Abstract
This work investigates the effect of the Palladium nanoparticles (PdNPs) on the characteristics and pH sensitivity of an interdigitated electrode extended-gate field-effect transistor (pH-IEGFET) sensors based on the bare n-type macro porous Si (n-macro PSi) layer. The synthesized bare porous layer was fabricated by photo electrochemical etching process of n-type (100) using short-wavelength 410 nm and intensive laser power density 80 mW/cm2. The surface morphology of n-macro PSi was modified by inserting PdNPs to form PdNPs/n-macro PSi hetero structure layers via simple and fast immersing process in PdCl2 solution for about 14 min and 20 min by the ion reduction process. The fabricated sensors have been verified in the range of pH from 3 to 11 at the ambient temperature. Specific features of n-macro PSi and hybrid structure were investigated using a scanning electron microscope, energy dispersive x-ray spectroscopy (EDX) analysis and x-ray diffraction. It was found that the morphology and the size of the deposited PdNPs intensely influence on the PdNPs/ n-macro PSi hetero structure pH-IEGFET sensor. The efficient pH sensing process was obtained for n-macro PSi pH sensor with the lowest size of PdNPs deposited on the external walls of the n-macro PSi pores. Significant enhancement of sensitivity of about 42.89 mV/pH and linearity of 0.9948 was achieved, compared with the bare n-macro PSi pH sensor having a sensitivity of 25.12 mV/pH and linearity of 0.9392. This sensitivity enhancement is due to the additional specific surface area of PdNPs which causes improved it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Porous silicon (PSi) is well-thought-out as a very effective material in the chemical sensor because of its exclusive properties, which depend on the large surface area (Alwan et al. 2018a; Pacholski 2013). Modification in the morphology of PSi can be attained via the change of etching conditions through a number of factors, such as current density, etching time, and illumination conditions (Alwan et al. 2018b; Jabbar, Alwan 2019). The activity of the PSi as a chemical sensor is influenced by the surface morphological features, comprising pore forms, homogeneity, and the dimension of the PSi, moreover, the depth of the porous layer (Mehdi, Alwan 2019; Dheyab et al. 2019). In specific, pH sensor is unique to the supreme sensors owing to the significance of pH measurement for biochemical and industrial applications (Harraz 2014; Yuqing et al. 2005). PSi layer can be used as a sensitive element for determining the pH level of buffer solution based on the variation of photoluminescence properties (Al-Hardan et al. 2016a; Benilov et al. 2007).Variations of the pH level lead to the regular variations of photoluminescence intensity, the high photoluminescence intensity corresponds to a lower pH level, whereas the low photoluminescence intensity corresponds to a higher pH level (Benilov et al. 2007). Bare PSi as a reference-less semiconductor sensing layer for pH sensing within several categories of field-effect transistor (FET) and ion-sensitive field-effect transistor (ISFET) (Bergveld 1970) has been investigated. PSi has formerly been used for sensing of pH in different platforms. Benilov et al. (Benilov et al. 2007) studied the radiative lifetime of the bare and modified PSi in liquid solutions with different pH, and it was shown that the photoluminescence (PL) intensity and the decay lifetime of modified PSi strongly depend on the value of pH. (Zehfroosh et al. 2010) composed the PSi with the gate region of ISFET, the obtained sensitivities for both types are higher than the Nernst theoretical limit of about 59.2 mV/pH. Naif H. (Al-Hardan et al. 2016b) prepared and tested PSi as an extended gate field-effect transistor (EGFET) for sensing of pH, and it was found that it has a sensitivity value of 66 mV/pH. Ahmed et al. (Ahmed et al. 2017) used (111) PSi as the basis of an EGFET pH sensor and obtained that pH is detectable with a pH sensitivity of 56.13 mV/pH. Zeng et al. (Zeng et al. 2018) showed that the sensitivity and linearity of pH for IEGFET are 22.4 mV/pH and 0.928, correspondingly. Nasser et al. (Nasser 2018a, b) reported on the PSi IEGFET pH sensor. They studied the effect of the crystalline orientation of P-type PSi (p-type < 100 > and p-type < 111 >) and of n-type PSi (n-type < 100 > and n-type < 111 >) on the IEGFET pH sensor performance in the range of pH from 3 to 11 at the ambient temperature. It was found that the performance improvement of the PSi at the crystalline orientation of < 100 > for both types of PSi sensors is higher than that at the crystalline orientation of < 111 > , owing to the high surface area to volume ratio.
The modification of bare PSi within the chemical and biological devices via incorporation of metallic nanoparticles has been found as an efficient method to increase the activity of porous layer (Wali et al. 2019a; Alwan,Dheyab 2017; Wali et al. 2019b). The PSi surface is usually terminated by hydrogen (Alwan,Abdulrazaq 2008). The existence of Si–Hx (x = 1,2,3) bonds on the PSi surface is proficient of formation metallic nanoparticles deposited over the PSi through the reduction process of metallic ions (Alwan et al. 2017). It is recognized that palladium freely absorbs efficiently the hydrogen molecules at room temperature (Das et al. 2014). Based on this exclusive feature of palladium, the palladium nanoparticles (PdNPs) layer as a sensitive element in the IEGFET structures within the bare PSi pH sensor represents an important case for developing the performance of the sensors. In the current research, n-type PSi layer-based interdigitated electrode extended-gate field-effect transistor (IEGFET) structure has been studied by a surface modification process through the incorporation of PdNPs with different morphologies and particle sizes via a simple and well-controlled method for pH detecting. The sensor performances have been studied and analyzed widely.
2 Fabrication and experimental
2.1 Formation of bare n-macro PSi
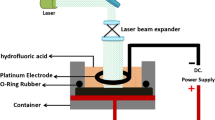
Bare n-macro PSi sample was fabricated via photo electrochemical etching process (PECEP) using a short wavelength of 410 nm and an intensive laser power density of 80 mW/cm2. n-type Si substrate of (100) crystalline orientation and 20 Ω.cm resistivity was used in the etching process .The silicon substrate was cut into pieces with a dimension of (2 cm × 2 cm) and then etched in 20% concentration; (1:1) electrolyte solution (HF 40%: C2H5OH 98.98%) at a current density of 18 mA/cm2 for 15 min etching time at the room temperature. Bare n-macro PSi samples were then reserved in methanol to evade the growth of the native oxide SiO2 layer. Fig. 1 shows the PECEP etching setup used in this work.
2.2 Modification of bare n-macro PSi samples with PdNPs and Electrode
PdCl2 salt having 98% purity was dissolved in the deionized water to prepare PdCl2 aqueous solution at a concentration of 10–3 M. The suitable PdCl2 solution concentrations were precisely using the formula (Alwan et al. 2017):
W (g) and M.wt (g/mol) are the weight and molecular weight of PdCl2 salt, respectively. V (liter) is the volume of the dissolved solution. 0.2 M HF and little drops of HCl (37wt %) were added to the 10-3 M PdCl2 aqueous solution at 55 °C with magnetic stirring for 25 min. Then, bare n-macro PSi samples were immersed in PdCl2 solution for about 14 min for sample (S1) and 20 min for sample (S2) to form PdNPs/ n-macro PSi hetero structure pH sensors. The modification process of bare PSi by incorporation of PdNPs was carried out according to the subsequent reactions at the room temperature (Wali et al. 2019a; Alwan et al. 2019):
To synthesize the interdigitated sensing structure with PdNPs/ n-macro PSi hetero structures, the Pd metal electrode with 250 nm thickness and 99% purity was selected to metallize the hetero structures through a suitable mask. Palladium is a metal of choice in devices for hydrogen sensing. Palladium electrode was attained over the PdNPs/n-macro Psi hetero structures by using a vacuum thermal evaporator system with a pressure of 10–5 mbar. Figure 2 illustrates the schematic representation of the palladium electrode above the PdNPs/n-macro PSi layer.
The pads of the electrode for the synthesized interdigitated Pd/ PdNPs/ n-macro PSi /Pd sensors were attached with a copper wire through Ag paste for connecting with the read out unit. The current–voltage (I-V) characteristics of the IEGFET were measured via external read out unit (616 kethily digital electrometer). The measurement setup is shown in Fig. 3. The first sensor terminal was linked to the FDC655BN, whereas the second was connected to the FDC655BN n-MOSFET gate terminal which acts as the FET in the IEGFET device. The interdigitated Pd/ PdNPs/n-macro PSi /Pd sensors were dipped in the buffer solution of pH at the range of (3–11) for two min. The measurements were recorded at the ambient temperature. After each test, the sensing gate was washed using deionized water for 1 min to remove the waste substances from the sensor. The morphological features of the bare n-macro PSi and PdNPs/n-macro PSi hetero structures were inspected by means of scanning electron microscope (SEM) image (JM-5600). The PdNPs size distribution was determined by special software program Image J version 7. The structural properties were measured using XRD-6000, Shimadzu x-ray diffractometer, and energy dispersive x-ray spectroscopy (EDX) analysis.
3 Results and discussion
3.1 Structural characterization of bare n-macro PSi and PdNPs/n-macro PSi hetero structures.
The surface morphology of the bare n-macro PSi layer before the deposition of nanoparticles is shown in Fig. 4; it can be noted that the n-macro PSi appears as a pore-like structure with circular connected pore shapes of different sizes. The range of the histogram for the sizes of PSi pores is around 0. 15 µm to 1.25 µm, and the histogram peak is about 0.15 µm. The porosity of the prepared n-macro PSi was computed using the gravimetrical method (Ahmed et al. 2013) and it was evaluated as 82%; the Si nanocrystallite sizes were assessed as approximately 75 nm.
Figure 5(a, b) displays the images of SEM for PdNPs deposited on the bare n-macro PSi layer. From this figure, it is shown that the PdNPs have various morphologies of different particle sizes and shapes. For sample S1, as exposed in Fig. 5a, the PdNPs are very consistently distributed outside the silicon nanocrystallites within the porous matrix. The histogram was wide-ranging from 25 to 75, nm and the peak of the histogram situated at 25 nm. Whereas, for sample S2 (Fig. 5b), the PdNPs layer partially covered the pores of the n-macro PSi due to the high agglomeration of the PdNPs as island regions over the bare PSi layer surface. The average area of the island regions was in the range of (1.5–13.5) µm2.
The EDX spectra of PdNPs deposited on the n-macro PSi layer are elicited in Fig. 6(a, b). This figure approves the development of PdNPs and the existence of silicon element, and no additional elements were realized. The intensity of PdNPs of sample S2 is greater than that of sample S1, signifying that the nucleation rate of the metallic nanoparticles in sample S1 is lower than that of sample S2 due to the higher immersion time.
The XRD pattern of PdNPs incorporated on the bare n-macro PSi layer is manifested in Fig. 7(a, b). It is realized that the grown-up PdNPs are polycrystalline and have particular diffraction peaks at 39.85° and 43.9° that correspond to (111) and (200) diffraction planes, respectively (reference code: 00–001-1201) belong to the PdNPs of F.C.C structure. The XRD peaks of the sample S1 are lesser than that of sample S2 due to the enhancement of the nucleation rate of PdNPs and then, increase their amount with the immersion time. The full width at half maximum (FWHM) of the peaks of the sample S1 is wider than that of sample S2 due to its lower value of grain sizes.
The average grain sizes (d) of PdNPs were assessed from the peak widening, and it can be obtained via Scherrer’s formula as follows (Alwan et al. 2017):
where, 0.9, λ and θ are the shape factor value, the wavelength of the employed radiation and the Bragg diffraction angle, respectively. The specific surface area (SSA) is unique of the particulars of the quality of the nanostructure materials, it was evaluated using the following equation (Alwan et al. 2017):
where, ρ is the density of the palladium (12.023) g/cm3. The average grain sizes of PdNPs and the specific surface area of the two samples (S1, S2) are calculated for the plane (111). For sample S1, they are about 28.2 nm and 16.2 m2/g, respectively, while they are about 33 nm and 11.2 m2/g, respectively for sample S2.
3.2 Electrical properties of the PdNPs/n-macro PSi hetero structure pH-IEGFET sensors
The variation in the threshold voltage (Vth (IEGFET)) and hence the current–voltage (I–V) characteristics of the hetero structure pH-IEGFET sensor was recorded as a function of the change in the pH level. The variation in Vth (IEGFET) with the change in pH follows the following formula (Chou and Chen 2009)
where Vth (MOSFET), ϕM, q, Vref, χSol and ψ are the metal oxide field effect transistor (MOSFET) threshold voltage, the metal gate work function, the electron charge, the reference electrode voltage, the electrolyte surface dipole-voltage and the surface voltage at the interface of the sensing layer/ electrolyte, respectively. The surface voltage (ψ) is the only variable term, it is a pH dependent factor (Nasser 2018b). Other terms of the Eq. (6) are constants. The operating of IEGFET is fairly like to that of the traditional MOSFET, but the metal gate is replaced by the interdigitated structure on the sensing layer. This layer can be assumed as PdNPs/n-macro Psi hetero structure. The IEGFET operation depends on the formation of double layers at the electrolyte / PdNPs/n-macro PSi hetero structure layer interface creating a surface voltage that changes the turn-on voltage (Nasser and Ali 2018a; Al-Hardan et al. 2016a). The effects of the incorporation of PdNPs on the n-macro PSi layer on the (IDS-VGS) characteristics of the hetero structures pH-IEGFET sensor were examined at a fixed value of VDS (0.2 V) in the linear part of MOSFET characteristics, as evinced in Fig. 8(b, c), whereas Fig. 8a depicts the characterization of n-macro PSi layer before inserting the PdNPs. From this figure, the increase of the pH value rises leads to an increase in the output voltage. This outcome can be clarified by Eq. (7), where the increase of the pH value leads to decrease ψ. This ψ decreasing is owing to the reduction in the aggregated hydrogen ions (H+) on the sensing layer (macro PSi or PdNPs/n-macro PSi hetero structure) surfaces and the solution. The relationship between the surface voltage and the values of pH may be expressed using the following equation (Nasser 2018b; Chou et al. 2003):
where, pHpzc, K, T and β are the value of the pH at the point of zero charges, the Boltzmann constant, the absolute temperature and the sensitivity parameter, respectively. The value of ψ is related to the properties of the base sensing material (PSi), especially the density of the dangling bonds (Si–H+) within the porous matrix in addition to the specific surface area of the resulting porous layer. The increasing of these bonds and the specific surface area leads to an increase in the surface voltage (ψ). Where, based on the theory of the site binding, the density of the aggregated charges H+ of the Si–H+ (binding sites) remaining over the sensing layer can modify the surface voltage in the sensing layer/solution interface.
Figure 8a, of the IEGFET pH sensor with the bare macro PSi displays a weak response to the variation in the pH level with a poor voltage sensitivity and linearity. The performance of this type of sensor is much less than that of the PdNPs/n-macro PSi hetero structure sample S1 (Fig. 8b), but it's better than that of PdNPs/n-macro PSi hetero structure sample S2 (Fig. 8c).Performance of the IEGFET pH sensor with the hetero structure (sample S1) may be clarified as follows: the pore size modification by incorporating the PdNPs on the pore walls improves the sensitivity performance of the device due to the enhanced specific surface area integrated (specific surface area of PSi layer and specific surface area of PdNPs). Increasing of the specific surface area improves the sensor’s sensitivity (Nasser 2018b; Harraz 2014). While for the IEGFET pH sensor with the hetero structure (sample S2), the decreasing device sensitivity is related to the morphology of the deposited PdNPs. Where, the high agglomeration of PdNPs (island regions of PdNPs) that partially covers the pores of the n-macro PSi lead is to reduce the activity of the pores, and hence the Si–H+, so the sensor’s sensitivity will decrease.
The sensitivity of the pH voltage was obtained from the IDS-VGS properties, and the relationship between VGS and pH at the fixed IDS current of about 200 μA is revealed in Fig. 9(a-c). The sensitivity of the pH voltage was computed using the following formula:
For sample S1, the achieved sensitivity and linearity for the IEGFET pH sensor with the hetero structure (Fig. 9b) are about 42.89 mV/pH and 0.9948, respectively. And, they are about 25.12 mV/pH and 0.9392, respectively for the bare n-macro PSi pH sensor (Fig. 9a). The sensitivity and the linearity of the IEGFET pH sensor with the hetero structure for sample S1 are high compared with those of the bare n-macro PSi.While for sample S2, the sensitivity for the IEGFET pH sensor with the hetero structure was dropped to a very poor value of about 19.07 mV/pH (Fig. 9c).
4 Conclusion
In this work, PdNPs/n-macro PSi hetero structure pH-IEGFET sensors were fabricated and tested successfully for a pH concentration ranging from 3 to 11. The surface modification process of the bare n-macro PSi was conducted by incorporating different sizes and morphologies of PdNPs. It has been distinguished that the performance of the hetero structure IEGFET pH sensor is governed by the morphology and the PdNPs sizes. An efficient pH sensing process was obtained for the n-macro PSi pH sensor with the lowest size of PdNPs deposited on the external walls of the pores due to the improvement of the integrated specific surface area.
References
Ahmed, N.M., Al-Douri, Y., Alwan, A.M., Jabbar, A.A., Arif, G.E.: Characteristics of nanostructure silicon photodiode using laser assisted etching. Procedia Engineering 53, 393–399 (2013)
Ahmed, N.M., Kabaa, E., Jaafar, M., Omar, A.: Characteristics of extended-gate field-effect transistor (EGFET) based on porous n-type (111) silicon for use in pH sensors. J. Electron. Mater. 46(10), 5804–5813 (2017)
Al-Hardan, N., Abdul Hamid, M., Ahmed, N., Jalar, A., Shamsudin, R., Othman, N., Kar Keng, L., Chiu, W., Al-Rawi, H.: High sensitivity pH sensor based on porous silicon (PSi) extended gate field-effect transistor. Sensors 16(6), 839 (2016a)
Al-Hardan, N.H., Hamid, A., Azmi, M., Ahmed, N.M., Jalar, A., Shamsudin, R., Othman, N.K., Kar Keng, L., Chiu, W., Al-Rawi, H.N.: High sensitivity pH sensor based on porous silicon (PSi) extended gate field-effect transistor. Sensors 16(6), 839 (2016b)
Alwan, A.M., Abdulrazaq, O.A.: Aging effect on the photosynthesized porous silicon. Int. J. Mod. Phys. B 22(04), 417–422 (2008)
Alwan, A.M., Dheyab, A.B.: Room temperature CO 2 gas sensors of AuNPs/mesoPSi hybrid structures. Applied Nanoscience 7(7), 335–341 (2017)
Alwan, A.M., Hashim, D.A., Jawad, M.F.: Efficient bimetallic nanoparticles embedded-porous silicon CO gas sensor. Solid-State Electronics 153, 37–45 (2019)
Alwan, A.M., Wali, L.A., Yousif, A.A.: Optimization of AgNPs/mesoPS active substrates for ultra–low molecule detection process. Silicon 10(5), 2241–2251 (2018a)
Alwan, A.M., Yousif, A.A., Wali, L.A.: The growth of the silver nanoparticles on the mesoporous silicon and macroporous silicon: a comparative study. Indian J. Pure Appl. Phys. (IJPAP) 55(11), 813–820 (2017)
Alwan, A.M., Yousif, A.A., Wali, L.A.: A study on the morphology of the silver nanoparticles deposited on the n-Type porous silicon prepared under different illumination types. Plasmonics 13(4), 1191–1199 (2018b)
Benilov, A., Gavrilchenko, I., Benilova, I., Skryshevsky, V., Cabrera, M.: Influence of pH solution on photoluminescence of porous silicon. Sens. Actuators, A 137(2), 345–349 (2007)
Bergveld, P.: Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Transactions on Biomedical Engineering(1), 70–71 (1970)
Chou, J.-C., Chen, C.-W.: Fabrication and application of ruthenium-doped titanium dioxide films as electrode material for ion-sensitive extended-gate FETs. IEEE Sens. J. 9(3), 277–284 (2009)
Chou, J.-C., Kwan, P.-K., Chen, Z.-J.: SnO2 separative structure extended gate H+-ion sensitive field effect transistor by the sol–gel technology and the readout circuit developed by source follower. Jpn. J. Appl. Phys. 42(11R), 6790 (2003)
Das, A., Ko, D.H., Chen, C.-H., Chang, L.-B., Lai, C.-S., Chu, F.-C., Chow, L., Lin, R.-M.: Highly sensitive palladium oxide thin film extended gate FETs as pH sensor. Sensors and Actuators B: Chemical 205, 199–205 (2014)
Dheyab, A.B., Alwan, A.M., Zayer, M.Q.: Optimizing of gold nanoparticles on porous silicon morphologies for a sensitive carbon monoxide gas sensor device. Plasmonics 14(2), 501–509 (2019)
Harraz, F.A.: Porous silicon chemical sensors and biosensors: A review. Sensors and Actuators B: Chemical 202, 897–912 (2014)
Jabbar, A.A., Alwan, A.M.: Efficient detecting of TNT molecules using Palladium nanoparticles/cross shape pores like structure porous silicon. Vibrational Spectroscopy, 102933 (2019)
Mehdi, Q., Alwan, M.: Active control of silver nanostructure aggregates for ultrahigh sensitive SERS detection of organic molecules: single molecule approach. Int J Anoelectron Mater 12(1), 55–66 (2019)
Nasser, A.R., Ali, G.M.: Orientation Effect of n-Type PSi on Integrated Interdigitated Electrode EGFET pH Sensor Performance. In: 2018 International Conference on Advanced Science and Engineering (ICOASE) 2018a, pp. 200–205. IEEE
Nasser, A.R., Ali, G.M.: A Porous Silicon P-Type Interdigitated Extended-Gate Field Effect Transistor pH Sensor. Silicon, 1–8 (2018b)
Pacholski, C.: Photonic crystal sensors based on porous silicon. Sensors 13(4), 4694–4713 (2013)
Wali, L.A., Alwan, A.M., Dheyab, A.B., Hashim, D.A.: Excellent fabrication of Pd-Ag NPs/PSi photocatalyst based on bimetallic nanoparticles for improving methylene blue photocatalytic degradation. Optik 179, 708–717 (2019)
Wali, L.A., Hasan, K.K., Alwan, A.M.: Rapid and highly efficient detection of ultra-low concentration of penicillin G by gold nanoparticles/porous silicon SERS active substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 206, 31–36 (2019)
Yuqing, M., Jianrong, C., Keming, F.: New technology for the detection of pH. J. Biochem. Biophys. Methods 63(1), 1–9 (2005)
Zehfroosh, N., Shahmohammadi, M., Mohajerzadeh, S.: High-sensitivity ion-selective field-effect transistors using nanoporous silicon. IEEE Electron Device Lett. 31(9), 1056–1058 (2010)
Zeng, R., Zhang, J., Sun, C., Xu, M., Zhang, S.-L., Wu, D.: A reference-less semiconductor ion sensor. Sensors and Actuators B: Chemical 254, 102–109 (2018)
Acknowledgements
The authors would like to thank the University of Technology, Baghdad-Iraq and Mustansiriyah University (www.uomustansiriyah.edu.iq), Baghdad-Iraq for their support in the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alwan, A.M., Wali, L.A. & Zayer, M.Q. A new approach of pH-IEGFET sensor based on the surface modification of macro porous silicon with palladium nanoparticles. Opt Quant Electron 52, 227 (2020). https://doi.org/10.1007/s11082-020-02346-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-020-02346-0