Abstract
Omega-3 (n3) is a polyunsaturated fatty acid well known for its anti-inflammatory and neuroprotective properties. Obesity is linked to chronic inflammation that disrupts metabolism, the intestine physiology and the central nervous system functioning. This study aims to determine if n3 supplementation can interfere with the effects of obesity on the mitochondrial activity, intestinal barrier, and neurotransmitter levels in the brain of Wistar rats that received cafeteria diet (CAF). We examined adipose tissue, skeletal muscle, plasma, intestine, and the cerebral cortex of four groups: CT (control diet), CTn3 (control diet with n3 supplementation), CAF, and CAFn3 (CAF and n3). Diets were offered for 13 weeks, with n3 supplementation in the final 5 weeks. Adipose tissue Electron Transport Chain complexes I, II, and III showed higher activity in CAF groups, as did complexes III and IV in skeletal muscle. Acetate levels in plasma were reduced in CAF groups, and Lipopolysaccharide (LPS) was higher in the CAF group but reduced in CAFn3 group. Claudin-5 in the intestine was lower in CAF groups, with no n3 supplementation effect. In the cerebral cortex, dopamine levels were decreased with CAF, which was reversed by n3. DOPAC, a dopamine metabolite, also showed a supplementation effect, and HVA, a diet effect. Serotonin levels increased in the CAF group that received supplementation. Therefore, we demonstrate disturbances in mitochondria, plasma, intestine and brain of rats submitted to CAF and the potential benefit of n3 supplementation in endotoxemia and neurotransmitter levels.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Omega-3 (n3) constitutes a group of polyunsaturated fatty acids, encompassing alpha-linolenic (ALA), eicosapentaenoic (EPA), and docosahexaenoic (DHA) acids. These fatty acids play a crucial role in the body as they are not internally synthesized. N3 demonstrates anti-inflammatory effects by inhibiting NFkB, a transcription factor associated with inflammatory gene expression. It also reduces the production of arachidonic acid-derived eicosanoids and decreases inflammatory markers such as cytokines, chemokines, acute-phase proteins, and adhesion molecules [1]. N3 fatty acids exhibit a positive correlation with anti-inflammatory and neuroprotective responses [2]. They contribute to the proper functioning of neurotransmitter synthesis, release, and reuptake. In the brain, the consumption of unsaturated fatty acids enhances spatial learning and memory while mitigating the negative impact of physiological stress and depressive symptoms on cognitive abilities [1, 3,4,5]. Previous studies from our research group have demonstrated the ability of n3 to reduce anxiety-like behavior in obese rats through n3 supplementation [6].
The exploration of anti-inflammatory compounds has intensified, particularly in addressing conditions and diseases linked to inflammatory responses. Obesity, recognized as a global pandemic due to the rising prevalence of overweight individuals, has been a focal point of this research [7].
Diet is a key factor for maintaining a diverse gut microbiota. An obesogenic diet leading to obesity can induce changes in gut microbiota, along with disruptions in the intestinal barrier, potentially resulting in endotoxemia. This condition involves an increase in LPS in the bloodstream, activating the immune system through TLR4 [8].
Moreover, obesity is frequently associated with metabolic syndrome [9], affecting adipose tissue and skeletal muscle physiology. Immune infiltration and insulin resistance impact their metabolic function, promoting an inflammatory response through adipokines and myokines [10,11,12].
Several studies have revealed the connection between obesity and alterations in neurotransmitter levels in rats, including elevated levels of dopamine and glutamate and reduced levels of serotonin in the whole brain [13, 14]. Obesity can disrupt neurotransmitter production and release through neuroinflammation and insulin resistance, as insulin predominantly signals to neurotransmission via anorexigenic effects. Preclinical studies on obesity often involve various dietary protocols, such as the cafeteria diet (CAF). This diet exposes animals to highly palatable and processed foods, resulting in persistent hyperphagia and increased energy intake. Consequently, it serves as a valuable model for understanding human obesity [6, 15, 16]. Therefore, the objective of this study is to determine whether n3 supplementation can mitigate the adverse effects of CAF-induced obesity on the metabolic function of adipose tissue and muscle, intestinal permeability and neurotransmitter levels in the cerebral cortex.
Methods
Animals
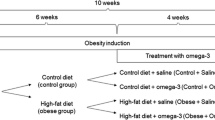
Three-month-old male Wistar rats were obtained from the animal facility of the Federal University of Health Sciences of Porto Alegre (UFCSPA). They were housed two per cage in a 12-h light/dark cycle environment at a temperature of 21 ºC +− 2 ºC. The animals were divided into 4 groups (n = 10/group): CT (control, standard chow), CTn3 (standard chow + omega-3 supplementation), CAF (cafeteria diet), and CAFn3 (cafeteria diet + omega-3 supplementation). This study was approved by UFCSPA Institutional Animal Care and Use Committee under protocol N° 570/18. All experiments were designed and performed to minimize the number and suffering of subjects, following the international laws that regulate the care of laboratory animals.
Diet
The two diets (standard, CT, and cafeteria, CAF) were administered for 13 weeks. CT consisted of the standard chow diet (Nuvilab CR-1, Nuvital®) which offered 3.4 kcal/g (63% carbohydrates, 26% proteins, 11% lipids). While the CAF diet consisted of three menus per week containing: bacon mortadella (Perdigão®), strawberry wafers (Isabela®), chocolate cookies (Isabela®), pizza-flavored crackers (Parati®), white chocolate (Harald®), sausage (Alibem®), and orange-flavored soda (Sukita®) offered concomitantly with ad libitum stan- dard chow and water. CAF diet provided 4.4 kcal/g (48% carbohydrates, 10% proteins, and 44% lipids). Food consumption was registered by weighing the leftovers every menu change. The animals were also weighed weekly to determine weight gain.
Omega-3
Omega-3 (n3) (Vitafor®) was administered by oral gavage to the CTn3 and CAFn3 groups. Water (Vehicle = VEH) was administered to the CT and CAF groups on the same protocol to equal the stress induced by the procedure on all groups. The gavage was performed 5 days per week for 5 weeks from the 9th week of diet. The n3 used had a concentration of 10% EPA and 50% DHA, and was administered at a dose of 500 mg/kg/day in a volume of 1 ml/kg.
Tissue and Blood Samples
The tissues were collected immediately after the euthanasia. Blood was collected and plasma was separated from whole blood by centrifugation and stored at − 80 °C. The cerebral cortex, hypothalamus, gastrocnemius muscle, intestine, and adipose tissue were dissected and quickly frozen in liquid nitrogen.
Electron Transport Chain (ETC) Analysis in Adipose and Skeletal Muscle Tissue
About 150–200 mg of frozen adipose and skeletal muscle tissue were weighed and homogenized in 1:20 ice-cold sucrose buffer in a 1 mL glass-glass tissue grinder. After that, the samples were transferred to a 1.5 mL microtube and centrifuged at 600G for 10 min at 4 ºC, the supernatant was collected for analysis. Total protein concentration was measured by Bradford method. After that, samples were pipetted into microplate wells with distilled water and reagents for each complex analyzed [17]. Complex I was read at 340 nm, Complex II at 600 nm and Complexes III and IV at 550 nm using the SpectraMax® M Series, SoftMax Pro 5.4.1. For the calculation of enzymatic activity, we employed the following formula: enzyme activity (nmol min−1 mg−1) = (∆ Absorbance/min × 1,000) / [(extinction coefficient × volume of sample used in ml) × (sample protein concentration in mg ml−1)].
Plasma LPS and Acetate Quantification
A volume of 150uL of plasma was hydrolyzed with 74 uL of NaCl 150 mM and 300 uL of HCl 8 M, and incubated for 4 h at 90 ºC. 3 mL of hexane was added and centrifuged at 3.500 rpm for 10 min. The upper phase achieved after the centrifugation was withdrawn, and the pellet was reconstituted in 50 uL of methanol and transferred to a vial. An aliquot of 3 uL was injected into the analytical system. The Nexera-i LC-2040C Plus system coupled to LCMS-8045 triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) was used for the analysis [18].
Neurotransmitters in the Cerebral Cortex
A 50 mg portion of cerebral cortex tissue was homogenized in 500 μL of 2% formic acid, and 50 μL of this homogenate was delicately transferred to plastic microtubes. To this mixture, 930 μL of acetone and 20 μL of an internal standard analyte (at a concentration of 250 ng/mL) were added. After vigorous vortexing for 30 s, the sample underwent centrifugation at 10.000G for 6 min. Subsequently, 880 μL of the resulting supernatant was carefully collected, followed by a drying process under a nitrogen flow at room temperature. The desiccated material was then reconstituted in 50 μL of ultrapure water. Of this solution, 20 μL was injected into the liquid chromatograph (Nexera UFLC) that was integrated with the LCMS-8045 triple quadrupole mass spectrometer (Shimadzu, Japan). Optimization of the mass spectrometer involved the assessment of parameters including source temperature, capillary energy, nebulization flow, and collision-induced dissociation gas flow. Moreover, MRM transition optimization was conducted for a range of analytes, namely dopamine, serotonin, glutamate, GABA, homovanillic acid (HVA), and acetylcholine [19].
Western Blot
To quantify the protein expression of claudin-5 in the intestine, the tissue samples were initially homogenized in a lysis buffer containing a protease inhibitor cocktail. Subsequently, the samples underwent centrifugation for 10 min at 8000 rpm, and the protein concentration was determined using the Bradford protein assay. A mixture of Laemmli buffer and 30 µg of proteins was heated to 100 °C for 3 min. Following this, the proteins were loaded onto a 10% SDS-PAGE gel and electrophoresed at 140 V for 2 h. The proteins were then transferred onto nitrocellulose membranes and blocked overnight at 4 °C with 5% nonfat milk in Tris-buffered solution (TBS) with Tween. The membranes were subsequently rinsed with distilled water and incubated overnight with primary antibodies against claudin-5 (1:1000, Cat# ABT45, RRID: AB_11205041; MilliporeSigma, Burlington, Massachusetts), and β-actin (1:30,000 Cat# HRP-660009; Proteintech).
The following day, the membranes were washed with TBS and then incubated for 2 h with a horseradish peroxidase-conjugated secondary antibody. Subsequently, a chemiluminescent signal was visualized using a Chemi-Doc MP Imaging System (Bio-Rad Laboratories, Hercules, California) after the exposure of membranes to an electrochemiluminescence solution (Bio-Rad Laboratories). The signal was measured and quantified utilizing ImageJ software.
Statistical Analysis
The data analysis was performed using GraphPad Prism 9 statistical software (GraphPad Software, San Diego, CA, USA) and SPSS v29 statistical software (IBM SPSS Statistics for Windows). Normality testing was conducted in Prism using the Kolmogorov–Smirnov test, and histograms were generated in SPSS to confirm the normal distribution of the data. Two-way ANOVA with a Bonferroni post hoc analysis was carried out, considering the effects of diet and n3 supplementation. The interaction between those variables was also analyzed. The outcomes were presented as the mean ± standard error of the mean (SEM). Outliers were eliminated using the ROUT test, and statistical significance was defined at a p-value of less than 0.05.
Results
The analysis of the Electron Transport Chain (ETC) in the mitochondria of adipose tissue showed higher activity of complexes I, II, and III. A diet effect was found in the activity of Complex I (F (1, 24) = 8,784, P = 0.0068), Complex II (F (1, 24) = 5,629, P = 0.0260), and Complex III (F (1, 24) = 27,33, P < 0.0001), showing an increase following CAF. Complex IV did not exhibit statistically significant differences among the groups. Additionally, n3 supplementation showed no effect. None post hoc differences were found, (Fig. 1).
Activity of the Electron Transport Chain (ETC) in mitochondria of adipose tissue. A Complex I, B Complex II, and C Complex III showed diet effect, with higher activity in CAF groups. D Complex IV did not show statistical significant differences. Data are presented as mean ± SEM. n = 7/group. CT = control diet, CAF = cafeteria diet, VEH = vehicle, n3 = omega-3, two-way ANOVA
We also evaluated the activity of ETC in the skeletal muscle. The evaluation of Complex I and Complex II did not show statistically significant differences. However, Complex III exhibited a diet effect (F (1, 20) = 11.14, P = 0.0033), with higher levels in the CAF groups, and also a n3 supplementation effect (F (1, 20) = 9.771, P = 0.0053), which n3 decreased complex III activity. Complex IV also showed a supplementation effect (F (1, 20) = 4.684, P = 0.0427), indicating a decrease of its activity. No differences in the post hoc tests were found, (Fig. 2).
Activity of the ETC on skeletal muscle. A Complex I and B Complex II did not show significant differences. C Complex III has shown higher levels in CAF groups showing a diet effect and lower levels in n3 groups showing a supplementation effect. D Complex IV shows a supplementation effect. Data are presented as mean ± SEM. n = 6/group. CT = control diet, CAF = cafeteria diet, VEH = vehicle, n3 = omega-3, two-way ANOVA, Bonferroni post hoc
Acetate levels in the plasma were diminished across all CAF groups, highlighting a significant diet effect (F (1, 28) = 8.979, P = 0.0057), (Fig. 3A).
Plasma levels of Acetate and Lipopolysaccharide (LPS). A The levels of Acetate in plasma were diminished in the CAF groups. B LPS in the plasma were elevated in the CAF groups, with higher levels in CAF, and lower levels in CAFn3. These results show an interaction between diet and supplementation. Data are presented as mean ± SEM. n = 8/group. *P < 0,05. CT = control diet, CAF = cafeteria diet, VEH = vehicle, n3 = omega-3, two-way ANOVA, Bonferroni post hoc
Plasma lipopolysaccharide (LPS) levels showed a significant interaction between diet and n3 supplementation (F (1, 28) = 5,609, P = 0,0250), a significant diet effect (F (1, 28) = 13.07, P = 0.0012), and a supplementation effect (F (1, 28) = 3,046, P = 0,0919), with a reduction observed in the CAFn3 group compared to CAF (CAF x CAFn3 P = 0,0141), (Fig. 3B).
We analyzed by Western blot the expression of claudin-5, a tight junction protein, in the intestine. We found reduced Claudin-5 expression in CAF-fed animals (diet effect: F (1, 16) = 6.238, P = 0.0238). However, no differences were found in multiple comparisons analysis, (Fig. 4).
We also evaluated neurotransmitter levels in the cerebral cortex. Dopamine levels showed a significant interaction between diet and n3 supplementation (F (1, 23) = 4.854, P = 0.0379). In CAF-fed animals, dopamine levels were significantly increased in the group that received n3 supplementation (CAF x CAFn3 P = 0,0355).
Additionally, DOPAC levels indicate a supplementation effect (F (1, 26) = 9.198, P = 0.0054), with a statistically significant difference between CAFn3 and CAF (CAFn3 x CAF P = 0.0125). HVA levels were higher in the CAF groups, indicating a diet effect (F (1, 26) = 5.343, P = 0.0290). Furthermore, HVA levels showed a supplementation effect (F (1, 26) = 8.881, P = 0.0062).
On the other hand, serotonin levels revealed a supplementation effect (F (1, 26) = 5.165, P = 0.0315), with serotonin levels in the CAFn3 group higher than those in the CAF group (CAFn3 vs CAF P = 0.0227). In contrast, acetylcholine, adrenaline, and glutamate did not show statistically significant results, (Fig. 5).
Levels of neurotransmitters analyzed in the cerebral cortex. A Dopamine levels showed interaction between diet and n3 supplementation and higher levels in the CAFn3 group. B DOPAC levels showed a n3 supplementation effect, and CAFn3 were higher than CAF group. C HVA levels were higher in CAF groups showing a diet effect, and also a supplementation effect. D Serotonin levels revealed a supplementation effect, CAFn3 levels were higher than CAF on post-hoc test. Acetylcholine E, Adrenalin F, Glutamate G, and GABA H did not show significant results. Data are presented as mean ± SEM. n = 3–8/group. *P < 0,05. CT = control diet, CAF = cafeteria diet, VEH = vehicle, n3 = omega-3, two-way ANOVA, Bonferroni post hoc
Discussion
One of the major disturbances in metabolism caused by obesity is mitochondrial dysfunction in both white adipose tissue and skeletal muscle [20,21,22,23,24], which disrupts energy expenditure and facilitates fat accumulation over time. In humans, it has been described a reduction in the activity of the ETC complexes I, II, III and IV in the subcutaneous white adipose tissue from the abdominal region of individuals with obesity and type 2 diabetes [25]. Interestingly, the present study showed higher activity of complexes I, II, and III in both adipose tissue and complex III in muscle of CAF animals. The increased activity of ETC complexes in muscle tissue following obesity was already shown elsewhere [25, 26]. It was described that increased mitochondrial activity following a high-fat diet may be due to the stimulation of a range of mitochondrial proteins, including enzymes of the fatty acid oxidation pathway, citrate cycle, and respiratory chain [26]. Also, overexpression of lipoprotein lipase in the skeletal muscle causes increased fatty acid influx, promoting extensive mitochondrial proliferation [27]. This compensation could result in a period during which the muscle tissue increases its metabolic activity before experiencing a permanent dysfunction. Indeed, our previous study using samples from the same animals as in the present study showed an increase in insulin resistance, indicated by an increase in the HOMA-IR index and elevated plasma triglyceride levels [28]. In those analyses, n3 was able to reduce insulin resistance and triglyceride levels, while our observations of ETC complexes showed an effect of n3 at complex III and IV in the muscle but not in adipose tissue; this observation may be related to the metabolic activity of each tissue. The duration of the obesogenic diet may explain this phenomenon of an initial increase in the mitochondrial activity, followed by mitochondrial dysfunction after long-term administration of a high-fat diet [29]. It’s worth noting that comparing enzymatic activity across different tissues—like adipose tissue and muscle—can be tricky because of methodological factors. As explained in the methodology section, the protocol's calculation accounts for total protein expression in the samples. As a result, muscle mitochondria might seem to have lower enzymatic activity compared to adipose tissue. This apparent discrepancy is due to the formula's adjustment for differences in protein concentration between tissues. Thus, it's crucial to exercise caution when interpreting and comparing enzymatic activity across different types of tissues.
Acetate stands as the most prevalent short-chain fatty acid (SCFA) generated by the gut microbiota, originating from the microbial fermentation of residual peptides and fats [30,31,32]. Rats subjected to a high-fat diet (comprising 60% fat) displayed an amplified turnover of acetate. This was coupled with a significant modification in the microbiota profile, resulting in an elevated Firmicutes/Bacteroidetes ratio. In prior studies, our research group observed an increase in acetate levels in rats exposed to a 20-week CAF protocol [33]. In another study, we found that zinc supplementation was effective in restoring the reduced concentration of acetate induced by CAF in the proximal part of the colon [34]. However, in the present study, a protocol of a 13-week diet yielded a reverse effect, with lower levels of plasmatic acetate in the CAF groups. The diet duration may be related to this finding, as SCFA levels are highly influenced by changes in the microbiota over time. This result also highlights the complexity of the effects of obesogenic diets on the organism since the findings of the studies should consider not only the composition but also the duration of the diets.
We also observed a reduction in the expression of claudin-5 in the intestine following CAF, suggesting a compromised intestinal barrier that allows the migration of compounds from the digestive tract into the bloodstream. This is an expected finding, as obesity can lead to a condition known as leaky gut, characterized by increased gut permeability, allowing the influx of pathogen-associated molecular patterns (PAMPs) into the bloodstream [8]. One of the common PAMPs found in the gut is LPS, a constituent of gram-negative bacteria in the gut microbiota [31]. Consistent with our current and previous studies, we found an increase in plasma LPS levels in CAF-fed animals [8, 34, 35]. LPS in the bloodstream can interact with Toll-like receptor 4 (TLR4) and trigger pro-inflammatory responses, even in the absence of an infection, a condition known as endotoxemia [35]. n3 supplementation was effective in reducing LPS levels in the CAF groups, consistent with prior findings [8, 34]. Interestingly, it did not impact the expression of claudin-5, suggesting that n3 may act not directly on the gut barrier. However, a previous microbiota analysis indicated that CAF promoted an increase in the Proteobacteria phylum (Gram-negative bacteria), and n3 significantly decreased it, highlighting the potential of n3 in reducing endotoxemia by modulating the gut microbiota [34].
There is growing evidence showing the effect of diet and obesity on neurotransmitter levels in the brain. Dopamine is a neurotransmitter that has been studied in obesity scenarios, once it is involved with behaviors that include food intake. In high fat diets, it was shown a decrease in dopamine receptor D2 (D2R) binding potential, and reduction in dopamine transporter (DAT) function and membrane localization. Additionally, obesogenic diets can disrupt satiety and reward signal in the brain through disrupting insulin sensitivity in striatal pathways; insulin induces dopamine release and reuptake, however, hyperinsulinemic conditions such as obesity and insulin resistance disrupt that system [36,37,38]. In CAF, there is evidence of lower dopamine concentration in the nucleus accumbens [37]. Two main metabolites of dopamine: 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) [39] are also affected by diverse conditions; in Parkinson’s disease for example, lower levels of HVA in cerebrospinal fluid of untreated patients are related to lower levels of dopamine in the nigrostriatal system [40, 41]. In the present study, CAF was able to decrease dopamine and increase HVA concentration in the cerebral cortex. We hypothesized that these findings are due to a reduction in dopamine transporter (DAT) function, shifting the dopamine metabolization through its principal metabolite: HVA. We also showed that n3 was able to reverse the decrease of dopamine in the cerebral cortex caused by the CAF, in the same way, HVA levels were higher with n3 supplementation. Serotonin levels were increased by n3 in obese rats. These findings corroborate the scientific literature that has been shown the positive effects of DHA and EPA on the central nervous system [42,43,44,45]. One of the mechanisms that n3, especially EPA, can interact with neurotransmitter levels is by inhibiting E2 series prostaglandins that, in turn can inhibit serotonin release [45]; indeed, plasma n3 levels was positively associated with the serotonin metabolite 5-HIAA in cerebrospinal fluid [46]. Another mechanism of n3 action is by optimizing cell membrane fluidity, particularly by DHA, once that n3 is a key component of cell membrane, and its well functioning prevents the ROS (reactive oxygen species) in the way that both of this alterations can highly impact dopaminergic neurons that are highly sensitive to those factors [47]. In the same way a more fluid membrane increases significantly the sensitivity of 5-HT, one of the most important serotonin receptors [48]. All of those factors modulate neurotransmitter levels since they are closely related to the homeostasis of brain cells [49, 50]. In humans, several studies have shown that insufficient n3 PUFAs are linked to depressive and anxious moods, decreased cognitive function, disrupted sleep, and increased tendencies toward aggression and impulsivity [51, 52]. In summary, n3 emerges as a crucial component for the proper functioning of the central nervous system.
Conclusion
In summary, our investigation unveils a compelling compensatory effect in the mitochondria of adipose tissue and skeletal muscle, despite the metabolic effects of obesity. This observed enhancement in ETC complexes' activity underscores the intricate relationship between metabolic pathways and mitochondrial function, providing valuable insights into potential avenues for therapeutic interventions. Our study also identifies the influence of the cafeteria diet on plasma acetate levels, coupled with an elevation in LPS. Notably, the successful reversal endotoxemia induced by LPS through n3 supplementation highlights the potential of this intervention in mitigating inflammatory responses associated with obesity. Additionally, our investigation sheds light on the impact of obesity induced by CAF on neurotransmitter levels, suggesting a potential link to behavioral changes observed in previous studies. Nevertheless, it is important to acknowledge the limitations of our research. Our data are derived from an animal model and may not be directly applicable to human physiology. Furthermore, our dietary and supplementation methodologies may not be universally applicable. As discussed, other studies utilizing similar protocols but extending the duration of the diet have yielded divergent results, underscoring the complexity of the variables involved in obesity research. In conclusion, while our study offers valuable insights, further research is warranted to fully elucidate the mechanisms underlying obesity-related metabolic alterations and to validate potential therapeutic interventions in human populations.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Djuricic I, Calder PC (2021) Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients 13:2421. https://doi.org/10.3390/nu13072421
Chalon S (2006) Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids 75:259–269. https://doi.org/10.1016/j.plefa.2006.07.005
Devarshi PP, Grant RW, Ikonte CJ, Hazels Mitmesser S (2019) Maternal omega-3 nutrition, placental transfer and fetal brain development in gestational diabetes and preeclampsia. Nutrients 11:1107. https://doi.org/10.3390/nu11051107
Shahidi F, Ambigaipalan P (2018) Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol 9:345–381. https://doi.org/10.1146/annurev-food-111317-095850
Liao Y, Xie B, Zhang H et al (2019) Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry 9:190. https://doi.org/10.1038/s41398-019-0515-5
Neto J, Jantsch J, de Oliveira S et al (2022) DHA/EPA supplementation decreases anxiety-like behaviour, but it does not ameliorate metabolic profile in obese male rats. Br J Nutr 128:964–974. https://doi.org/10.1017/S0007114521003998
Caballero B (2019) Humans against obesity: Who will win? Adv Nutr 10:S4–S9. https://doi.org/10.1093/advances/nmy055
de Vos WM, Tilg H, Van Hul M, Cani PD (2022) Gut microbiome and health: mechanistic insights. Gut 71:1020–1032. https://doi.org/10.1136/gutjnl-2021-326789
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K (2021) Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health—pathophysiology and therapeutic strategies. Gastroenterology 160:573–599. https://doi.org/10.1053/j.gastro.2020.10.057
Mengeste AM, Rustan AC, Lund J (2021) Skeletal muscle energy metabolism in obesity. Obesity 29:1582–1595. https://doi.org/10.1002/oby.23227
Kawai T, Autieri MV, Scalia R (2021) Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 320:C375–C391. https://doi.org/10.1152/ajpcell.00379.2020
Sabaratnam R, Hansen DR, Svenningsen P (2023) White adipose tissue mitochondrial bioenergetics in metabolic diseases. Rev Endocr Metab Disord. https://doi.org/10.1007/s11154-023-09827-z
Labban RSM, Alfawaz H, Almnaizel AT et al (2020) High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl Neurosci 11:147–160. https://doi.org/10.1515/tnsci-2020-0099
Hersey M, Woodruff JL, Maxwell N et al (2021) High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav Immun 96:63–72. https://doi.org/10.1016/j.bbi.2021.05.010
de Macedo IC, de Freitas JS, da Silva Torres IL (2016) The influence of palatable diets in reward system activation: a mini review. Adv Pharmacol Sci 2016:7238679. https://doi.org/10.1155/2016/7238679
Leigh S-J, Kendig MD, Morris MJ (2019) Palatable western-style cafeteria diet as a reliable method for modeling diet-induced obesity in rodents. J Vis Exp. https://doi.org/10.3791/60262
Spinazzi M, Casarin A, Pertegato V et al (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7:1235–1246. https://doi.org/10.1038/nprot.2012.058
Teixeira PC, Dorneles GP, Santana Filho PC et al (2021) Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. Int Immunopharmacol 100:108125. https://doi.org/10.1016/j.intimp.2021.108125
Viana RR, Pego AMF, de Oliveira TF et al (2022) Liquid chromatography-tandem mass spectrometry method for simultaneous quantification of neurotransmitters in rat brain tissue exposed to 4’-fluoro-α-PHP. Biomed Chromatogr 36:e5487. https://doi.org/10.1002/bmc.5487
Brestoff JR, Wilen CB, Moley JR et al (2021) Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab 33:270-282.e8. https://doi.org/10.1016/j.cmet.2020.11.008
Sarparanta J, García-Macia M, Singh R (2017) Autophagy and mitochondria in obesity and type 2 diabetes. Curr Diabetes Rev 13:352–369. https://doi.org/10.2174/1573399812666160217122530
Heinonen S, Jokinen R, Rissanen A, Pietiläinen KH (2020) White adipose tissue mitochondrial metabolism in health and in obesity. Obes Rev 21:e12958. https://doi.org/10.1111/obr.12958
Pileggi CA, Parmar G, Harper M-E (2021) The lifecycle of skeletal muscle mitochondria in obesity. Obes Rev 22:e13164. https://doi.org/10.1111/obr.13164
Chattopadhyay M, Guhathakurta I, Behera P et al (2011) Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 60:1702–1710. https://doi.org/10.1016/j.metabol.2011.04.015
Turner N, Bruce CR, Beale SM et al (2007) Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56:2085–2092. https://doi.org/10.2337/db07-0093
Garcia-Roves P, Huss JM, Han D-H et al (2007) Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci U S A 104:10709–10713. https://doi.org/10.1073/pnas.0704024104
Levak-Frank S, Radner H, Walsh A et al (1995) Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest 96:976–986. https://doi.org/10.1172/JCI118145
González LPF, da Rodrigues F, S, Jantsch J, et al (2023) Effects of omega-3 supplementation on anxiety-like behaviors and neuroinflammation in Wistar rats following cafeteria diet-induced obesity. Nutr Neurosci. https://doi.org/10.1080/1028415X.2023.2168229
Di Meo S, Iossa S, Venditti P (2017) Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol 233:R15–R42. https://doi.org/10.1530/JOE-16-0598
Hernández MAG, Canfora EE, Jocken JWE, Blaak EE (2019) The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11:1943. https://doi.org/10.3390/nu11081943
Cummings JH, Pomare EW, Branch WJ et al (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. https://doi.org/10.1136/gut.28.10.1221
Perry RJ, Peng L, Barry NA et al (2016) Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534:213–217. https://doi.org/10.1038/nature18309
Neto J, Jantsch J, Rodrigues F et al (2023) Impact of cafeteria diet and n3 supplementation on the intestinal microbiota, fatty acids levels, neuroinflammatory markers and social memory in male rats. Physiol Behav 260:114068. https://doi.org/10.1016/j.physbeh.2022.114068
Squizani S, Jantsch J, da Rodrigues F, S, et al (2022) Zinc supplementation partially decreases the harmful effects of a cafeteria diet in rats but does not prevent intestinal dysbiosis. Nutrients 14:3921. https://doi.org/10.3390/nu14193921
Fuke N, Nagata N, Suganuma H, Ota T (2019) Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 11:2277. https://doi.org/10.3390/nu11102277
Stouffer MA, Woods CA, Patel JC et al (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 6:8543. https://doi.org/10.1038/ncomms9543
Kleinridders A, Pothos EN (2019) Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Curr Nutr Rep 8:83–91. https://doi.org/10.1007/s13668-019-0276-z
Jantsch J, da Rodrigues F, S, Fraga G de F, et al (2023) Calorie restriction mitigates metabolic, behavioral and neurochemical effects of cafeteria diet in aged male rats. J Nutr Biochem 119:109371. https://doi.org/10.1016/j.jnutbio.2023.109371
Soares-da-Silva P, Garrett MC (1990) A kinetic study of the rate of formation of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the brain of the rat: implications for the origin of DOPAC. Neuropharmacology 29:869–874. https://doi.org/10.1016/0028-3908(90)90135-e
Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG (2014) Cerebrospinal fluid biochemical studies in patients with Parkinson’s disease: toward a potential search for biomarkers for this disease. Front Cell Neurosci 8:369. https://doi.org/10.3389/fncel.2014.00369
Wallace CW, Fordahl SC (2022) Obesity and dietary fat influence dopamine neurotransmission: exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake. Nutr Res Rev 35:236–251. https://doi.org/10.1017/S0954422421000196
Zhou L, Xiong J-Y, Chai Y-Q et al (2022) Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front Psychiat. https://doi.org/10.3389/fpsyt.2022.933704
Patrick RP, Ames BN (2015) Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 29:2207–2222. https://doi.org/10.1096/fj.14-268342
DiNicolantonio JJ, O’Keefe JH (2020) The importance of marine omega-3s for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients 12:2333. https://doi.org/10.3390/nu12082333
Günther J, Schulte K, Wenzel D et al (2010) Prostaglandins of the E series inhibit monoamine release via EP3 receptors: proof with the competitive EP3 receptor antagonist L-826,266. Naunyn Schmiedebergs Arch Pharmacol 381:21–31. https://doi.org/10.1007/s00210-009-0478-9
Hibbeln JR, Linnoila M, Umhau JC et al (1998) Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early-and late-onset alcoholics. Biol Psychiat 44:235–242. https://doi.org/10.1016/s0006-3223(98)00141-3
Li P, Song C (2022) Potential treatment of Parkinson’s disease with omega-3 polyunsaturated fatty acids. Nutr Neurosci 25:180–191. https://doi.org/10.1080/1028415X.2020.1735143
Carabelli B, Delattre AM, Waltrick APF et al (2020) Fish-oil supplementation decreases Indoleamine-2,3-Dioxygenase expression and increases hippocampal serotonin levels in the LPS depression model. Behav Brain Res 390:112675. https://doi.org/10.1016/j.bbr.2020.112675
Heinrichs SC (2010) Dietary omega-3 fatty acid supplementation for optimizing neuronal structure and function. Mol Nutr Food Res 54:447–456. https://doi.org/10.1002/mnfr.200900201
Pinot M, Vanni S, Pagnotta S et al (2014) Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 345:693–697. https://doi.org/10.1126/science.1255288
Jahangard L, Sadeghi A, Ahmadpanah M et al (2018) Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—Results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res 107:48–56. https://doi.org/10.1016/j.jpsychires.2018.09.016
Emery S, Häberling I, Berger G et al (2020) Verbal memory performance in depressed children and adolescents: associations with epa but not dha and depression severity. Nutrients 12:3630. https://doi.org/10.3390/nu12123630
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council for Scientific and Technological Development (CNPq), the Federal University of Health Sciences of Porto Alegre (UFCSPA) and the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Contributions
GF and RG contributed to the conceptualization of the study. JJ and FR conducted the in vivo experimental procedures. GF, FR, JJ, VD, VM, FW, CM, AB, SE participated in ex vivo tissue and sample analysis. GF, JJ, FR and RG performed the statistical analysis. FR, JJ and RG provided critical revisions to the manuscript. All authors participated in writing, reading, editing, and approving the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
This study was approved by UFCSPA Institutional Animal Care and Use Committee under protocol N° 570/18.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Farias Fraga, G., da Silva Rodrigues, F., Jantsch, J. et al. Omega-3 Attenuates Disrupted Neurotransmission and Partially Protects Metabolic Dysfunction Caused by Obesity in Wistar Rats. Neurochem Res 49, 2763–2773 (2024). https://doi.org/10.1007/s11064-024-04201-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04201-0