2 in the placenta decreases from the mid stage to the end of gestation. Biosci. Biotechnol. Biochem. 69:1871–1876 (2005).
Y. Imai, E. Ishikawa, S. Asada, and Y. Sugimoto. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. 65:596–604 (2005).
J. Cao, M. Wood, Y. Liu, T. Hoffman, J. Hyde, O. K. Park-Sarge, and M. Vore. Estradiol represses prolactin-induced expression of Na+/taurocholate cotransporting polypeptide in liver cells through estrogen receptor-alpha and signal transducers and activators of transcription 5a. Endocrinology. 145:1739–1749 (2004).
Acknowledgments
The authors acknowledge financial support from NIH grants HD044404 (to QM and JDU) and GM073715 (to QM). We also thank Yi Zhang, Lin Zhou, Eun-Woo Lee, and Weibin Zhou (Department of Pharmaceutics, University of Washington) for technical assistance in cell culture and immunoblotting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Unadkat, J.D. & Mao, Q. Hormonal Regulation of BCRP Expression in Human Placental BeWo Cells. Pharm Res 25, 444–452 (2008). https://doi.org/10.1007/s11095-007-9432-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9432-z