Abstract
Currently, accumulating evidence has indicated that overnutrition-associated obesity may result in not only metabolic dysregulations, but also cognitive impairments. This study aimed to investigate the protective effects of Diosmetin, a bioflavonoid compound with multiple biological functions, on cognitive deficits induced by a high fat diet (HFD) and the potential mechanisms. In the present study, oral administration of Diosmetin (25, 50 and 100 mg/kg) for 12 weeks significantly reduced the body weight, restored glucose tolerance and normalized lipid profiles in the serum and liver in HFD-induced obese rats. Diosmetin also significantly ameliorated depression-like behaviors and impaired spatial memory in multiple behavioral tests, including the open field test, elevated plus-maze and Morris water maze, which was in accordance with the decreased pathological changes and neuronal damage in different regions of hippocampus as suggested by H&E and Nissl staining. Notably, our results also indicated that Diosmetin could significantly improve mitochondrial dysfunction induced by HFD through upregulating genes involved in mitochondrial biogenesis and dynamics, increasing mitochondrial ATP levels and inhibiting oxidative stress. Moreover, the levels of key enzymes involved in the TCA cycle were also significantly increased upon Diosmetin treatment. Meanwhile, Diosmetin inhibited HFD-induced microglial overactivation and down-regulated inflammatory cytokines both in the serum and hippocampus. In conclusion, these results indicated that Diosmetin might be a novel nutritional intervention to prevent the occurrence and development of obesity-associated cognitive dysfunction via metabolic regulation and anti-inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Along with the economic development and technological advancement in the contemporary society, people’s lifestyles and dietary structure have drastically changed [1]. A nutrition transition from a traditional agricultural-based diet to a westernized high-fat diet (HFD) has gradually become one of the major drives of the global epidemics of obesity [2]. Obesity, characterized by the excessive accumulation of adipose tissue, is a complex multifactorial disease which contribute to the increased likelihood of Type 2 diabetes mellitus [3], cardiovascular disease [4], metabolic syndrome [5] and cognitive impairments [6, 7]. In a previous dose-response meta-analysis, the risk of all cause dementia, Alzheimer’s disease and vascular dementia in midlife was remarkably increased when body mass index surpassed 29, 30 and 32 kg/m2 respectively [8]. Hippocampus is part of the temporal lobe and plays an indispensable role in memory, learning, and emotion in the brain [9]. Previous studies have indicated that excessive intake of HFD could impair hippocampus function via metabolic disturbances, neuroinflammation and mitochondrial dysfunction, thus inducing or accelerating the development of cognitive decline [10,11,12,13].
In the central nervous system, brain mitochondria are responsible for energy production, maintenance of intracellular calcium homeostasis and apoptosis regulation, the dysregulation of which is highly associated with the increased production of reactive oxygen species, decreased cerebral energy gain and impaired learning and memory abilities [13, 14]. There is accumulating evidence demonstrating that Sirtuin1 (SIRT1), an NAD+-dependent deacetylase, incorporates a protective role in mitochondria-related energy metabolism and controls the expression of brain-derived neurotrophic factor (BDNF), a critical factor for neuronal development and synaptic plasticity, via the activation of peroxisome proliferator activated receptor co-activator-1α (PGC-1α) [15,16,17]. Meanwhile, high circulating proinflammatory adipocytokines and elevated hippocampal neuroinflammation are also potential mechanisms for cognitive impairments induced by obesity, involving the disturbed blood-brain barrier, the activation of microglia and subsequent synaptic alterations [18, 19]. Therefore, it is of great significance to develop an ideal intervention strategy for early prevention of obesity-related metabolic dysfunctions and pro-inflammatory risk factors.
Diosmetin (3’, 5, 7-trihydroxy-4’-methoxyflavone), is a bioflavonoid compound extracted from legumes, olive leaves, citrus fruits, and Menthae Haplocalycis herba [20, 21]. Pharmacologically, diosmetin was found to possess broad activities, including anticancer [22], anti-inflammatory [23], antioxidant [21], and anti-apoptosis activities [24]. Recently, it has been reported that diosmetin could mitigate cognitive and memory impairment in mice exposed to chronic unpredictable mild stress paradigm via increasing the antioxidant capacity and improving serum corticosterone levels [25]. It has also been proved that, in in vivo and in vitro models of cerebral ischemia/reperfusion injury, diosmetin inhibited oxidative stress through the activation of SIRT signaling [21]. However, the role of diosmetin in alleviating hippocampal neuroinflammation and metabolic disorders induced by HFD remains unclear.
Based on the research studies mentioned above, we hypothesized that diosmetin may be promising as a therapeutic agent and nutritional intervention to alleviate HFD-induced cognitive impairments via its metabolic modulation and anti-inflammation properties. In this study, we used HFD-induced obese rat model to teste our hypothesis and evidenced the protective effects of Diosmetin on cognitive deficits.
Materials and Methods
Reagents and Antibodies
Diosmetin (purity > 98%) was purchased from Meryer Chemical Technology Co., Ltd (Shanghai, China). Antibodies against ionized calcium binding adapter molecule 1 (IBA-1, A19776), SIRT1 (A11267), PGC-1α (A11971), BDNF (A4873) and GAPDH (AC001) were purchased from ABclonal (Wuhan, China).
Animals
40 male 4-week-old Sprague-Dawley (SD) rats were purchased from Sanxia University (Yichang, China). The animals were maintained at 22 ± 2 °C and 50–65% relative humidity on a 12 h:12 h light-dark cycle (06:00–18:00 h) with free access to conventional standard rodent chow and water. Protocols were conducted according to the Regulations of the Chinese Council on Animal Care and approved by the Ethics Committee of Wuhan University Center for Animal Experiment, Wuhan, China (NO. WP20210532).
Group and Treatment
After 1-week acclimation, animals were randomized into 5 groups (n = 8/group): control group, HFD group (45% kcal from fat, Keao Xieli, Beijing, China), HFD + low dose Diosmetin (25 mg/kg) group, HFD + middle dose Diosmetin (50 mg/kg) group and HFD + high dose Diosmetin (100 mg/kg) group [26,27,28,29] (For the composition of the normal chow diet and high fat diet, please see Supplementary Table 1). The rats in the HFD + Diosmetin treatment (25, 50 and 100 mg/kg) groups were orally administrated with the corresponding drug solution dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na) once a day. The control and HFD group received an equivalent volume of vehicle by gavage. Body weight and food intake were monitored daily throughout the whole experiment. At the end of the 12-week experiment, the animals were fasted for 12 h (20:00–08:00 h) and sacrificed by cervical dislocation post anaesthetization with sodium pentobarbital. Serum was collected by centrifugation of blood at 3000× g for 10 min and stored at -80℃. The livers were rapidly removed, fast frozen with liquid nitrogen and stored at -80℃ for lipid profile measurement. The brains were dissected on ice to collect hippocampus tissues, fast frozen with liquid nitrogen and stored at -80℃ for western blot and quantitative real-time polymerase chain reaction analysis, or fixed with 4% paraformaldehyde for immunofluorescence and histopathology observation.
Oral Glucose Tolerance test (OGTT)
Rats were fasted overnight for 14 h (20:00–10:00 h) before OGTT and then orally administrated 50% glucose at a dose of 2 g/kg body weight post 11 weeks of treatment. Blood samples were collected from the tail by making a cut at 1–2 mm from the tail end of rats and blood glucose levels were measured at 0 (before gavage), 30, 60, 90 and 120 min post oral glucose administration using a glucometer (Roche, Shanghai, China) [30].
Biochemical Parameters
Measurements for biochemical parameters were performed as previously described with minor modifications [12, 31, 32]. For tissue sample preparation, liver and hippocampus samples were homogenized in a 9-fold volume of precooled physiological saline and centrifuged at 10,000× g for 10 min at 4℃ to collect the supernatant for subsequent measurements. Glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC) and triglyceride (TG) levels in the serum and liver were determined using reagent kits (Jiancheng, Nanjing, China). Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) levels in the serum and hippocampus were assessed via ELISA kits (Elk Biotechnology, Wuhan, China). Hippocampal levels of malondialdehyde (MDA) and activities of superoxide dismutase (SOD) and catalase (CAT) were measured using reagent kits (Jiancheng, Nanjing, China). A BCA kit was used to determine protein levels in the liver and hippocampus (Beyotime, Shanghai, China). All the experimental procedures were performed in accordance with the manufacturer’s instructions.
Behavioral Testing
To investigate the behavioral changes post 10 weeks of treatment, the open field test (OFT), elevated plus-maze (EPM) and Morris water maze (MWM) test were performed as previously described with minor modifications [32, 33]. Trials were recorded via a video tracking system (Techman, Chengdu, China).
OFT is an experiment serving to assess locomotion, anxiety and stereotypical behaviors [34]. The test apparatus consists of a 1000 × 1000 × 400 mm black acrylic box, with the base equally sectioned into 25 squares. Each rat was initially placed alone in the center of the arena and allowed free movement for 5 min. During the test, movement distance and time spent in the center, corner and sides were recorded and analyzed.
EPM is also an experiment to evaluate anxiety-like behavior in rodents [35]. The test apparatus consists of two open (420 mm x 100 mm) and two closed (420 mm x 100 mm x 225 mm) arms extending from a central platform and is elevated 700 mm above the floor. Each rat was initially placed in the center of the maze, facing one of the open arms, and allowed to explore freely for 5 min. During the test, the frequency of open arm entries and the percentage of time spent in the open and closed arms respectively were calculated and analyzed.
MWM serves to assess spatial learning and memory [36]. The test was conducted in a dark circular pool (diameter: 160 cm and height: 55 cm), which was equally divided into four quadrants with extra-maze cues on each side of the four quadrants. A platform was centered in one of the four quadrants and submerged 1.5 cm beneath the water surface for escape. During the learning phase, 4 training trials to search for the hidden platform were performed on each animal for 5 consecutive days. The spatial probe trial was performed on the sixth day, in which each rat was given 60 s to swim freely after the escape platform was removed.
Hematoxylin and Eosin (H&E) and Nissl Staining
H&E and Nissl staining were performed according to the previously described methods [37]. Paraffin-embedded brain tissues were coronally sectioned into 4–6 μm thick sections with a microtome. Tissue sections were routinely dewaxed with xylene, hydrated with gradient alcohol (100%-75%) and washed with tap water. For H&E staining, sections were stained with hematoxylin for 2–3 min and rinsed with tap water for 10 min. Then, the sections were immersed in 0.5% hydrochloric acid for 10s, immersed in water for another 10 min and stained in eosin solution for 2–3 min. For Nissl staining, sections were stained with toluidine blue staining solution for 5 min, rinsed with tap water, differentiated by 1% glacial acetic acid and washed with water again to end reaction. Finally, sections were dehydrated and dewaxed again and sealed with neutral gum. Histopathological changes in the hippocampus were observed using a microscope (Olympus, Tokyo, Japan).
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described with minor modifications [31]. Hippocampal slices were washed by PBS (PH7.4) three times for 15 min, followed by incubation for 30 min in 10% goat serum. Then, the slices were incubated overnight with primary antibodies IBA-1 (1:200) and BDNF (1:200) respectively. Following washing with PBS three times for 15 min, the slices were incubated for 50 min with FITC-labeled goat anti-rabbit antibodies (SeraCare, USA, 1:400) in the dark. Finally, the slices were mounted with 40,60-diamidino-2-phenylindole (DAPI) and antifade reagent (Baiqiandu, Wuhan, China). Images were captured with a fluorescence microscope (Olympus, Tokyo, Japan).
Quantitative Real-time Polymerase Chain Reaction Analysis
Quantitative real-time polymerase chain reaction analysis was performed as previously described [31]. Hippocampal total RNA was extracted using AFTSpin Tissue/Cell Fast RNA Extraction Kit (ABclonal, Wuhan, China). cDNA was obtained by reverse transcription of total RNA using ABScript III RT Master Mix (ABclonal, Wuhan, China). mRNA expression of target genes was quantified by real-time quantitative PCR using 2X Universal SYBR Green Fast qPCR Mix (ABclonal, Wuhan, China) in the CFX96™ real-time system (Bio-Rad, USA). All the experimental procedures were performed according to the manufacturer’s instructions. The Ct values were normalized to GAPDH and the relative expression of target genes was calculated by the 2−ΔΔCt method. Gene-specific rat primers used are shown in Supplementary Table 2.
Isolation of Hippocampal Mitochondria
Extraction of hippocampal mitochondria was performed via a mitochondria isolation kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. Briefly, freshly removed hippocampal samples were homogenized in precooled isolation buffer A (1:10, w/v) and centrifuged at 600 g for 5 min at 4 °C. Supernatants were then transferred to other centrifuge tubes and centrifuged at 11,000 g for 10 min at 4 °C. The sediment consisted of isolated mitochondria after removing the supernatants and was resuspended in appropriate amounts of mitochondria stock solution. A BCA kit was used to determine mitochondrial protein levels (Beyotime, Shanghai, China). Quantitative measurements of mitochondrial levels of pyruvate dehydrogenase (PDH), citrate synthase (CS) and α-ketoglutarate dehydrogenase (OGDH) were conducted using ELISA kits (Elk Biotechnology, Wuhan, China). Mitochondrial samples were also assayed for ATP content using a reagent kit (Jiancheng, Nanjing, China).
Western Blotting
Western blotting was performed according to the previously described methods with minor modifications [31]. In brief, protein samples were extracted from hippocampus tissues of rats, which were dissolved in RIPA lysate (Beyotime, Shanghai, China) with protease inhibitor cocktail (Bioss, Beijing, China) and homogenized. Then, the prepared samples were separated by SDS-PAGE eletrophoresis and transferred to a PVDF membrane activated in soaking and activation buffer for 30 s (Beyotime, Shanghai, China). Membranes were blocked with blocking buffer for 2 h and incubated in the corresponding primary antibodies with suggested dilution ratio by the manufacturers overnight at 4 °C. The next day, membranes were incubated in the secondary antibodies for 1.5 h at room temperature and visualized with chemiluminescence imaging system (ChemiDoc XRS, BioRad, USA) after treatment with chemiluminescence reagents (Meilunbio, Dalian, China).
Statistical Analysis
Quantitative analysis of images was performed using image J software. Data in this study was expressed as mean ± SEM and analyzed using GraphPad Prism 8.0. Significant difference was determined by one-way ANOVA followed by Tukey’s multiple comparison test. p-Values < 0.05 were considered to be statistically significant.
Results
Dietary Supplementation of Diosmetin Reduced HFD-induced body Weight gain and Improved Biochemical Parameters
To investigate whether Diosmetin could exert protective effects on HFD-induced metabolic dysregulation, we initially examined the alterations in body weight, calorie intake and metabolic parameters in experimental animals. Compared with the control group, HFD consumption markedly increased body weight, while 25, 50 and 100 mg/kg Diosmetin treatment had an obvious inhibitory effect on the body weight gain without significant difference in calorie intake. Meanwhile, the occurrence of impaired glucose metabolism in HFD-induced obese rats indicated by significantly increased AUCOGTT and fasting serum glucose concentrations was also evidently reversed by 25, 50 and 100 mg/kg Diosmetin treatment. For serum and liver lipid parameters, the results showed that HFD consumption significantly increased TG, TC and LDL-C, but decreased HDL-C levels both in the serum and liver. However, 25, 50 and 100 mg/kg Diosmetin treatment effectively normalized the serum and liver lipid profiles (Table 1). Taken together, these data indicated that Diosmetin treatment has the capacity to protect against HFD-induced obesity and disturbances in glucose and lipid metabolism.
Dietary Supplementation of Diosmetin Improved HFD-induced Anxiety-like Behaviors and Cognitive Deficits
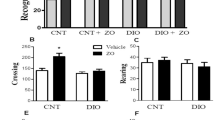
Previous researches have indicated that long-term consumption of HFD could result in an increase in anxiety-like behaviors and accelerate cognitive decline [38, 39]. Therefore, we conducted 3 behavioral tests, including OFT, EPM and MWM to provide a comprehensive evaluation on the effects of Diosmetin treatment on cognitive impairments induced by HFD. In OFT, rats under HFD feeding spent significantly less time in the center but more time in the corner throughout the experiment (Fig. 1B). Accordingly, markedly decreased movement distance in the center was also observed (Fig. 1C). Consistent with the findings in OFT, rats in the HFD group spent significantly less time in the open arms but more time in the closed arms (Fig. 1D) and showed evidently lower frequencies of entries into the open arms in EPM (Fig. 1E). As expected, 50 and 100 mg/kg Diosmetin treatment could effectively reversed the above anxiogenic phenotypes observed in OFT and EPM. Next, MWM was also performed to examine whether Diosmetin treatment could improve spatial learning and memory. In MWM, during the learning phase, 50 and 100 mg/kg Diosmetin treatment significantly shortened the latency to find the platform on day 3 and 5 compared with the HFD group (Fig. 2B). Furthermore, in the spatial probe trial on day 6, the HFD group showed significantly decreased residence time and distance in the target quadrant (Fig. 2, C and D) and passed through the platform for markedly fewer times (Fig. 2E). However, all these results were also effectively reversed by 100 mg/kg Diosmetin treatment. Taken together, performance in OFT, EPM and MWM successfully confirmed the protective effects of Diosmetin on HFD-induced anxiety-like behaviors and deficits in spatial learning and memory.
Effects of dietary supplementation of Diosmetin on performance in OFT and EPM in HFD-induced obese rats. (A) Representative heat map of trajectory in OFT; (B) Time spent in the center, corner and sides in OFT; (C) Distance in the center, corner and sides and the total distance in OFT; (D) The percentage of time spent in the open and closed arms in EPM; (E) Number of entries into the open arms in EPM. All data are expressed as mean ± SEM, n = 8/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Effects of dietary supplementation of Diosmetin on impaired spatial memory of HFD-induced obese rats. (A) Trajectory map in the spatial probe trial on the 6th day (circle 3 is the initial position of the platform); (B) The escape latency on the 1st, 3rd and 5th day; (C-E) Time spent in the target quadrant, the proportion of distance in the target quadrant and number of platform crossings in the spatial probe trial on the 6th day. All data are expressed as mean ± SEM, n = 8/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Dietary Supplementation of Diosmetin Ameliorated HFD-induced Hippocampal Pathological Changes and Neuronal Damage
Next, we used H&E staining to observe the neuronal pathological morphology in dentate gyrus (DG) region, hippocampal cornuammonis region 3 (CA3) and hippocampal cornuammonis region 1 (CA1) of the hippocampus. In the control group, large, polygonal, and closely arranged hippocampal neurons with normal cell morphology and uniform nuclear staining were observed. By contrast, most of the hippocampal neurons in the HFD group were disorderly arranged with enlarged intercellular space and fewer layers, indicating severe nuclear pyknosis and neuronal degeneration. However, these histological features were visually improved by 25, 50 and 100 mg/kg Diosmetin treatment (Fig. 3A). Nissl staining was also used to analyze neuronal injury degree (Fig. 3B). As expected, 50 and 100 mg/kg Diosmetin treatment successfully inhibited HFD-induced hippocampal neuron death in the DG, CA3 and CA1 regions (Fig. 4A). In addition, we further evaluated the mRNA expression of antiapoptotic protein B-cell lymphoma-2 (Bcl-2) and pro-apoptotic protein Bcl-2 Associated X (Bax) and found that 100 mg/kg Diosmetin effectively reversed HFD-induced down-regulation of Bcl-2 and up-regulation of Bax (Fig. 4, B and C). Similar results were also observed in the mRNA expression levels of synaptic-related markers postsynaptic density protein-95 (PSD95) and synaptosome associated protein 25 (SNAP25) that the down-regulation of PSD95 and SNAP25 induced by HFD was significantly inhibited by 25 and 100 mg/kg Diosmetin treatment (Fig. 4, D and E). Taken together, these results suggested that Diosmetin could evidently ameliorate HFD-induced hippocampal pathological injuries and neuronal damage.
Dietary supplementation of Diosmetin ameliorated HFD-induced hippocampal pathological changes. (A) Representative images of H&E staining in the DG, CA3, and CA1 regions of the hippocampus (200×); (B) Representative images of Nissl staining in the DG, CA3, and CA1 regions (200×) (n = 3). Black arrows indicate shrunken and deformed neurons; Black squares indicate extensive atrophy and deformation of neurons in the hippocampus
Dietary supplementation of Diosmetin improved HFD-induced hippocampal neuronal damage. (A) The neuronal density ratio (% of control) in the hippocampal DG, CA3, and CA1 regions respectively; (B-E) Relative mRNA expressions of Bcl-2, Bax, PSD95 and SNAP25 in the hippocampus. All data are expressed as mean ± SEM, n = 3–5/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Dietary Supplementation of Diosmetin Inhibited the Activation of Microglia and Reduced the Neuroinflammation in the Brains of Obese Rats Induced by HFD
Microglia are key regulators of inflammatory responses in the central nervous system [40]. Since IBA-1 is a marker of microglia, an immunofluorescence staining experiment was further performed to exam the inhibitory effect of diosmetin on HFD-induced activation of microglia in the DG (Fig. 5A), CA3 (Fig. 5B) and CA1 (Fig. 6A) regions. Immunofluorescence results revealed that over-activation of microglia induced by HFD consumption was significantly suppressed by 50 and 100 mg/kg Diosmetin treatment in the CA3 and CA1 regions of hippocampus (Fig. 6B). The mRNA expression levels of genes involved in neuroinflammation were also quantified. As expected, 25, 50 and 100 mg/kg Diosmetin treatment effectively reversed HFD-induced up-regulation of matrix metalloproteinase-3 (MMP3), cyclooxygenase-2 (COX2) and inducible nitric oxide synthase (iNOS) in a dose-dependent manner, while no significant difference was observed in mRNA expression levels of matrix metalloproteinase-9 (MMP9) among groups (Fig. 6C). Next, our study further examined whether Diosmetin treatment could suppress HFD-triggered release of high levels of pro-inflammatory mediators. Consistent with the above findings, significantly elevated concentrations of TNF-α, IL-6 and IL-1β in the serum and hippocampus induced by HFD were successfully decreased by 50 and 100 mg/kg Diosmetin treatment (Fig. 6, D and E). Taken together, these results suggested that Diosmetin treatment could exert an inhibitory effect on the activation of microglia and the occurrence of systematic and hippocampal inflammatory response.
Dietary supplementation of Diosmetin reduced the neuronal and systematic inflammation in the obese rats induced by HFD. (A) Representative immunofluorescence images of IBA-1 (green) in the CA1 regions of the hippocampus (200×); (B) Quantification of IBA-1 (green) fluorescence intensity ratio (% of control) in the DG, CA3 and CA1 regions; (C) Relative mRNA expressions of MMP3, MMP9, COX2 and iNOS in the hippocampus; (D and E) TNF-α, IL-6 and IL-1β concentrations in the serum and hippocampus respectively. All data are expressed as mean ± SEM, n = 3–5/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Dietary Supplementation of Diosmetin Inhibited Mitochondrial Dysfunction and Oxidative Stress Induced by HFD
To evaluate the changes in mitochondrial function, we first analyzed the mRNA expression of key regulatory genes involved in mitochondrial biogenesis and dynamics. The results revealed that rats under HFD feeding showed significantly down-regulated expression of PGC-1α, mitochondrial transcription factor A (Tfam), nuclear respiratory factor-1 (Nrf1), nuclear factor erythroid2-related factor 2 (Nrf2), mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and cytochrome c (cycs) but up-regulated expression of pyruvate dehydrogenase kinase (PDK), which was effectively reversed by 50 and 100 mg/kg Diosmetin treatment. Notably, the expression levels of PGC-1α, Nrf1, Nrf2, Mfn1 and Mfn2 in 100 mg/kg Diosmetin group were also significantly higher compared with the other two groups under Diosmetin treatment (Fig. 7A). In addition, 25, 50 and 100 mg/kg Diosmetin treatment successfully restored mitochondrial capacity to generate ATP (Fig. 7B). Meanwhile, the levels of key enzymes involved in the tricarboxylic acid (TCA) cycle, including PDH, CS and OGDH were quantified. The results showed that HFD consumption significantly decreased PDH, CS and OGDH levels, while 100 mg/kg Diosmetin treatment exhibited the most significant effects on increasing the levels of these enzymes (Fig. 7, C-E). Next, we further investigated the effects of Diosmetin treatment on oxidative stress parameters and activities of antioxidant enzymes. As expected, Diosmetin markedly suppressed HFD-induced MDA production (Fig. 7F) and increased the activities of SOD and CAT in a dose-dependent manner (Fig. 7, G and H). Taken together, these results suggested that Diosmetin treatment could effectively ameliorate mitochondrial dysfunction and prevent oxidative damage induced by HFD.
Dietary supplementation of Diosmetin inhibited mitochondrial dysfunction and oxidative stress induced by HFD. (A) mRNA expressions of PGC-1α, Tfam, Nrf1, Nrf2, Mfn1, Mfn2, PDK and cycs in the hippocampus; (B) Mitochondrial ATP levels; (C-E) Mitochondrial PDH, CS and OGDH levels respectively; (F) MDA levels; (G-H) Enzyme activities of SOD and CAT in the hippocampus. All data are expressed as mean ± SEM, n = 5/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Dietary Supplementation of Diosmetin Activated SIRT1/PGC-1α Signaling and Increased Hippocampal Neurotrophic Factors in HFD-induced Obese Rats
It has been reported that SIRT1/PGC-1α signaling pathway not only plays a vital role in the regulation of oxidative stress and mitochondrial energy metabolism [41], but also affects the expression of BDNF [42]. Thus, the activation of SIRT1/PGC-1α signaling pathway in the hippocampus was evaluated by western blot analysis. The results revealed that 25 and 100 mg/kg Diosmetin treatment significantly increased the expression levels of SIRT1 and PGC-1α compared with the HFD group (Fig. 8, A-C). Furthermore, immunofluorescence staining of BDNF showed that 100 mg/kg Diosmetin markedly increased BDNF expression in the DG (Fig. 8D), CA3 (Fig. 9A) and CA1 (Fig. 9B) regions of hippocampus compared with the HFD group (Fig. 9C). Consistent results were also observed for mRNA expression levels of BDNF. We also measured mRNA expression of other neurotrophic factors, including neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), and nerve growth factor (NGF). As expected, 100 mg/kg Diosmetin treatment effectively up-regulated the expression of NT-4 and NGF compared with the HFD group, while no significant difference was observed in the expression of NT-3 among groups (Fig. 9D). Taken together, these results suggested that Diosmetin treatment could activate SIRT1/PGC-1α signaling pathway and increase hippocampal neurotrophic factors, including BDNF, NT-4 and NGF in HFD-induced obese rats.
Dietary supplementation of Diosmetin activated SIRT1/PGC-1α signaling in HFD-induced obese rats. (A) Representative gel images of western blot analysis for SIRT1 and PGC-1α protein expression; (B and C) Quantification of western blot analysis for SIRT1 and PGC-1α protein expression; (D) Representative immunofluorescence images of BDNF (red) in the DG regions of the hippocampus (200×). All data are expressed as mean ± SEM, n = 3/group. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Dietary supplementation of Diosmetin increased hippocampal neurotrophic factors in HFD-induced obese rats. (A-B) Representative immunofluorescence images of BDNF (red) in the CA3 and CA1 regions of the hippocampus (200×); (C) Quantification of BDNF (red) fluorescence intensity ratio (% of control) in the DG, CA3 and CA1 regions; (D) Relative mRNA expressions of BDNF, NT-3, NT-4 and NGF in the hippocampus. Results are considered significant at p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the HFD group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared with 25 mg/kg Diosmetin administration group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared with 50 mg/kg Diosmetin administration group
Discussion
In this study, we found that Diosmetin could improve the performance of rats in multiple behavioral tests, including OFT, EPM and MWM, ameliorate HFD-induced damage to neuronal morphology and function and upregulate the hippocampal neurotrophic factors. In addition, Diosmetin could also reduce systematic and hippocampal inflammation and inhibit HFD-induced over-activation of microglia, especially in the CA3 and CA1 regions of hippocampi in rats. Diosmetin, which also act as a potent metabolic regulator, is able to reverse HFD-induced metabolic disturbances including overweight, glucose tolerance impairment, hyperlipidemia and mitochondrial dysfunction in the hippocampus. Overall, Diosmetin effectively improved the learning abilities and long-term memory and attenuated cognitive impairments in HFD-induced obese rats.
Glucose tolerance impairment and disordered lipid profiles are typical features of the obesity-related metabolic dysregulations and associated with the development and progression of neuropathy [11, 43]. In this study, we observed significantly decreased body weight, AUCOGTT and fasting serum glucose levels in all dose groups under Diosmetin treatment without significant difference in calorie intake. In addition, we also found that all doses of Diosmetin treatment could normalized the serum and liver lipid profiles indicated by lower levels of TG and LDL-C but higher levels of HDL-C in the serum and liver compared with the HFD group, while the most significant effect was reached in 100 mg/kg Diosmetin treatment group.
Consistent with previous findings [12, 44], HFD consumption resulted in obvious anxiety-like behaviors and impaired spatial memory in the behavioral tests conducted to evaluate the alterations in cognitive function, while 50 and 100 mg/kg Diosmetin treatment successfully improved the performance of experimental rats. DG, CA1 and CA3 regions play a crucial role in hippocampus-dependent mnemonic formation, and degenerative pathological changes of hippocampal neurons in these regions has been shown to cause severe impairments in hippocampal memory function [45, 46]. Intriguingly, the results in H&E and Nissl staining further confirmed the protective effects of Diosmetin on HFD-induced extensive atrophy, deformation and apoptosis of hippocampal neurons. Meanwhile, our results also showed that Diosmetin effectively reversed the down-regulation of synaptic-related markers PSD95 and SNAP25, indicating a significant improvement in HFD-induced deficits in hippocampal synaptic plasticity and structure integrity.
The induction of low-grade inflammation in the periphery and central nervous system, especially in the hippocampus, plays a crucial role in the obesity-associated cognitive impairment [47]. In the past few years, numerous studies have explored the anti-inflammation effects of Diosmetin on multiple inflammatory illnesses, including chronic and acute colitis [28], nonalcoholic steatohepatitis [27] and H1N1 influenza virus-induced acute lung injury [48]. Consistent with these results, we found that HFD-triggered elevation of proinflammatory cytokines levels, including TNF-α, IL-6 and IL-1β was effectively inhibited by Diosmetin treatment both in the serum and hippocampus. Microglia are one of the primary participants in the brain inflammatory response, the over-activation of which could prompt the release of multiple pro-inflammatory cytokines and cause neuroinflammation [40]. As expected, immunofluorescence staining results indicated that 50 and 100 mg/kg Diosmetin markedly decreased the expression of IBA-1, a marker of microglia in CA1 and CA3 regions of hippocampus and suppressed the over-activation of microglia compared with the HFD group. Additionally, we also detected the mRNA expression of important regulators in inflammatory responses [12, 49], and found that HFD-induced up-regulation of pro-inflammatory genes, including MMP3, COX-2 and iNOS, was successfully reversed by all doses of Diosmetin treatment in a dose-dependent manner, further confirming the effectiveness of Diosmetin in attenuating the neuroinflammation caused by long-term HFD consumption.
The maintenance of proper functioning of brain requires a large amount of energy. Mitochondria are highly dynamically active organelles and require a balanced status between fusion and fission for the quality control of a mitochondrial network [50, 51]. However, in addition to causing excessive neuroinflammation, HFD consumption also impaired brain mitochondrial function including biogenesis, dynamics, bioenergetics and oxidative status, thus resulting in lower neuronal ATP generation and poorer cognitive capabilities [52]. Data from in vivo experiments supported the protective role of Diosmetin in ameliorating HFD-induced brain mitochondrial dysfunction. To be specific, Diosmetin treatment effectively reversed HFD-induced down-regulation of genes involved in mitochondrial biogenesis and dynamics, including PGC-1α, Tfam, Nrf1, Nrf2, Mfn1, Mfn2 and cycs, and restored mitochondrial ATP levels in the hippocampus. Notably, oxidative stress injuries were also successfully attenuated by Diosmetin treatment. TCA cycle is the primary source of cellular energy and provides intermediates utilized in multiple metabolic pathways, while the dysfunction of TCA cycle-related enzymes could result in neurometabolic disorders [53]. Surprisingly, the levels of key enzymes involved in the TCA cycle, including PDH, CS and OGDH were evidently increased by 100 mg/kg Diosmetin treatment. Increasing evidence has indicated that SIRT1 is involved in a series of vital biological functions, including energy metabolism, apoptosis and autophagy, and regulates the transcriptional activity of PGC-1α via deacetylation. PGC-1α is a key regulator of mitochondrial function and structure and highly expressed in tissues with high-energy demands [32, 54, 55]. We further investigated the effects of Diosmetin on SIRT1/PGC-1α signaling pathway. Western blotting showed that 25 and 100 mg/kg Diosmetin treatment significantly activated SIRT1/PGC-1α signaling pathway compared with the HFD group. Besides, the activation of PGC-1α has also been shown to augment the expression of neurotrophic factor BDNF [17, 42], which was consistent with our results in immunofluorescence that 100 mg/kg Diosmetin treatment markedly increased BDNF expression in the DG, CA3 and CA1 regions. Similarly, Diosmetin treatment also effectively reversed HFD-induced down-regulation of other neurotrophic factors, such as NT-4 and NGF.
There are some limitations of our study. First, we evaluated the protective effects of Diosmetin only on male SD rats, while the effectiveness of Diosmetin on HFD-induced obese female rats needs further clarification. Second, although overall our study indicated that 100 mg/kg Diosmetin exhibited the most significant effects on HFD-induced cognitive impairments, not all results were altered in a dose-dependent manner and future studies with larger sample size and more doses of Diosmetin are needed to screen the optimal concentration of Diosmetin treatment.
Conclusion
In conclusion, the present study revealed that Diosmetin could alleviate HFD-induced cognitive impairments via inhibiting metabolic disorders, mitochondrial dysfunction and neuroinflammation in the hippocampus. Therefore, Diosmetin might be a novel nutritional intervention to prevent the occurrence and development of obesity-associated cognitive dysfunction, which is worth further investigating.
Data Availability
The data that supports the findings of this study are available on reasonable request from the corresponding author.
Abbreviations
- Bax:
-
pro-apoptotic protein Bcl-2 Associated X
- Bcl-2:
-
B-cell lymphoma-2
- BDNF:
-
brain-derived neurotrophic factor
- CA1:
-
cornuammonis region 1
- CA3:
-
cornuammonis region 3
- CAT:
-
catalase
- COX2:
-
cyclooxygenase-2
- CS:
-
citrate synthase
- cycs:
-
cytochrome c
- DAPI:
-
40,60-diamidino-2-phenylindole
- DG:
-
dentate gyrus region
- EPM:
-
elevated plus-maze
- HDL-C:
-
high-density lipoprotein cholesterol
- HFD:
-
high fat diet
- IBA-1:
-
Ionized calcium binding adapter molecule 1
- IL-1β:
-
interleukin-1β
- IL-6:
-
interleukin-6
- iNOS:
-
inducible nitric oxide synthase
- LDL-C:
-
low-density lipoprotein cholesterol
- MDA:
-
malondialdehyde
- Mfn1:
-
Mitofusin 1
- Mfn2:
-
Mitofusin 2
- MMP3:
-
matrix metalloproteinase-3
- MMP9:
-
matrix metalloproteinase-9
- MWM:
-
Morris water maze
- NGF:
-
nerve growth factor
- Nrf1:
-
nuclear respiratory factor-1
- Nrf2:
-
Nuclear factor erythroid2-related factor 2
- NT-3:
-
neurotrophin-3
- NT-4:
-
neurotrophin-4
- OFT:
-
open field test
- OGDH:
-
α-ketoglutarate dehydrogenase
- OGTT:
-
Oral glucose tolerance test
- PDH:
-
pyruvate dehydrogenase
- PDK:
-
pyruvate dehydrogenase kinase
- PGC-1α:
-
peroxisome proliferator activated receptor co-activator-1α
- PSD95:
-
postsynaptic density protein-95
- SD:
-
Sprague-Dawley
- SIRT1:
-
Sirtuin1
- SNAP25:
-
synaptosome associated protein 25
- SOD:
-
superoxide dismutase
- TC:
-
total cholesterol
- TCA:
-
tricarboxylic acid
- TNF-α:
-
Tumor necrosis factor-α
- Tfam:
-
transcription factor A
- TG:
-
triglyceride
References
Bluher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15:288–298
Popkin BM, Ng SW (2022) The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev 23:e13366
Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R et al (2023) Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne) 14:1161521
Koliaki C, Liatis S, Kokkinos A (2019) Obesity and cardiovascular disease: revisiting an old relationship. Metabolism 92:98–107
Duan Y, Zhang W, Li Z, Niu Y, Chen Y, Liu X et al (2022) Predictive ability of obesity- and lipid-related indicators for metabolic syndrome in relatively healthy Chinese adults. Front Endocrinol (Lausanne) 13:1016581
Li H, Ren J, Li Y, Wu Q, Wei J (2023) Oxidative stress: the nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol (Lausanne) 14:1134025
Wieckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U (2021) Western diet as a trigger of Alzheimer’s disease: from metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev 70:101397
Qu Y, Hu HY, Ou YN, Shen XN, Xu W, Wang ZT et al (2020) Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev 115:189–198
Fares J, Bou DZ, Nabha S, Fares Y (2019) Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci 129:598–611
Fado R, Molins A, Rojas R, Casals N (2022) Feeding the brain: effect of nutrients on cognition, synaptic function, and AMPA receptors. Nutrients.;14
Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z et al (2018) FGF21 attenuates high-Fat Diet-Induced Cognitive Impairment via Metabolic Regulation and anti-inflammation of obese mice. Mol Neurobiol 55:4702–4717
Mulati A, Zhang X, Zhao T, Ren B, Wang L, Liu X et al (2021) Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFkappaB signaling pathways. Food Funct 12:9261–9272
Wang D, Yan J, Chen J, Wu W, Zhu X, Wang Y (2015) Naringin improves neuronal insulin signaling, brain mitochondrial function, and cognitive function in High-Fat Diet-Induced obese mice. Cell Mol Neurobiol 35:1061–1071
Schmitt LO, Gaspar JM (2023) Obesity-Induced Brain Neuroinflammatory and mitochondrial changes. Metabolites.;13
Cheng CF, Ku HC, Lin H (2018) PGC-1alpha as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci.;19
Wang CS, Kavalali ET, Monteggia LM (2022) BDNF signaling in context: from synaptic regulation to psychiatric disorders. Cell 185:62–76
Lang X, Zhao N, He Q, Li X, Li X, Sun C et al (2020) Treadmill exercise mitigates neuroinflammation and increases BDNF via activation of SIRT1 signaling in a mouse model of T2DM. Brain Res Bull 165:30–39
Hao S, Dey A, Yu X, Stranahan AM (2016) Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 51:230–239
Salas-Venegas V, Flores-Torres RP, Rodriguez-Cortes YM, Rodriguez-Retana D, Ramirez-Carreto RJ, Concepcion-Carrillo LE et al (2022) The obese brain: mechanisms of systemic and local inflammation, and interventions to reverse the cognitive deficit. Front Integr Neurosci 16:798995
Patel K, Gadewar M, Tahilyani V, Patel DK (2013) A review on pharmacological and analytical aspects of diosmetin: a concise report. Chin J Integr Med 19:792–800
Mei Z, Du L, Liu X, Chen X, Tian H, Deng Y et al (2022) Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct 13:198–212
Choi J, Lee DH, Park SY, Seol JW (2019) Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed Pharmacother 117:109091
Lee DH, Park JK, Choi J, Jang H, Seol JW (2020) Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int Immunopharmacol 89:107046
Si Q, Shi Y, Huang D, Zhang N (2020) Diosmetin alleviates hypoxia–induced myocardial apoptosis by inducing autophagy through AMPK activation. Mol Med Rep 22:1335–1341
Saghaei E, Nasiri BS, Safavi P, Borjian BZ, Bijad E (2020) Diosmetin mitigates cognitive and memory impairment provoked by chronic unpredictable mild stress in mice. Evid Based Complement Alternat Med 2020:5725361
Shi M, Wang J, Bi F, Bai Z (2022) Diosmetin alleviates cerebral ischemia-reperfusion injury through Keap1-mediated Nrf2/ARE signaling pathway activation and NLRP3 inflammasome inhibition. Environ Toxicol 37:1529–1542
Luo N, Yang C, Zhu Y, Chen Q, Zhang B (2021) Diosmetin Ameliorates Nonalcoholic Steatohepatitis through modulating lipogenesis and inflammatory response in a STAT1/CXCL10-Dependent manner. J Agric Food Chem 69:655–667
Li HL, Wei YY, Li XH, Zhang SS, Zhang RT, Li JH et al (2022) Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol Sin 43:919–932
Mo G, He Y, Zhang X, Lei X, Luo Q (2020) Diosmetin exerts cardioprotective effect on myocardial ischaemia injury in neonatal rats by decreasing oxidative stress and myocardial apoptosis. Clin Exp Pharmacol Physiol 47:1713–1722
Abdelmageed ME, Shehatou GS, Abdelsalam RA, Suddek GM, Salem HA (2019) Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn Schmiedebergs Arch Pharmacol 392:243–258
Gao JF, Tang L, Luo F, Chen L, Zhang YY, Ding H (2023) Myricetin treatment has ameliorative effects in DNFB-induced atopic dermatitis mice under high-fat conditions. Toxicol Sci 191:308–320
Cheng Y, Zeng X, Mai Q, Bai X, Jiang Y, Li J et al (2021) Insulin injections inhibits PTZ-induced mitochondrial dysfunction, oxidative stress and neurological deficits via the SIRT1/PGC-1alpha/SIRT3 pathway. Biochim Biophys Acta Mol Basis Dis 1867:166124
Raymond J, Morin A, Plamondon H (2022) Delivery method matters: omega-3 supplementation by restricted feeding period and oral gavage has a distinct impact on corticosterone secretion and anxious behavior in adolescent rats. Nutr Neurosci 25:169–179
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Open Field Test for measuring locomotor activity and Anxiety-like Behavior. Methods Mol Biol 1916:99–103
Kraeuter AK, Guest PC, Sarnyai Z (2019) The elevated plus maze test for measuring anxiety-like Behavior in rodents. Methods Mol Biol 1916:69–74
Othman MZ, Hassan Z, Che HA (2022) Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory. Exp Anim 71:264–280
Yang S, Wang L, Zeng Y, Wang Y, Pei T, Xie Z et al (2023) Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine 114:154762
Zhuang H, Yao X, Li H, Li Q, Yang C, Wang C et al (2022) Long-term high-fat diet consumption by mice throughout adulthood induces neurobehavioral alterations and hippocampal neuronal remodeling accompanied by augmented microglial lipid accumulation. Brain Behav Immun 100:155–171
Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM et al (2015) Spatial cognition in adult and aged mice exposed to High-Fat Diet. PLoS ONE 10:e0140034
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9:42
Yao P, Li Y, Yang Y, Yu S, Chen Y (2019) Triptolide improves cognitive dysfunction in rats with vascular dementia by activating the SIRT1/PGC-1alpha signaling pathway. Neurochem Res 44:1977–1985
Zhao Z, Yao M, Wei L, Ge S (2020) Obesity caused by a high-fat diet regulates the Sirt1/PGC-1alpha/FNDC5/BDNF pathway to exacerbate isoflurane-induced postoperative cognitive dysfunction in older mice. Nutr Neurosci 23:971–982
Iqbal Z, Bashir B, Ferdousi M, Kalteniece A, Alam U, Malik RA et al (2021) Lipids and peripheral neuropathy. Curr Opin Lipidol 32:249–257
Ji Y, Lang X, Wang W, Li S, Zhao C, Shen X et al (2021) Lactobacillus paracasei ameliorates cognitive impairment in high-fat induced obese mice via insulin signaling and neuroinflammation pathways. Food Funct 12:8728–8737
Hainmueller T, Bartos M (2020) Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci 21:153–168
Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Satjaritanun P, Wang X, Liang G et al (2016) FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav 85:86–95
Leigh SJ, Morris MJ (2020) Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis 1866:165767
Zhou B, Wang L, Yang S, Liang Y, Zhang Y, Pan X et al (2023) Diosmetin alleviates benzo[a]pyrene-exacerbated H1N1 influenza virus-induced acute lung injury and dysregulation of inflammation through modulation of the PPAR-gamma-NF-kappaB/P38 MAPK signaling axis. Food Funct 14:3357–3378
Aggarwal A, Sharma N, Khera A, Sandhir R, Rishi V (2020) Quercetin alleviates cognitive decline in ovariectomized mice by potentially modulating histone acetylation homeostasis. J Nutr Biochem 84:108439
Maneechote C, Chunchai T, Apaijai N, Chattipakorn N, Chattipakorn SC (2022) Pharmacological targeting of mitochondrial fission and Fusion alleviates cognitive impairment and brain pathologies in pre-diabetic rats. Mol Neurobiol 59:3690–3702
Yapa N, Lisnyak V, Reljic B, Ryan MT (2021) Mitochondrial dynamics in health and disease. Febs Lett 595:1184–1204
Vilela WR, Bellozi P, Picolo VL, Cavadas BN, Marques K, Pereira L et al (2023) Early-life metabolic dysfunction impairs cognition and mitochondrial function in mice. J Nutr Biochem 117:109352
Kang W, Suzuki M, Saito T, Miyado K (2021) Emerging role of TCA cycle-related enzymes in Human diseases. Int J Mol Sci.;22
Guo Z, Fan D, Liu FY, Ma SQ, An P, Yang D et al (2022) NEU1 regulates mitochondrial energy metabolism and oxidative stress post-myocardial infarction in mice via the SIRT1/PGC-1 alpha Axis. Front Cardiovasc Med 9:821317
Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin D et al (2019) Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci 139:352–360
Acknowledgements and Contributions
The authors’ responsibilities were as followed. Y. Z. and H. D. designed research; Y. Z., C.L., P.H., Y.C., Y.M. and J.G. conducted research; Y. Z. and H. D. analyzed data; Y. Z. wrote the paper; Y. Z. and H. D. had primary responsibility for the final content. All authors read and approved the final version of the paper.
Funding
No.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Protocols were conducted according to the Regulations of the Chinese Council on Animal Care and approved by the Ethics Committee of Wuhan University Center for Animal Experiment, Wuhan, China (NO. WP20210532).
Consent to Participate
Not applicable.
Consent for Publication
All authors give their consent to publish this manuscript.
Conflict of Interest
Y. Zhang, C. Luo, P. Huang, Y. Cheng, Y. Ma, J. Gao, H. Ding, no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Luo, C., Huang, P. et al. Diosmetin Ameliorates HFD-induced Cognitive Impairments via Inhibiting Metabolic Disorders, Mitochondrial Dysfunction and Neuroinflammation in Male SD Rats. Mol Neurobiol 61, 8069–8085 (2024). https://doi.org/10.1007/s12035-024-04083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-04083-x