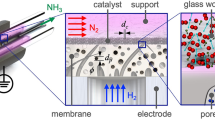
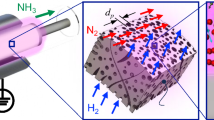
Abstract
The plasma nitrogen fixation for NOx synthesis from N2 and O2 in MnOx/Al2O3 packed-bed dielectric barrier discharge (DBD) and an enhanced effect of MnOx/Al2O3 catalyst are reported. At N2 content of 50% and SEI of ~ 16 kJ/mol (flow rate of 800 SCCM and discharge power of ~ 9.5 W), NOx production rates are 0.28 SCCM for Al2O3 and 0.42 SCCM for MnOx/Al2O3, and improved by ~ 60% due to the enhanced effect of MnOx/Al2O3. The enhanced effect becomes more significant at lower specific energy input (SEI) or higher N2 content (lower O2 content). The MnOx/Al2O3-packed DBD features much more and lower-intensity micro-discharges, larger total capacitance, greater peak-to-peak charge, and higher vibrational temperature of N2 than the Al2O3-packed DBD. The surface role of MnOx/Al2O3 catalyst in the enhanced effect was disclosed by two-step surface reaction processes and in-situ temperature programed desorption for the adsorbed species of the first step.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of plasma for nitrogen fixation has attracted great attention in reduction of N2 to ammonia with hydrogen or oxidation of N2 to nitrogen oxides (NOx, NO + NO2) with oxygen [1,2,3,4,5,6,7,8,9,10]. The oxidative nitrogen fixation is superior to reductive nitrogen fixation in the cost of feed gas, because air can be directly used for NOx synthesis. The NOx production rate is limited by N2 and O2 dissociation due to high bond energy of N2 (9.8 eV) and O2 (5.2 eV). Due to the relatively low electron energy in atmospheric-pressure plasma [11], most of the molecules are in electronically and vibrationally excited states (O2*, N2*), rather than dissociation to N and O atoms, despite they are considered as the important species from a plausible plasma radical-involving mechanism [12].
The early attempt to promote the reaction of excited molecules was made by the combination of plasma with catalysts using inductively coupled plasma (ICP) at low pressure. For example, Rapakoulias et al. [13] found the enhanced effect of WO3 and MoO3 catalysts on NO synthesis and suggested that vibrationally excited nitrogen molecule underwent dissociative adsorption on the catalyst surface and then reacted with oxygen. Gicquel et al. [14] proposed that N atoms or excited N2 molecules reacted with WO3 and MoO3 catalysts to form MoO3–N (adsorbed) or MoO3–N2*(adsorbed) and then NO was released. However, the excited N2 molecules collided with the oxide surface would prefer energy relaxation instead of dissociation due to the very low sticking probability on oxides.
Recently, dielectric barrier discharge (DBD) plasma has been used to study the plasma catalytic synthesis of NOx. Patil et al. [15] investigated the effect of various metal oxides (WO3, PbO, CuO, Co3O4, NiO, MoO3 and V2O5) supported on Al2O3 on NOx synthesis in a DBD reactor. The WO3/Al2O3 catalyst increased the NOx concentration by about 10% compared to Al2O3 and the authors thought that the vibrationally excited nitrogen molecules have a reaction with the mobile oxygen species on the catalyst surface. Ma et al. [16] investigated the effect of Al2O3 and BaTiO3 packing on NOx synthesis in a DBD reactor. They proposed that the enhanced production of NOx was attributed to the increased electron energy due to the presence of packing materials instead of surface-reaction contribution. In our previous work [17], it was found that higher temperatures (> 623 K) were beneficial for the formation of NOx on Cu-ZSM-5 catalyst in a DBD reactor. The reaction of nitrogen species with the activated O2 or adsorbed atomic oxygen on Cu active sites at higher temperatures may improve the NO formation. Taken together, the studies on plasma nitrogen fixation for NOx synthesis have rather little reported, and the contribution of surface reaction is still hitherto unknown.
The aim of this work is to study, for the first time, NOx synthesis from N2 and O2 in MnOx/Al2O3-packed DBD plasma, because MnOx with strong reducibility, multiple oxidation states and oxygen vacancies [18], has a potential to activate the oxygen species. In this work, based upon the comparison with the empty and Al2O3-packed DBD, an enhanced effect on NOx production in MnOx/Al2O3-packed DBD is found. The enhanced effect becomes more significant at lower specific energy input (SEI) or higher N2 content (lower O2 content). To gain an insight on the enhanced effect of MnOx/Al2O3 catalyst, its electrical discharge characteristics, optical emission spectra and surface role of MnOx/Al2O3 catalyst were further investigated.
Experimental Methods
Figure 1 is the schematic diagram of the experimental setup. The DBD reactor is coaxial and consists of a quartz tube (10 mm outer diameter and 8 mm inner diameter), a high voltage electrode (3-mm-diameter stainless steel rod) and a ground electrode (30 mm-height stainless steel mesh). The high voltage electrode was powered by an AC power supply with frequency of 13.3 kHz (CTP‐2000 K, Nanjing Suman). The digital oscilloscope (Tektronix MDO3014) recorded signals from a high voltage probe (Tektronix P6015A) and a passive probe connected with a 51 Ω sampling resistor or a 0.22 µF sampling capacitor, then voltage-current waveforms or charge–voltage Lissajous figures were obtained. The input power (Pin) was directly measured by a wattmeter from the power supply, and the discharge power was calculated from the Lissajous figure. One of three types of circuits, including a sampling resistor, sampling capacitor and short circuit, was chosen to connect the switch without stopping discharge. The specific energy input (SEI) is defined as,
where Pdis and Fin are the discharge power and the gas inlet flow rate, respectively.
Optical emission spectroscopy (OES) was used for the plasma diagnostic. The fiber-optic probe is perpendicular to the DBD reactor, and the distance between the reactor wall and fiber is 45 mm. The light emitted from the DBD plasma was measured by a high-resolution spectrometer (Andor Shamrock SR-750, 1200 grooves/mm grating, 50 μm width slit) along with an intensified charge-coupled device (ICCD) detector (Andor iStar DH334). The spectrum was recorded with exposure time of 0.5 s and 20 accumulations. The second positive system of N2 (C3Пu → B3Пg) was used to evaluate the rotational (Tr) and vibrational (Tv) temperatures by the method reported previously [19]. Fig. S1 gives an example of experimental and fitted spectra for evaluating the rotational and vibrational temperatures.
N2 and O2 gases (purity 99.999%), whose flow rates were adjusted by mass flow controllers, were fed separately or mixed into the reactor. The outlet gas from the reactor flowed into the gas cell of the Fourier transform infrared (FT-IR) spectrometer (IGS gas analyzer, Thermo Fisher, USA). The concentrations of NO, NO2, N2O and O3 were monitored online by the FT-IR spectrometer, equipped with an MCT detector, in a scanning range of 600–4000 cm−1 and at a resolution of 0.5 cm−1. N2 was used to purge the gas cell and gas pipelines for removing air and water before measurement. The time for data collection is more than 20 min, which is enough to reach a steady concentration of the products even under a minimal flow rate (400 SCCM).
It is needed to note, there exist N2O and NO2 impurities in NO standard gas due to NO decomposition at high pressures of gas cylinder. Hence, the calibration curve of NO was obtained by using N2-balanced NO2 standard gas, which went through a molybdenum converter to obtain accurate concentrations of NO for NO calibration. The concentrations of NO and N2O were quantified in the range of 1880–1960 cm−1 and 2140–2280 cm−1, respectively. To quantify NO2, 1880–1960 cm−1 (below 1000 ppm) and 2820–2950 cm−1 (between 1000 and 10,000 ppm) were employed. The concentration calibration curves of NO and NO2 are shown in Fig. S2. Figure 2 shows a typical FTIR spectra. Because NO is easily oxidized to NO2 in O2-containing gas, the concentration of NOx is defined as the sum of NO and NO2 and it was adopted to evaluate the reaction performance.
The MnOx/Al2O3 catalysts were prepared by incipient wetness impregnation of γ-Al2O3 pellets (diameter ~ 1 mm) in manganese nitrate solution for 12 h at room temperature. The impregnated samples were dried at 120 °C for 4 h and then calcined at 450 °C in static air for 4 h. The nominal content in Mn of the MnOx/Al2O3 catalyst is 9 wt%. The X-ray diffraction (XRD) patterns were obtained by using D8 Advance X-ray Diffractometer (Bruker, Germany, Cu Kα, 0.154056 nm). The XRD profile of the as-prepared MnOx/Al2O3 is shown in Fig. S3, showing that the MnOx with weak crystallinity of β-MnO2. The morphology of MnOx/Al2O3 catalysts was observed by a field emission scanning electron microscopy (FE-SEM, JSM-7900F, JEOL LTD, Japan). The SEM images with energy dispersive spectroscopy (EDS) analysis of MnOx/Al2O3, as shown in Fig. S4, indicate an even dispersion of MnOx on the Al2O3 support. Before plasma reaction, the catalysts packed into the DBD reactor were in-situ purified with 100 SCCM N2 at 400 °C for 0.5 h using a tubular furnace, then cooled down to room temperature for starting the plasma catalytic reaction.
To gain an insight into the MnOx/Al2O3 catalyst enhanced NOx synthesis, the plasma catalytic reaction was separated into two steps. At the first step, the catalyst was treated by one of N2 and O2 plasmas for 20 min with a flow rate of 100 SCCM at Pin = 20 W; at the second step, the catalyst was treated by the other one at the same flow rate and Pin as the first step. For the MnOx/Al2O3 catalyst, the gaseous and surface products of the second step (N2 plasma) after O2 adsorption or O2 plasma for the first step were compared. Moreover, the adsorbed species of the first step were analyzed by in-situ temperature programed desorption (TPD). After the first step, the in-situ TPD was carried out from room temperature to 400 °C at a ramp rate of 10 °C/min, and kept at 400 °C for 30 min in Ar gas of 100 SCCM. A mass spectrometer (MS, Hiden HPR20, UK) with a heated (at 393 K) quartz inert capillary inlet was used to monitor online the products during the two-step surface reaction processes and the in-situ TPD.
Results and Discussion
Enhanced Effect of MnOx/Al2O3 Catalyst on NOx Synthesis
Figure 3 shows the NOx concentrations produced in the empty, Al2O3-packed, or MnOx/Al2O3-packed DBD reactor at flow rate of 600 SCCM and 50% nitrogen content. The NOx concentrations of the empty and Al2O3-packed DBD are very close at discharge power of about 9.5 W (Case A in Fig. 3) being 506 and 513 ppm, respectively. Nonetheless, the NOx concentration increases significantly to 755 ppm in MnOx/Al2O3-packed DBD, which is much higher than those of the empty and Al2O3-packed DBD. Likewise, at discharge power of about 17 W (Case B in Fig. 3), NOx concentration of the MnOx/Al2O3-packed DBD is the highest, reaching 1300 ppm. This demonstrates that MnOx/Al2O3 catalyst has an enhanced effect on NOx production.
The NOx concentration produced in the DBD reactor. Conditions: Fin = 600 SCCM, 50% N2 + 50% O2. Case A: Pdis of empty, Al2O3-packed and MnOx/Al2O3-packed DBD at Pin = 20 W are 9.6, 9.5 and 9.7 W, respectively; Case B: Pdis of empty, Al2O3-packed and MnOx/Al2O3-packed DBD at Pin = 30 W are 16.8, 16.9 and 17.3 W, respectively
By varying discharge power or flow rate, the effect of SEI on NOx concentration and production rate in the Al2O3-packed and MnOx/Al2O3-packed DBD at 50% nitrogen content is presented in Fig. 4. At a fixed flow rate, the residence time is kept at constant and thus the effect of SEI by changing discharge power is a single-factor examination. The SEI means the average energy absorbed by each molecule for activation and reaction in plasma. The increase of SEI leads to a rise in electron density, so higher SEI is benefit to the formation of more active species of nitrogen and oxygen to promote the production of NOx. As a result, NOx concentration and production rate increase rapidly with SEI by changing discharge power (Fig. 4a), which accords with the literature reported in plasma catalytic ammonia synthesis [20]. The SEI can also be varied by adjusting flow rate when the power is fixed, however, the residence time is changed accordingly. Interestingly, as shown in Fig. 4b (by changing flow rate), the NOx production rate rises very slow with SEI for Al2O3-packed, and even turns to decrease slightly after a small increase for MnOx/Al2O3-packed. Figure 4b is a result of combined effect of SEI and residence time. The long residence time brings about further oxidation of NOx–N2O5 [21], or NO decomposition into N2 and O2 [22, 23], which weakens or overwhelms the positive effect of SEI on NOx production.
NOx concentration and production rate versus SEI by changing, a discharge power and b flow rate. Conditions: 50% N2 + 50% O2. a Fin = 400 SCCM, Pdis of MnOx/Al2O3 (Al2O3) are 5.2 (5.2), 9.6 (9.4) and 17.3 (17.1) W at Pin of 15, 20 and 30 W, respectively; b Pin = 20 W, Pdis of MnOx/Al2O3 (Al2O3) are 9.9 (9.4), 9.7 (9.5) and 9.5 (9.1) W for Fin of 400, 600 and 800 SCCM, respectively
Moreover, Fig. 4 shows that NOx concentration and production rate of MnOx/Al2O3 are higher than that of Al2O3 at the same SEI, supporting again the enhanced effect of MnOx/Al2O3 catalyst. Especially at lower SEI, MnOx/Al2O3 catalyst provides greater enhanced effect on NOx production rate. For example, at SEI of ~ 16 kJ/mol (Fig. 4b), NOx production rates are 0.26 SCCM for Al2O3 and 0.42 SCCM for MnOx/Al2O3. The MnOx/Al2O3 catalyst improves the NOx production rate by ~ 60% compared to Al2O3, which is much higher than the reported enhancement (at most 10%) of active metal oxides (e.g., WO3) supported on Al2O3 [15].
Figure 5 shows the effect of N2 content on NOx production in the packed-bed DBD reactor. The discharge powers are very close at the same input power, as shown in Fig. S5. With the increase of N2 content from 40 to 90%, NOx concentrations present volcano-shaped variation, and the peak concentrations of NOx fall in the N2 content range from 50 to 70%. Within the investigated range of N2 content, MnOx/Al2O3 catalyst exhibits much higher NOx concentration than Al2O3.
According to NOx concentration and total flow rate in Fig. 5a, the ratio of NOx production rate of MnOx/Al2O3 to Al2O3 versus N2 content is shown in Fig. 5b. As N2 content increases from 40 to 90%, NOx production rate ratio of MnOx/Al2O3 to Al2O3 climbs linearly from 1.3 to 1.6. It can be concluded that MnOx/Al2O3 catalyst displays greater enhancement on NOx production at higher N2 content (lower O2 content).
To gain an insight on the enhanced effect of MnOx/Al2O3 catalyst, its electrical discharge characteristics, OES and surface role of the catalyst were further investigated.
Electrical Discharge Characteristics and OES Diagnostic
Figure 6 shows the waveforms of current–voltage for the empty, Al2O3-packed, or MnOx/Al2O3-packed DBD reactors. The corresponding charge–voltage Lissajous figures for the three cases are displayed in Fig. 7, obtaining their discharge powers of about 17 W. The current pulses variation with time in Fig. 6 and the discharge appearance in Fig. S6 indicate that the discharge is in the typical filamentary mode with a large amount of micro-discharges [24]. In the case of the MnOx/Al2O3-packed DBD, the filamentary discharge becomes weak micro-discharge along with surface discharge. The current waveforms between empty DBD and Al2O3-packed DBD are very similar, there existing tens of current pulses in a quarter cycle of applied voltage, and the Lissajous figures are similar to parallelogram (Fig. 7). In contrast, the MnOx/Al2O3-packed DBD exhibits more current pulses with lower intensity (Fig. S7) and the Lissajous figure becomes a distorted parallelogram. The Lissajous figures show the relationship between the transferred charge in the circuit and the voltage applied to the DBD plasma. From the slope of the upper and lower edges of the parallelogram, the total capacitance of the DBD can be estimated. The MnOx/Al2O3-packed DBD has larger total capacitance than the empty and Al2O3-packed DBD, which is caused by that MnOx is a kind of semiconductor material. The peak-to-peak voltages are almost equal in the three cases in Fig. 7, but the peak-to-peak charge in the case of MnOx/Al2O3 is greater than the other two cases. A similar phenomenon was observed in the BaTiO3-packed DBD [16]. Compared with the empty and Al2O3-packed DBD, the MnOx/Al2O3-packed DBD shows special discharge characteristics and features much more and lower-intensity micro-discharges, leading to an increase in collision probability of electrons and reactant molecules. This is advantageous to the generation of more active species and promotes the subsequent reaction for NOx formation.
At the same conditions as Fig. 5, the variations in rotational temperature (Tr) and vibrational (Tv) temperatures of N2 molecule from fitting the OES results with N2 content are shown in Fig. 8. In the packed-bed DBD, the vibrational temperatures of MnOx/Al2O3 are higher than those of Al2O3, with an average increase of about 230 K. The MnOx/Al2O3-packed DBD features much more and lower-intensity micro-discharges, larger total capacitance and greater peak-to-peak charge than the Al2O3-packed DBD, leading to a somewhat increase in electron density. This is favorable to forming more vibrationally-excited nitrogen molecules with high vibration quantum number, thus bring about a rise in the vibrational temperature of MnOx/Al2O3. The differences in rotational temperature between MnOx/Al2O3 and Al2O3 are not significant, and they are equivalent if considering fitting deviation.
For the packed-bed DBD, the rotational temperature is independent of N2 content. By contrast, the vibrational temperature decreases with N2 content probably due to the decrease of average energy per N2 molecule from vibrational excitation. The lower Tv at higher N2 content suggests that the populations of the high-lying vibrational levels of N2 decline with the number of N2 molecules.
Insight into Surface Role of MnOx/Al2O3 Catalyst in the NOx Production
To gain an insight into surface role of MnOx/Al2O3 catalyst in the NOx production, the two-step surface reaction processes and in-situ TPD for the adsorbed species of the first step were implemented. For MnOx/Al2O3, after the first step of O2 adsorption or O2 plasma, the MS signal during the second step of N2 plasma is shown in Fig. 9a, b, respectively. MnOx/Al2O3 catalyst in N2 plasma after O2 adsorption is unable to produce NO (Fig. 9a). However, after O2 plasma, NO can be produced significantly in N2 plasma and plenty of O2 is released from the oxygen species adsorbed on MnOx/Al2O3 surface (Fig. 9b). This indicates that the oxygen species involved in production of NO and O2 in Fig. 9b derive from O2 plasma, rather than from O2 adsorption. As expected, on the Al2O3 surface, there is no production of NO and O2 even after O2 plasma (Fig. 9c). It can be concluded that O2 plasma results in the production of oxygen species on MnOx surface, providing the required oxygen source for the formation of NO in N2 plasma. The conclusion is confirmed again by in-situ TPD for the adsorbed species of the first step. The MnOx/Al2O3 catalyst after O2 plasma presents obviously a desorption peak of O2 at 652 K, compared with that after O2 adsorption (Fig. 10a). In contrast, there is no desorption peak of N2 on the MnOx/Al2O3 catalyst, whether after N2 plasma or after N2 adsorption (Fig. 10b).
MS signals of m/z = 30 (NO) and m/z = 32 (O2) from N2 plasma: a MnOx/Al2O3 after O2 adsorption, b MnOx/Al2O3 after O2 plasma, and c Al2O3 after O2 plasma. Conditions: O2 adsorption (Fin = 100 SCCM, 20 min); O2 plasma (Fin = 100 SCCM, Pin = 20 W, 20 min); N2 plasma (Fin = 100 SCCM, Pin = 20 W, 45 min)
When MnOx/Al2O3 catalyst is exposed to O2 plasma, the oxygen vacancies on MnOx surface may serve as the active sites of the excited-state oxygen molecules (O2*) produced from plasma, which may be electronically (e.g., metastable-state O2(1Δg)) and vibrationally excited. The O2* species on MnOx/Al2O3 catalyst becomes adsorbed oxygen atoms (Oad) by dissociation adsorption.
If the MnOx/Al2O3 catalyst is only exposed to N2 gas after O2 plasma, there is no NO production, as shown in Fig. S8a, confirming that the adsorbed oxygen atoms (Oad) on MnOx/Al2O3 catalyst has no efficient energy to react with ground-state N2 molecule. This implies that the Oad species on MnOx/Al2O3 catalyst can only react with the excited-state nitrogen molecules (N2*) produced from plasma, including electronically (e.g., metastable-state N2(A3 \({\Sigma }_{u}^{+}\)), N2(B3 \({\Pi }_{g}\)), N2(C3 \({\Pi }_{u}\))) and vibrationally (N2 (v)) excited nitrogen molecules.
Furthermore, if the MnOx/Al2O3 catalyst, after exposure to N2 plasma of the first step, is incapable of producing NO in O2 plasma of the second step, as shown in Fig. S8b. It is evidenced that the N2* species are unable to adsorb on MnOx/Al2O3 catalyst (Fig. 10b), due to the very low dissociative sticking probability [25]. It can be concluded that the O2* species in plasma may turn into the atomic oxygen species (Oad) adsorbed on MnOx/Al2O3 catalyst by means of dissociation adsorption, and then the Oad species react with the N2* species coming from plasma to form NO, as illustrated in Fig. 11. The contribution of MnOx/Al2O3 catalyst to NOx formation becomes greater under lower O2 content (higher N2 content), which is consistent with the dependence of enhancement of MnOx/Al2O3 on N2 content in Fig. 5b.
Conclusions
Based upon the comparison with the empty and Al2O3-packed DBD, an enhanced effect on NOx production in MnOx/Al2O3-packed DBD is reported. At N2 content of 50% and SEI of ~ 16 kJ/mol (flow rate of 800 SCCM and discharge power of ~ 9.5 W), NOx production rates are 0.28 SCCM for Al2O3-packed and 0.42 SCCM for MnOx/Al2O3-packed.
When the flow rate is fixed, the NOx concentration and production rate increase rapidly with SEI. In contrast, when the flow rate is changed, the NOx production rate rises very slow with SEI for Al2O3-packed, and even turns to decrease slightly after a small increase for MnOx/Al2O3-packed. NOx concentration presents volcano-shaped variation with N2 content and the peak concentrations of NOx fall in N2 contents of 50–70%. As N2 content increases from 40 to 90%, NOx production rate ratio of MnOx/Al2O3 to Al2O3 grows linearly from 1.3 to 1.6. In brief, the enhanced effect becomes more significant at lower SEI or higher N2 content (lower O2 content).
The MnOx/Al2O3-packed DBD features much more and lower-intensity micro-discharges, larger total capacitance and greater peak-to-peak charge than the Al2O3-packed DBD, forming more vibrationally-excited nitrogen molecules with high vibration quantum number, thus bring about a rise in the vibrational temperature of MnOx/Al2O3. The surface role of MnOx/Al2O3 catalyst in the enhanced effect was disclosed by two-step surface reaction processes and in-situ TPD for the adsorbed species of the first step. The O2* species in plasma may turn into the Oad species on MnOx/Al2O3 catalyst by means of dissociation adsorption, and then the Oad species react with the N2* species coming from plasma to form NO.
Data Availability
Data are available on request from the authors.
References
Patil BS, Wang Q, Hessel V, Lang J (2015) Plasma N2-fixation: 1900–2014. Catal Today 256:49–66
Abdelaziz AA, Ishijima T, Osawa N, Seto T (2019) Quantitative Analysis of ozone and nitrogen oxides produced by a low power miniaturized surface dielectric barrier discharge: effect of oxygen content and humidity level. Plasma Chem Plasma Process 39:165–185
Abdelaziz AA, Kim HH (2020) Temperature-dependent behavior of nitrogen fixation in nanopulsed dielectric barrier discharge operated at different humidity levels and oxygen contents. J Phys D Appl Phys 53:17
Han YF, Wen SY, Tang HW, Wang XH, Zhong CS (2018) Influences of frequency on nitrogen fixation of dielectric barrier discharge in air. Plasma Sci Technol 20:7
Zhang S, Zong LJ, Zeng X, Zhou RW, Liu Y, Zhang C, Pan J, Cullen PJ, Ostrikov K, Shao T (2022) Sustainable nitrogen fixation with nanosecond pulsed spark discharges: insights into free-radical-chain reactions. Green Chem 24:1534–1544
Van Alphen S, Eshtehardi HA, O’Modhrain C, Bogaerts J, Van Poyer H, Creel J, Delplancke MP, Snyders R, Bogaerts A (2022) Effusion nozzle for energy-efficient NOx production in a rotating gliding arc plasma reactor. Chem Eng J 443:12
Jardali F, Van Alphen S, Creel J, Eshtehardi HA, Axelsson M, Ingels R, Snyders R, Bogaerts A (2021) NOx production in a rotating gliding arc plasma: potential avenue for sustainable nitrogen fixation. Green Chem 23:1748–1757
Vervloessem E, Aghaei M, Jardali F, Hafezkhiabani N, Bogaerts A (2020) Plasma-based N2 fixation into NOx: insights from modeling toward optimum yields and energy costs in a gliding arc plasmatron. ACS Sustain Chem Eng 8:9711–9720
Gorbanev Y, Vervloessem E, Nikiforov A, Bogaerts A (2020) Nitrogen fixation with water vapor by nonequilibrium plasma: toward sustainable ammonia production. ACS Sustain Chem Eng 8:2996–3004
Wang WZ, Patil B, Heijkers S, Hessel V, Bogaerts A (2017) Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. Chemsuschem 10:2145–2157
Lu X, Bruggeman PJ, Reuter S, Naidis G, Bogaerts A, Laroussi M, Keidar M, Robert E, Pouvesle J-M, Liu D, Ostrikov K (2022) Grand challenges in low temperature plasmas. Front Phys 10:1040658
Liu TW, Gorky F, Carreon ML, Gomez-Gualdron DA (2022) Energetics of reaction pathways enabled by N and H radicals during catalytic, plasma-assisted NH3 synthesis. ACS Sustain Chem Eng 10:2034–2051
Rapakoulias D, Cavadias S, Amouroux J (1980) Processus catalytiques dans un réacteur à plasma hors d’équilibre II. Fixation de l’azote dans le système N2–O2. Rev Phys Appl 15:1261–1265
Gicquel A, Cavadias S, Amouroux J (1986) Heterogeneous catalysis in low-pressure plasmas. J Phys D Appl Phys 19:2013
Patil BS, Cherkasov N, Lang J, Ibhadon AO, Hessel V, Wang Q (2016) Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: effect of support materials and supported active metal oxides. Appl Catal B 194:123–133
Ma Y, Wang Y, Harding J, Tu X (2021) Plasma-enhanced N2 fixation in a dielectric barrier discharge reactor: effect of packing materials. Plasma Sources Sci Technol 30:105002
Sun Q, Zhu AM, Yang XF, Niu JH, Xu Y (2003) Formation of NOx from N2 and O2 in catalyst-pellet filled dielectric barrier discharges at atmospheric pressure. Chem Commun 9:1418–1419
Dong Y, Sun J, Ma X, Wang W, Song Z, Zhao X, Mao Y, Li W (2022) Study on the synergy effect of MnOx and support on catalytic ozonation of toluene. Chemosphere 303:134991
Zhao TL, Xu Y, Song YH, Li XS, Liu JL, Liu JB, Zhu AM (2013) Determination of vibrational and rotational temperatures in a gliding arc discharge by using overlapped molecular emission spectra. J Phys D Appl Phys 46:345201
Gershman S, Fetsch H, Gorky F, Carreon ML (2022) Identifying regimes during plasma catalytic ammonia synthesis. Plasma Chem Plasma Process 42:731–757
Cheng H, Li Y, Zheng K, Liu D, Lu X (2021) Numerical analysis of nitrogen fixation by nanosecond pulse plasma. J Phys D Appl Phys 54:184003
Katabathini N, Maksod IHAE, Mokhtar M (2021) Cu, Fe and Mn oxides intercalated SiO2 pillared magadiite and ilerite catalysts for NO decomposition. Appl Catal A 616:118100
Goto N, Kudo S, Motoyama H, Ohyama S (2002) Direct decomposition technique for NO in O2–N2 mixture using barrier discharge and Cu zeolite. Jpn J Appl Phys Part 2 41:L64–L66
van’t Veer K, van Alphen S, Remy A, Gorbanev Y, De Geyter N, Snyders R, Reniers F, Bogaerts A (2021) Spatially and temporally non-uniform plasmas: microdischarges from the perspective of molecules in a packed bed plasma reactor. J Phys D Appl Phys 54:174002
Rouwenhorst KHR, Jardali F, Bogaerts A, Lefferts L (2021) From the Birkeland-Eyde process towards energy-efficient plasma-based NOx synthesis: a techno-economic analysis. Energy Environ Sci 14:2520–2534
Funding
This work is supported by the National Natural Science Foundation of China (22278052, 11975069) and the Liaoning Revitalization Talent Program (XLYC2008032).
Author information
Authors and Affiliations
Contributions
TQZ: Investigation, Data curation, Validation, Writing—Original draft. XSL, JLL and XQW: Methodology, Formal analysis, Visualization, Writing—Reviewing and Editing. AMZ: Conceptualization, Supervision, Resources, Funding acquisition, Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Conflict of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, TQ., Li, XS., Liu, JL. et al. Plasma Nitrogen Fixation: NOx Synthesis in MnOx/Al2O3 Packed-Bed Dielectric Barrier Discharge. Plasma Chem Plasma Process 43, 1907–1919 (2023). https://doi.org/10.1007/s11090-023-10345-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10345-8