Abstract
Mounting evidences have demonstrated that diet-induced obesity is associated with cognition impairment via increasing oxidative stress and inflammation in the brain. Atorvastatin (Ator, a HMG-CoA reductase inhibitor) is a cholesterol lowering drug. Studies have reported that Ator can ameliorate the development and progression of cognition impairment. Additionally, silent information regulator 1 (SIRT1) has been demonstrated to be beneficial in cognition impairment. However, the interaction between Ator and SIRT1 activation for cognition impairment remains unclear. This study aimed to identify a relationship between the use of Ator and cognition impairment induced by high-fat diet via Sirt1 activation. A total of 60 healthy male C57BL/6J mice were purchased and then divided into 6 groups, including normal diet group (control), a high-fat diet group (40%HFD, 40% energy from fat), a model group (60%HFD, 60% energy from fat), and model group treated with different doses of Ator (high-dose (80 mg), moderate-dose (40 mg), and low-dose (20 mg) groups). All interventions took place for 7 months. Metabolic phenotypes were characterized for body weight and analysis of serum lipid level. The level of cognition development was examined by Morris water maze (MWM) approach and novel object recognition test (NORT); besides, the expression of Creb1, Gap-43, BDNF, CaMKII, and ERKs of frontal cortex and hippocampus was determined by reverse transcription polymerase chain reaction (RT-PCR). Then, the levels of factors related to inflammation (TNF-a, IL-1β, HMGB1 and IL-6) and oxidation stress (SOD, MDA, CAT and GSH-Px) were assessed using commercially available kits. Finally, SIRT1 and its downstream molecules (Ac-FoxO1, Ac-p53, Ac-NF-κB, Bcl-2 and Bax) were evaluated by Western blot analysis. Compared with the 60% HFD group, body weight and serum lipid levels were significantly decreased in the Ator treated groups. The results of MWM and NORT, as well as the levels of Creb1, Gap-43, BDNF, CaMKII, and ERKs were markedly reversed in the moderate- and low-dose of Ator treated groups. Meanwhile, the expression of IL-1β, TNF-a, IL-6, HMGB1, and MDA was notably decreased, whereas the activity of SOD, CAT, and GSH-Px was increased. It was also revealed that the expression of SIRT1 was remarkably unregulated, the level of Bcl-2 was upregulated, and the content of Ac-FoxO1, Ac-p53, Ac-NF-κB, and Bax was downregulated in the moderate- and low-dose of Ator. Furthermore, results showed that the effect of moderate-dose of Ator was significantly greater than the low-dose of Ator. However, these effects were not observed in the high-dose of Ator. Our results showed that moderate- and low-dose of Ator can significantly attenuate cognition impairment induced by HFD through its antioxidant and anti-inflammatory functions related to SIRT1 activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of obesity continues to rapidly grow with at least two-third of the adult population characterized as obese and another one-third labeled with overweight [1,2,3]. Current diet content and the amount of fat intake can be altered, contributing to the increasing incidence of obesity. Moreover, a recent study demonstrated that objective hypercholesterolemia is a significant risk factor for cognition impairment [4]. Cognition impairment is a progressively neurodegenerative disease, influencing ability necessary to intelligent maintenance and development. The number of patients suffering from dementia worldwide was more than 46.8 million in 2015. Unfortunately, the incidence will almost double every 20 years, reaching 74.7 and 131.5 million in 2030 and 2050, respectively [5]. Indeed, several studies suggested that a diet rich in fat can disrupt cognition function [6,7,8]. Mechanistically, the increased risk of cognition impairment in obesity can be associated with chronic hyperglycemia, reductions in molecules regarding memory formation, brain vascular damage, accumulation of advanced glycation end products, peripheral insulin resistance, and oxidative and inflammatory stress [9, 10]. However, to date, in depth analysis of biological mechanism for cognition impairment observed with high-fat diet (HFD) has not been fully perceived.
Statins are clinically used to decrease blood cholesterol levels through inhibiting the enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, being the most potent agents for reduction of cholesterol serum levels among all hypolipidemic drugs. Furthermore, statins can exert other pleiotropic effects being independent of cholesterol reduction, including regulation of immune function, maintaining plaque stability, anti-inflammatory, anti-platelet, anti-fibrotic, and anti-oxidant effects [11, 12]. Statins are also associated with a reduced incidence of dementia and slow progression of Alzheimer’s disease (AD) [13]. Atorvastatin (Ator) is a commonly available member of statins family. Experimental data indicated that Ator intervention can ameliorate cognition impairment through manipulating cholesterol metabolism, improving endothelial function, as well as increasing synaptogenesis in hippocampus [14, 15]. An in vitro experiment also suggested that statin can modulate amyloid-β deposition and tau metabolism in AD model [16]. However, a number of researches warned about statin-related reversible cognition impairment or memory loss [17]. Moreover, in 2012, the United States Food and Drug Administration (FDA) addressed the potential cognitive-impairing effects caused by statin [18]. Randomized controlled trials evaluating the effects of statins on cognition with AD patients failed to show positive outcomes [19]. A case report also linked cognition impairment with use of high-dose of statin [20]. Cholesterol is vital for learning and memory formation, and cholesterol at low level may be associated with cognition dysfunction [21]. Increased exposure to statin may lead to increased diffusion and decreased cholesterol level locally in brain, thereby causing cognitive side effects [17, 22, 23]. The objective of this study is to clarify statins’ effects on cognition and some potential mechanisms.
Sirtuins, also called HDACs III, are a class of proteins that possess either mono-ADP-ribosyltransferase, or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity. Silent information regulator type-1 (SIRT1) is known to be protective against cognition impairment [24]. SIRT1 contains some protective cellular responses, including antioxidant, anti-inflammatory, and anti-apoptotic via de-acetylation of a variety of proteins [25,26,27]. The available clinical trials, monitoring the beneficial effects of resveratrol on patients with AD showed positive results [28]. Additionally, the beneficial effects of SIRT1 activation will certainly be of great therapeutic importance in the future. However, the interaction between Ator and SIRT1 activation in cognition development has still remained unclear.
This study aimed to identify a relationship between use of Ator and cognition impairment induced by high-fat diet via Sirt1 activation. Furthermore, the potential influence of different doses of Ator on cognition function is a hot research topic as well. Moreover, it was attempted to clarify the effects of SIRT1 activation on the process. In the present study, we hypothesized that moderate- and low-dose of Ator may suppress cognition impairment induced by HFD via SIRT1 activation.
Materials and Methods
Animals and Diets
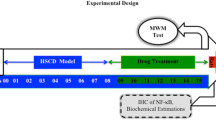
A total of 60 healthy male C57BL/6J mice (age, 6 weeks) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and maintained in specific pathogen-free conditions. Mice were randomly divided into the following 6 groups: control, 40% HFD, 60% HFD (the model), 60% HFD + 20 mg Ator, 60% HFD + 40 mg Ator, and 60% HFD + 80 mg Ator (n = 10, equal in each group). The mice in control group were fed ad libitum with standard rodent diet as previously described. The mice in HFD-based groups were given either 40% HFD (40% kJ from fat) or 60% HFD (60% kJ from fat) (Xietong Organism, Jiangsu, China). Mice fed with 60% HFD were partially given different doses of Ator (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9% physiological saline by oral gavage at the same time. The dosage of Ator was 3, 6 or 12 mg/kg/day, which was equivalent to the regular oral dosage appropriate for human (20, 40 or 80 mg/day). All interventions took place for 7 months. All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Body Weight and Serum Lipid Analysis
Body weight was monthly monitored, and blood samples were collected from eye angular vein after intraperitoneal injection of 10% chloral hydrate 7 months later. Then, the samples were collected and kept at room temperature for 30 min and centrifuged at 12,000 g for 15 min at 4 °C to collect the serums. The serums were assayed to indicate the levels of triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDLC), and high-density lipoprotein cholesterol (HDLC) conducted by Shanghai Rongsheng BioTech Co., Ltd. (Shanghai, China) through an automatic biochemical analyzer (7020; Hitachi, Tokyo, Japan).
Novel object recognition test (NORT)
The NORT was carried out in a black acrylic chamber (44 × 44 cm2) and the objects to be discriminated were different in shape, color, size, and material (objects A and B). Mice were individually adapted to the chamber in which two identical novel objects (A + A or B + B) were placed at the center for 20 min for 4 consecutive days. Then, mice were submitted to an acquisition trial for 5 min in maze where a familiar and a novel object (A + B) were placed at the 5th day. Discrimination index (DI) were calculated as follows: \({\text{DI}} = \left( {{\text{novel}}\;{\text{object}}\;{\text{exploration}}\;{\text{time}}/{\text{total}}\;{\text{exploration}}\;{\text{time}} \times 100} \right){-}\left( {{\text{familiar}}\;{\text{object}}\;{\text{exploration}}\;{\text{time}}/{\text{total}}\;{\text{exploration}}\;{\text{time}} \times 100} \right).\)
Morris Water Maze (MWM) Test
A 120 × 50 cm open circular pool was filled halfway with water and maintained at 22 ± 1 °C. Water surface was divided into four quadrants (NE, SE, SW, and NW) and four visual clues (N, E, S, and W) were placed on the curtains of the tank. A white escape platform submerged in the middle of one of the quadrants was approximately 1 cm below the water level. Each mouse was submitted to 5 trials from 5 starting points (NE, E, SE, S, and SW) randomly on 5 consecutive days. Mice were allowed to swim for 60 s to find the platform, and then rested for 20 s. Those mice which couldn’t locate the platform were guided to the platform. After that, the platform was removed at the 6th day, and mice were placed from the opposite side of the original platform to measure latency time and number of entries into the prior platform zone during 60 s.
RNA Isolation and Determination of Cognition-Associated Markers
Total RNA in frontal cortex and hippocampus was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and 1 g RNA was reversely transcribed using cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Then, reverse transcription polymerase chain reaction (RT-PCR) was undertaken by QuantStudio™ 6 Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Relative gene expressions were calculated and normalized using the 2−ΔΔCt method. Primers used are shown in Table 1 (Sangon Biotech, Shanghai, China).
Evaluation of Inflammatory Cytokines
The frontal cortex and hippocampus were collected to be measured using commercially available immunoassay to determine the level of interleukin-6 (IL-6) (Cat#BMS603-2, Thermo Fisher Scientific, Waltham, MA, USA), High-Mobility Group Box 1 protein (HMGB1) (Cat#ST51011; IBL International GmbH, Hamburg, Germany), tumor necrosis factor-α (TNF-α) (Cat#BMS607-3; Thermo Fisher Scientific, Waltham, MA, USA), and interleukin-1β (IL-1β) (Cat#KMC0011; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Measurement of CAT, GSH-Px, SOD, and MDA
The activities of CAT (Cat#S0082; Beyotime Institute of Biotechnology, Shanghai, China), glutathione peroxidase (GSH-Px) (Cat#A005; Jiancheng, Nanjing, China,), superoxide dismutase (SOD) (Cat#19160-1KT-F; Sigma-Aldrich, St. Louis, MO, USA), and MDA (Cat#MAK085-1KT; Sigma-Aldrich, St. Louis, MO, USA) content in frontal cortex and hippocampus were measured by commercially available kits.
Western Blot Analysis
Frontal cortex and hippocampus were separately homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) followed with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), and then quantified by bicinchoninic acid assay (BCA assay) (Beyotime Institute of Biotechnology, Shanghai, China). After that, 30 µg of protein was loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene difluoride (PVDF) membranes. Then, the membranes were then blocked with 5% skim milk in Tris-buffered saline, 0.1% Tween 20 (TBST), and incubated overnight at 4 °C with primary antibodies as follows: SIRT1 (1:1000; Cat#ab12193; Abcam, Cambridge, UK), acetylated-forkhead box class O1 (1:500; Ac-FoxO1; Cat#sc-49437; Santa Cruz Biotechnology, Dallas, TX, USA), acetylated-p53 (1:1000; Ac-p53; Cat#ab75754; Abcam, Cambridge, UK), acetylated-NF-κB p65 (1:1000; Ac-NF-Κb; Cat#ab19870; Abcam, Cambridge, UK), Bax (1:1000; Cat#ab32503; Abcam, Cambridge, UK), Bcl-2 (1:1000; Cat#ab182858; Abcam, Cambridge, UK), and β-actin (1:2000; Cat#66009-1-Ig; Proteintech, Rosemont, Chicago, IL, USA). Then, the membranes were incubated with secondary anti-rabbit-horseradish peroxidase (HRP) (1:2000; Cat#ab6721; Abcam, Cambridge, UK) or anti-mouse-HRP antibodies (1:2000; Cat#ab6728; Abcam, Cambridge, UK) at normal temperature for 2 h. The enhanced chemiluminescence reagent (Eecl; Millipore, Bedford, MA, USA) and gel imaging system (Bio-Rad Laboratories, Hercules, CA, USA) were used to detect bands. Protein levels were normalized to β-actin levels in respective blots.
Immunofluorescence
After deparaffinized and antigen retrieval, the sections (3 µm) of frontal cortex and hippocampus were processed and incubated with primary antibodies as follows: SIRT1 (1:50; Cat#9475; Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C, followed application of Cy3-conjugated (1:300; Jackson Immuno Research Laboratories, West Grove, PA, USA) for 1 h at room temperature. Slides were counterstained with DAPI (4,6-diamidino-2-phenylindole) (Sigma-Aldrich, St. Louis, MO, USA) and acquired by Leica microscope (Leica Microsystems GmbH, Wetzlar, Germany). Each group was scored from 3 random images of 5 mice and counted by an observer blinded to the treatment.
Statistical Analysis
In this study, SPSS 19.0 software (IBM, Armonk, NY, USA) was used to statistically analyze the data. One-way analysis of variance (ANOVA) and Student–Newman–Keuls test were used for making comparison among the groups. Data were expressed as the mean ± standard error of the mean (SEM). A p-value < 0.05 was considered statistically significant.
Results
Effects of HFD and Ator on Metabolic Phenotypes
There were significant increases in body weight fed by HFD over the 7-month period. Serum lipid parameters in HFD-based groups, including TG, TC, and LDLC, were drastically increased, while there were significant decreases in the level of HDLC compared with control. Meanwhile, results of these parameters were significantly different between 40% HFD and 60%HFD groups (Fig. 1).
As indicated in Fig. 2, body weight was significantly decreased in those treated with Ator compared with mice in 60% HFD group. Moreover, dyslipidemia could be reversed by Ator treatment in a dose-dependent manner.
Effects of Ator on metabolic phenotypes. Body weight and assay of serum lipid parameters. Data are presented as mean ± SEM (n = 10) (***p < 0.001, **p < 0.01, *p < 0.05 vs. 60%HFD group; &&&p < 0.001, &&p < 0.01, &p < 0.05 vs. 20 mg of Ator group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. 40 mg of Ator group)
Effects of HFD and Ator on Behavioral Tests
Results of MWM test revealed that mice in all groups were able to reduce the latency time spent to find the platform during trial days. There were less time and fewer number of entries into the platform zone induced by HFD and the results of 60% HFD group were even lower than the 40% HFD group (Fig. 3a). In NORT, HFD-based groups showed a lower DI than control group, and the level of DI was significantly lower in 60% HFD group compared with 40% HFD group (Fig. 3b).
Effects of HFD on behavioral tests. a Results of escape latency time to the platform during training days, number of entries, and time spent into hidden platform zone. b Results of discrimination index (DI) by novel object recognition test (NORT). Data are presented as mean ± SEM (n = 10) (***p < 0.001, **p < 0.01, *p < 0.05 vs. control group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. 40% HFD group)
As shown in Fig. 4a, moderate- and low-dose of Ator remarkably increased the time and entry number into the quadrant compared with model group, and those were even higher increased in the moderate group. In addition, results of NORT demonstrated that moderate- and low-dose of Ator- showed a significant preference for novel object than the old one, while high-dose of Ator did not conclude the similar results. Meanwhile, DI in moderate-dose group was higher than the low-dose group (Fig. 4b).
Effects of HFD and Ator on Cognition-Associated Creb1, Gap-43, BDNF, CaMKII, and ERKs
Expressions of cognition-associated markers in frontal cortex and hippocampus were significantly decreased in HFD-based groups in comparison with control, and the decreases were remarkably lower in 60% HFD group than those in 40% HFD group (Fig. 5).
Following moderate- and low-dose of Ator, contents of the five cognition-associated parameters were notably increased compared with the model group, and the increases were higher in moderate-dose group than those in the low-group. In contrast, compared with the model group, the expressions in the high-dose group were not increased (Fig. 6).
Effects of HFD and Ator on Inflammation and Oxidative Stress
Our data demonstrated that levels of TNF-α, IL-1β, HMGB1, and IL-6 in HFD-based groups revealed a pronounced increment compared with control group, and the levels of these factors were higher in 60% HFD group than those in the 40% HFD group (Fig. 7). Alternatively, expressions of SOD, catalyse (CAT), and GSH-Px were markedly decreased, while the content of malondialdehyde (MDA) was significantly increased in HFD-based groups compared with control group. Additionally, compared with the 40% HFD group, expression levels of SOD, CAT, and GSH-Px were significantly downregulated, whereas expression level of MDA was significantly upregulated in 60% HFD group (Fig. 8).
The moderate- and low-dose of Ator resulted in the increase of TNF-α, IL-1β, HMGB1, and IL-6 induced by 60% HFD; and the rate of decrease in moderate-dose group was higher than the low-dose group. In contrast, high-dose of Ator did not result in similar conclusion (Fig. 9). Levels of SOD, CAT, and GSH-Px rose progressively, and content of MDA remarkably decreased treated with moderate- and low-dose of Ator compared with the model group; besides, the rates of increment and decrement in the moderate-dose group were both greater than the low-dose group (Fig. 10).
Effects of HFD and Ator on SIRT1-Dependent Mechanism
As illustrated in Fig. 11, the percentage of SIRT1-positive staining cells was significantly lower in HFD-based groups compared with control group, and the rate of decrease was even higher in 60% HFD group. Moreover, results obtained from Western blotting revealed that there was significant downregulation in the expression level of SIRT1 and Bcl-2, and upregulation in the expression level of Ac-FoxO1, Ac-p53, Ac-NF-κB, and Bax in HFD-based groups compared with control group; also, the inhibition of SIRT1 in 60% HFD group was greater (Fig. 12).
Effects of HFD on SIRT1 immunofluorescence staining. Assay and quantification of SIRT1 immunofluorescence staining. Nucleus were stained with DAPI as shown in blue. SIRT1-positive staining cells were visualized in red (magnification, × 400). Data are presented as mean ± SEM (n = 5) (***p < 0.001, **p < 0.01, *p < 0.05 vs. control group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. 40% HFD group)
Effects of Ator on SIRT1 immunofluorescence staining. Assay and quantification of SIRT1 immunofluorescence staining. Nucleus were stained with DAPI as shown in blue. SIRT-positive staining cells were visualized in red (magnification, × 400). Data are presented as mean ± SEM (n = 5) (***p < 0.001, **p < 0.01, *p < 0.05 vs. 60% HFD group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. 40% HFD group)
The decreased number of SIRT1-positive staining cells was significantly increased in those cells treated with moderate- and low-dose of Ator compared with 60% HFD group, and the rate of increase was lower in the low-dose group. However, regional analysis of the high-dose group did not reveal such increase (Fig. 13). Results of Western blotting analysis showed that the inhibition of SIRT1 was activated in moderate- and low-dose of Ator; and the activation in the moderate-dose group was stronger. In contrast, the high-dose of Ator on 60% HFD group showed no significant effect on SIRT1 activation (Fig. 14).
Discussion
Growing evidences have demonstrated that obesity can be associated with cognition impairment. In this study, we successfully established a diet-induced obesity model in C57BL6J mice via 40% HFD or 60% HFD fed for 7 months. We observed the effects of HFD and Ator on cognition-associated markers, in addition to the expression of a variety of molecules involved in the processes of learning and memory formation. Finally, we measured the results of SIRT1 mediated inflammation and oxidative reaction in the processes. The main findings are as follows: (i) HFD induces cognition impairment in a dose-dependent manner; (ii) moderate- and low-dose of Ator can protect mice against cognition impairment; (iii) moderate- and low-dose of Ator can attenuate neuroinflammation and oxidative stress; (iv) SIRT1 activation is involved in the protective effect; and (v) high-dose of Ator has no protective effect. To our knowledge, the present study, for the first time, provides a direct evidence that high-dose of Ator may not play a protective role in mice fed with HFD.
The hippocampus and frontal cortex are critical brain regions for learning and memory processing. Animal-based studies have shown that high-calorie diet can damage the structure and function of brain [29]. Moreover, changes of molecular levels associated with cognition development and maintenance in frontal cortex and hippocampus are important neurochemical foundations for learning and memory processing, such as growth-associated protein-43 (Gap-43), brain-derived neurotrophic factor (BDNF), extracellular signal-regulated kinases (ERKs), CaMKII, and Creb1 [30,31,32,33,34,35]. In our study, we found that HFD decreased the expression levels of these molecules, and the effect of 60% HFD was greater than 40%HFD. Furthermore, the results of MWM and NORT were generally used to assess cognition level. Our findings therefore suggest that HFD has a direct negative influence on cognition in a dose-dependent manner, and previous studies have shown the same results as well [36,37,38].
Neuroinflammation has been widely reported to play a key role in diverse pathological events related to neurodegenerative disorders. Additionally, HFD is known to increase the burden of inflammatory stress in hippocampus and cortex, including IL-1β, TNF-α, HMGB1, and IL-6 [39,40,41,42,43,44,45,46]. Our results were correlated with previous researches that the expression of these molecules was significantly increased in frontal cortex and hippocampus in HFD-based groups. Oxidative-dependent injury has been well-known to be highly involved in cognition impairment [47, 48]. Furthermore, MDA is directly produced by lipid peroxidation, and also can be used to indicate the degree of lipid peroxidation. The major antioxidant enzymes are SOD, CAT, and GSH-Px [47, 49,50,51]. In this study, we found that HFD increased lipid peroxidation and caused an imbalance in antioxidant system, as demonstrated by decreased activities of the CAT, SOD, and GPx, and the increased content of MDA. These results suggest that HFD can induce oxidative stress, and then may increase to a higher vulnerability to cognition impairment.
An earlier evidence also suggested that HFD may induce cognition impairment by inhibiting SIRT1 activation [52]. Previous studies showed that SIRT1 was critically associated with learning and memory process, and the inhibition of SIRT1 led to acetylation of some essential molecules, such as FoxO1, NF-κB, and p53. The acetylation of these molecules was closely involved in brain inflammation, oxidative stress, and apoptosis [53]. As shown in the present study, the expression of SIRT1 and Bcl-2 was decreased, while the expression of Bax was increased in the HFD-based groups, however, these changes were reversed by moderate- and low-dose of Ator. Our results suggest that the protective effects provided by moderate- and low-dose of Ator could be associated with SIRT1 activation, resulting in enhanced anti-oxidative, anti-inflammatory, and anti-apoptotic effects. These findings lend credence to studies of statins in previous researches. For instance, statin can increase the expression of SIRT1, thereby inhibiting the p53-dependent apoptosis in endothelial progenitor cells [54]. Statin attenuates cell apoptosis via the elevation of SIRT1 and subsequent inactivation of NF-κB activity [55]. Additionally, treatment with statins is also mediated by SIRT1 activation to reduce oxidative stress and inflammation response [56, 57].
Treatment with Ator can alleviate weight gain and improve serum lipid level compared with 60% HFD group in the present study. Moreover, mice treated with moderate- and low-dose of Ator showed that cognition impairment is significantly ameliorated, as reflected by a superior performance in behavioral tests and higher expression of those cognition markers. As reported in previous studies concerning the potential effect of statin on the treatment of cognition impairment, some evidences have reported beneficial effects [14,15,16]. In vitro experiments have also shown that Ator can attenuate hippocampal inflammatory and oxidation response mediated by injection of Aβ [16, 58]. Our results also suggest that level of inflammation and oxidation markers can be decreased through treatment with moderate- and low-dose of Ator.
However, high-dose of Ator (80 mg) did not demonstrate such effects in our study. High-dose statins are defined as atorvastatin (80 mg), simvastatin (80 mg), pravastatin (40 mg), and rosuvastatin (20 mg) per day [59]. A study concentrated on different effects of high-dose statin (80 mg) versus low-dose one (10 mg) in patients with no priority of high-dose statin [60]. A previous clinical study revealed that high-dose of Ator was not effective in decreasing the levels of circulating inflammation-related biomarkers [61]. Statin may also increase glucose level and decrease HDL level, thereby increasing the risk of mild cognition impairment [62].
However, the present study contains a number of limitations. Firstly, we didn’t discuss the side effects of high-dose of Ator, and the single effect of high-dose on cognition without the effect of high-fat diet. As reported in previous studies, statins decreased levels of some fat-soluble substances, including vitamin A, vitamin E, and ubiquinone related to subtle impairments in mental processing [63]. Occasional event reported that treatment with high-dose of statin might increases the risk of hepatotoxicity and intracerebral hemorrhage [64]. Furthermore, in the present study, we demonstrated that the protective effects of low- and moderate dose of Ator are associated with SIRT1 activation. However, the exact mechanistic role of SIRT1 by loss- or gain- of function was not performed in our study. Thus, in the future study, we will pay a great attention to these issues.
In conclusion, the present study suggests that treatment with moderate- and low-dose of Ator treatment results in a significant neuroprotective effect against cognition impairment induced by HFD through modulation of SIRT1 activation, reducing oxidation production, as well as inhibiting inflammation. Our study may provide strong evidences for the clinical administration of statins on the treatment of obesity associated with dementia risk.
References
Traill WB, Mazzocchi M, Shankar B et al (2014) Importance of government policies and other influences in transforming global diets. Nutr Rev 72:591–604
Martin KA, Mani MV, Mani A (2015) New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol 763:64–74
Keihani S, Hosseinpanah F, Barzin M et al (2015) Abdominal obesity phenotypes and risk of cardiovascular disease in a decade of follow-up: the Tehran Lipid and Glucose Study. Atherosclerosis 238(2):256–263
Kullmann S, Heni M, Hallschmid M et al (2016) Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96:1169–1209
World Alzheimer Report (2015) The global impact of dementia/Alzheimer’s disease International. http://www.alz.co.uk/research/world-report-2015
Norton S, Matthews FE, Barnes DE et al (2014) Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13:819–828
Hao S, Dey A, Yu X et al (2016) Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 51:230–239
Moreira EL, de Oliveira J, Engel DF et al (2014) Hypercholesterolemia induces short-term spatial memory impairments in mice: up-regulation of acetylcholinesterase activity as an early and causal event. J Neural Transm (Vienna) 121(4):415–426
Alosco ML, Gunstad J (2014) The negative effects of obesity and poor glycemic control on cognitive function: a proposed model for possible mechanisms. Curr Diab Rep 14(6):495
Miller AA, Spencer SJ (2014) Obesity and neuroinflammation: a pathway to cognition impairment. Brain Behav Immun 42:10–21
Haines BE, Wiest O, Stauffacher CV (2013) The increasingly complex mechanism of HMG-CoA reductase. Acc Chem Res 46:2416–2426
Everett BM, Smith RJ, Hiatt. WR (2015) Reducing LDL with PCSK9 inhibitors—the clinical benefit of lipid drugs. N Engl J Med 373:1588–1591
Lilly SM, Mortensen EM, Frei CR et al (2014) Comparison of the risk of psychological and cognitive disorders between persistent and nonpersistent statin users. Am J Cardiol 114:1035–1039
Reis PA, Alexandre PCB, D’Avila JC et al (2017) Statins prevent cognition impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun 60:293–303
Lu D, Goussev A, Chen J et al (2004) Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma 21(1):21–32
Boimel M, Grigoriadis N, Lourbopoulos A et al (2009) Statins reduce the neurofibrillary tangle burden in a mouse model of tauopathy. J Neuropathol Exp Neurol 68(3):314–325
Banach M, Rizzo M, Nikolic D et al (2017) Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther 170:181–191
U.S. Food and Drug Administration (2012) FDA Consumer Health Information: FDA expands advice on statin risks. http://www.fda.gov/consumer
Hammer GP, du Prel J-B, Blettner M (2009) Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int 106:664–668
Okeahialam BN, Isiguzoro I (2012) Statin related memory dysfunction in a Nigerian woman: a case report. Curr Drug Saf 7:33–34
Orth M, Bellosta S (2012) Cholesterol: Its regulation and role in central nervous system disorders. Cholesterol 2012:292598
van Vliet P (2012) Cholesterol and late-life cognitive decline. J Alzheimers Dis 30(Suppl 2):S147–S162
Kobalava ZD, Villevalde SV, Vorobyeva MA (2017) Effects of high-dose statin therapy on cognitive functions and quality of life in very high cardiovascular risk patients. Kardiologiia 57(9):34–41
Bonda DJ, Lee H, Camins A et al (2011) The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol 10:275–279
Sathya M, Moorthi P, Premkumar P et al (2017) Resveratrol intervenes cholesterol- and isoprenoid-mediated amyloidogenic processing of AβPP in Familial Alzheimer’s disease. J Alzheimer’s Dis 60:S3–S23
Turner RS, Thomas RG, Craft S et al (2015) A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85:1383–1391
Kreusser MM, Backs J (2014) Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front Pharmacol 5:36
Sawda C, Moussa C, Turner RS (2017) Resveratrol for Alzheimer’s disease. Ann N Y Acad Sci 1403(1):142–149
Treviño S, Aguilar-Alonso P, Flores Hernandez JA et al (2015) A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse 69(9):421–433
Rao R, Tkac I, Unger EL et al (2013) Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatr Res 73(1):31–37
Latchney SE, Masiulis I, Zaccaria KJ et al (2014) Developmental and adult GAP-43 deficiency in mice dynamically alters hippocampal neurogenesis and mossy fiber volume. Dev Neurosci 36:44–63
Cortese GP, Barrientos RM, Maier SF et al (2011) Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci 31(11):4274–4279
Jiang X, Chai GS, Wang ZH et al (2015) CaMKII-dependent dendrite ramification and spine generation promote spatial training-induced memory improvement in a rat model of sporadic Alzheimer’s disease. Neurobiol Aging 36(2):867–876
Xie H, She GM, Wang C et al (2015) The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci 19(4):647–657
Lee B, Sur B, Shim J et al (2014) Acupuncture stimulation improves scopolamine-induced cognition impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med 14:338
Cordner ZA, Tamashiro KL (2015) Effects of high-fat diet exposure on learning & memory. Physiol Behav 152(Pt B):363–371
Diba R, Mohaddes G, Mirzaie Bavil F et al (2018) Protective effects of troxerutin on maternal high-fat diet-induced impairments of spatial memory and apelin in the male offspring. Iran J Basic Med Sci 21(7):682–687
Alzoubi KH, Mayyas FA, Mahafzah R et al (2018) Melatonin prevents memory impairment induced by high-fat diet: role of oxidative stress. Behav Brain Res 336:93–98
Jayaraman A, Lent-Schochet D, Pike CJ (2014) Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflamm 11:162
Yang J, Huang C, Yang J et al (2010) Statins attenuate high mobility group box-1 protein induced vascular endothelial activation: a key role for TLR4/NF-κB signaling pathway. Mol Cell Biochem 345:189–195
Hu H, Zhou S, Liu Q (2015) The magic and mystery of statins in aging: The potent preventive and therapeutic agent. Int J Cardiol 187:58–59
Kumar A, Babu GN (2010) In vivo neuroprotective effects of peripheral kynurenine on acute neurotoxicity induced by glutamate in rat cerebral cortex. Neurochem Res 35(4):636–644
Dursun E, Gezen-Ak D, Hanağası H et al (2015) The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognition impairment or Parkinson’s disease. J Neuroimmunol 283:50–57
Osso LA, Chan JR (2015) Astrocytes underlie neuroinflammatory memory impairment. Cell 163:1574–1576
Xu M, Zhou GM, Wang LH et al (2016) Inhibiting High-Mobility Group Box 1 (HMGB1) attenuates inflammatory cytokine expression and neurological deficit in ischemic brain injury following cardiac arrest in rats. Inflammation 39(4):1594–1602
Wei H, Zou H, Sheikh AM et al (2011) IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflamm 8:52
Tucsek Z, Toth P, Sosnowska D et al (2014) Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A 69(10):1212–1226
Luo Y, Yue W, Quan X et al (2015) Asymmetric dimethylarginine exacerbates Aβ-induced toxicity and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med 79:117–126
Lu P, Mamiya T, Lu LL et al (2009) Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol 157(7):1270–1277
Kim M, Paik JK, Kang R et al (2013) Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metabolism 62(4):554–560
Ledreux A, Wang X, Schultzberg M et al., Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res 312:294–304
de Kreutzenberg SV, Ceolotto G, Papparella I et al (2010) Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes 59:1006–1015
Herskovits AZ, Guarente L (2014) SIRT1 in Neurodevelopment and Brain Senescence. Neuron 81:471–483
Du G, Song Y, Zhang T et al (2014) Simvastatin attenuates TNFαinduced apoptosis in endothelial progenitor cells via theupregulation of SIRT1. Int J Mol Med 34:177–182
Kilic U, Gok O, Elibol-Can B et al (2015) Efficacy of statins on sirtuin 1 and endothelial nitric oxide synthase expression: the role of sirtuin 1 gene variants in human coronary atherosclerosis. Clin Exp Pharmacol Physiol 42(4):321–330
Singh I, Samuvel DJ, Choi S et al (2018) Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model. Immunology 154(3):434–451
Hu HJ, Zhou SH, Liu QM (2015) The magic and mystery of statins in aging: The potent preventive and therapeutic agent. Int J Cardiol 187:58–59
Sirtori CR (2014) The pharmacology of statins. Pharmacol Res 88:3–11
Pandit AK, Kumar P, Kumar A et al (2016) High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand 134:22–28
Priti K, Agrawal A, Ranwa BL (2017) High versus low dose statin therapy in Indian patients with acute ST-segment elevation myocardial infarction undergoing thrombolysis. Indian Heart J 69:453–457
Oh J, Kang S, Hong N et al (2014) Effect of high-dose statin loading on biomarkers related to inflammation and renal injury in patients hospitalized with acute heart failure. CIRC J 78:2447–2454
Beydoun MA, Beason-Held LL, Kitner-Triolo MH et al (2011) Statins and serum cholesterol’s associations with incident dementia and mild cognition impairment. J Epidemiol Community Health 65:949–957
McGuinness B, Craig D, Bullock R et al (2016) Statins for the prevention of dementia. Cochrane Database Syst Rev 2(2):CD003160
Clarke AT, Johnson PCD, Hall GC et al (2016) High dose atorvastatin associated with increased risk of significant hepatotoxicity in comparison to simvastatin in UK GPRD Cohort. PLoS ONE 11:e0151587
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81771258).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Yang, J., Wang, K. et al. Moderate- and Low-Dose of Atorvastatin Alleviate Cognition Impairment Induced by High-Fat Diet via Sirt1 Activation. Neurochem Res 44, 1065–1078 (2019). https://doi.org/10.1007/s11064-019-02738-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02738-z