Abstract
Recent attention is focused on the impact of diet on health and mental well-being. High-salt and cholesterol diet (HSCD) is known to be associated with neuroinflammation which is the predominant factor for neurodegenerative disease like Alzheimer disease (AD). In the present study, we examined the neuroprotective potential of rosuvastatin, an HMG-CoA reductase inhibitor against HSCD induced neuroinflammation and cognitive impairment. Our results demonstrated that HSCD-induced cognitive impairment as determined by Morris water maze (MWM) task. HSCD also activated nuclear factor kappaB (NF-kB) signaling pathway. The cytokine response was measured using a cytometric bead-based assay quantified by flow cytometry. Treatment with rosuvastatin decreased the production of nitric oxide (NO), tumor necrosis factor alpha (TNF-α) and increased interleukin-10 (IL-10) in a dose-dependent manner. Our results also demonstrated that the rosuvastatin modulates neuronal cell death by inhibiting the overexpression of NF-kB in the CA1 region of hippocampus. In addition, molecular docking study of rosuvastatin indicated high affinity and tighter binding capacity for the active site of the NF-kB. These results suggest that HSCD-triggered inflammatory response and cognitive impairment may be associated with NF-κB signaling pathway. Therefore, treatment with rosuvastatin could be a potential new therapeutic strategy for sporadic dementia of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased intake of high-salt and cholesterol diet (HSCD) today, has become a worldwide health problem, predisposing individuals to coronary, cerebrovascular, and peripheral vascular diseases, as well as type-2 diabetes [1, 2]. In addition, accumulated evidences from preclinical and clinical studies had shown that HSCD may develop significant cognitive impairment [3–5]. HSCD can trigger neuroinflammation and influence amyloidogenesis in neuronal cells [6, 7]. In addition, levels of pro-inflammatory mediators or cytokines which include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors are found elevated in the brains of patients with Alzheimer disease (AD) [8]. Furthermore, nuclear translocation of nuclear factor kappaB (NF-κB), transcription factors involved in AD, indicate the presence of inflammatory processes [9]. Diet associated neuroinflammation is a potential contributor to the accumulation of beta-amyloid (Aβ) and other risk factors of AD [10–12]. AD is a devastating neurodegenerative disorder that influences the daily lives of many patients through memory loss as well as behavioral and cognitive changes [13, 14].
Nuclear factor kappaB (NF-κB) is a transcription factor that is involved in regulating a variety of key biological processes including inflammatory responses and the induction of apoptosis. NF-κB can be activated by HSCD [11]. The NF-κB family consists of p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel (Rel), and RelB. These proteins can form homo- or heterodimers which often are held captive in cytoplasm, inactive form [15]. The activated NF-κB translocates to the nucleus triggering expression of a number of inflammatory genes such as tumor necrosis factor-a (TNF-α), interleukins (IL-1β, IL-6, and IL-18), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [16]. Enhanced immunoreactivity was observed in neurons surrounding amyloid plaques in the brains of AD patients [17]. In in-vitro studies, NF-κB was shown to be activated by Aβ in both neuronal and microglial cells [18]. NF-κB signaling had been proven to be involved in AD [19]. Therefore, inhibiting NF-κB pathways could interrupt generation of Aβ as well as neuroinflammation. Together, it is suggested that activation of NF-κB plays an important role in mediating neuroinflammation in AD [20, 21].
Drug discovery for AD has been strongly focused on β-amyloid (initially plaques, then soluble oligomers), as genetic evidence from the familial cases have been supported by the hypothesis that β-amyloid must be driving the disease process [22]. Based on the “amyloid cascade hypothesis,” anti-amyloid therapies provided a hope to deliver a cure for AD. Unfortunately, numerous clinical trials with active and passive amyloid vaccines as well as γ -secretase inhibitors had failed. Pharmacological treatment of AD currently involves NMDA receptor antagonists and cholinesterase inhibitors. Piracetam is a commonly prescribed drug for cognitive impairment in Europe, Asia, and South America [23, 24]. Currently, there are no disease-modifying drugs available for AD. Consequently, alternative therapeutic targets such as neuroinflammation have been suggested for the prevention and treatment of AD [25]. As the expression of many pro-inflammatory cytokines is driven by the transcription factor NF-κB therefore modulation of HSCD-induced activation of NF-κB pathway could be a potential therapeutic strategy for the treatment of AD.
Statins, competitive inhibitors of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase are widely used as cholesterol-lowering drugs and have been indicated to have pleiotropic effects including anti-oxidative stress and anti-inflammatory properties [26]. Recently, the neuroprotective effects of statins have become an area of intense investigations [27]. Rosuvastatin, a new HMG-CoA reductase inhibitor, has exhibited a more potent affinity for the active site of HMG-CoA reductase than other statins [28]. The above fact has also been supported by the STELLAR, the DISCOVERY and the Discovery Belux studies [29]. Moreover, rosuvastatin is reported to possess neuroprotective effects in experimental stroke models including the focal cerebral ischemia model and the stroke-prone hypertensive rat [30]. In addition, an in vitro study showed that rosuvastatin protected cortical neurons from N-methyl-d-aspartate–induced excitotoxicity [31]. However, limited research is available that examines the effects of rosuvastatin in the context of neuroinflammation associated cognitive impairment. Therefore, the present study evaluated the neuroprotective potential of rosuvastatin against HSCD induced neuroinflammation and cognitive impairment via NF-κB pathway.
Materials and Methods
Rosuvastatin was obtained as a gift sample from Sun Pharmaceutical Industries Limited, Gurgaon, India. Piracetam was obtained as a gift sample from Arbro Pharmaceuticals Limited, New Delhi, India. Griess reagent was procured from Sigma Aldrich (Bangalore, India). NF-κB protein was purchased from Abcam, Cambridge, MA, USA. Goat anti rabbit secondary antibody was purchased from Jackson Immuno Research, West Grove, PA, USA. Cytokines kit was purchased from BioHouse Solution Private Limited, India.
Suspension of rosuvastatin and piracetam were prepared by triturating the weighed amount of rosuvastatin (5, 10 and 15 mg/kg) and piracetam (200 mg/kg) in 0.5% carboxy methyl cellulose (CMC) suspension (w/v) in normal saline respectively [32, 33]. High salt saline was prepared freshly by adding 2% w/v NaCl in water. Pellets of high cholesterol diet were prepared freshly by adding 1.25% cholesterol and 10% coconut oil in standard diet pellets and dried at room temperature.
The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) of Jamia Hamdard (Hamdard University), New Delhi, India (Registration No. JH/993/CPCSEA) as per the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. Female Wistar rats (8–12 weeks old, 150–200 g body weight) were issued from Central Animal House Facility, Jamia Hamdard, New Delhi and housed in standard polypropylene cages (six in each cage) and had access to commercial standard pellet diet (Amrut rat feed, Mfd by: Nav Maharashtra Chakan Oil Mills Ltd, New Delhi, India) and water ad libitum. The rats were maintained under controlled room temperature (20–25 ± 2 °C) and relative humidity (50 ± 15%) with 12 h light/12 h darkness (day/night) cycle in the animal house.
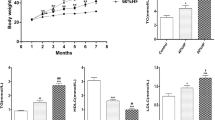
Prior to the commencement of experimental studies, animals were fed with standard rat food pellets for 2 days for acclimatization. Total duration of study was 15 weeks. Animals were fed a HSCD ad libitum for 8 weeks to induce neuroinflammation and cognitive impairment [5, 34]. After that, rats were treated with rosuvastatin (p.o.), piracetam (i.p.) and a combination of both for 7 weeks in different doses (Fig. 1). In this study piracetam and rosuvastatin served as a standard and test drugs respectively. The animals were randomly divided into nine groups (six rats in each group) for treatment schedule as described in Table 1. The rats were observed for behavioral parameters and then immediately sacrificed for estimation of biochemical parameters and immunohistochemistry analysis.
Molecular Docking Study
To predict binding modes of ligands to receptor on the basis of structures, molecular docking studies of the compounds were carried out on Maestro 10.5 program (Schrodinger Inc. USA). Three-dimensional structure of NF-κB-DNA complexes was retrieved from protein data bank (PDB code: 1VKX) to be used for the present docking study [17]. Molecular docking studies mainly involve selection and preparation of appropriate protein, grid generation, ligand preparation followed by docking and its analysis. The protein preparation was done in three steps i.e., preprocess, review and modify, followed by refinement using ‘protein preparation wizard’ in Maestro 10.5. In these steps water molecules were deleted and hydrogen atoms were added. Energy of the structure was minimized using OPLS 2005 force field. Similarly, ligands were prepared again using Force Field 2005. Receptor grid generation program was run by clicking any atom of the ligand and the default box was prepared. The ligand was docked into the grid generated from the protein using extra precision. The docking score, binding free energy and hydrogen bonds and pi–pi interaction formed with the surrounding amino acids are used to predict their binding affinities and proper alignment of these compounds at the active site of the NF-κB p50. The results were evaluated by docking score. Higher the docking score indicates more the binding affinity [35].
MM-GBSA Binding Free Energy
Prime molecular mechanics-generalized born surface area (MM-GBSA) was calculated using Maestro 10.5. It is a tool to calculate ligand binding free energy. The test compound (rosuvastatin) along with the standard (piracetam) was used against the NF-κB-DNA complexes (PDB code: 1VKX). Protein preparation and the ligand preparation were done from the above described methods. Alternatively the MM-GBSA results may be procured running the MM-GBSA program directly from the file generated by running the docking protocol. The docking score, binding free energy and hydrogen bonds and pi–pi interaction formed with the surrounding amino acids are used to envisage their binding affinities and proper alignment of these compounds at the active site of the NF-κB-DNA [36].
Neurobehavioral Assessment by Morris Water Maze Task
Neurobehavioral assessment was done by Morris water maze task, which is commonly used to evaluate spatial learning and memory in rodent [37, 38]. The experimental apparatus consisted of a black circular water pool (diameter 150 cm; height 60 cm; containing water at 24 ± 2 °C) with a featureless inner surface and divided into four equally spaced quadrants (NE, SE, NW, SW). A translucent 10 × 10 cm platform, submerged 1 cm below the water surface, was hidden in the center of quadrant NE (target quadrant) during the training period and was then removed at the time of the probe task. The training was conducted four times a day for five consecutive days before the probe task. Each rat was allowed to swim until they found the platform or until 120 s elapsed. Then, the rat was left on the platform for 10 s. During the spatial probe task, the platform was removed from the pool and the rats were allowed to swim for 120 s. The swim escape latency, path length and time spent in the target quadrant were recorded by a video tracking system (SMART v3.0.03 software, Panlab Harvard, USA).
Analysis of Nitrite Estimation by a Colorimetric Assay
The accumulation of nitrite in the brain tissue supernatant, an indicator of the production of nitric oxide (NO), was determined by a colorimetric assay with Griess reagent (0.1% N-(1-naphthyl) ethylene diamine dihydrochloride, 1% sulfanilamide and 2.5% phosphoric acid). In brief, equal volumes of Griess reagent and supernatant were mixed, after that the mixture was incubated for 10 min at room temperature in the dark. The absorbance of the supernatant was recorded at 540 nm with Perkin Elmer lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and calculated in terms of μg/mL [39].
Analysis of Cytokines Levels by a Bead-Based Multiplex Assay
Pro-inflammatory cytokine (TNF-α) and anti-inflammatory cytokine (IL-10) concentrations in the serum from different groups of rats were measured by a bead-based multiplex assay [40]. This assay used microspheres as the solid support and allowed simultaneous quantification of cytokines in a flow cytometer according to the manufacturer’s instructions. Briefly serum, or the cytokine standards were mixed with equal volume of antibody-coated capture beads and subsequently incubated with biotin-conjugated secondary antibody mixture (anti-rat) for 2 h at room temperature in the dark. Beads were then washed (400 g, 4 °C, 5 min) and the supernatant was discarded carefully, leaving approximately 100 ml sample in each tube. This was repeated once and the samples were further incubated with streptavidin–PE for 1 h at room temperature in the dark. After two further centrifugation steps as mentioned above, the beads were resuspended in assay buffer and read on a BD FACS Calibur LSRII (BD Biosciences). FCAP Array software (BD version 3.0) was used to create the standard curves for each cytokine and convert the fluorescent MFI values into cytokine concentrations.
Immunohistochemistry Analysis of NF-κB Protein
For Immunohistochemistry analysis, paraffin sections of the brains were deparaffinized in xylene and then with acetone for 5 min each. Samples were rehydrated with a graded series of ethanol. After washing under running double distilled water, antigen retrieval was performed by citrate buffer (pH 6). Three changes of section were done with TBS buffer solution. These sections were then blocked with 1.5% normal goat serum for 1 h. Sections were then incubated with purified goat polyclonal antibody raised against a peptide mapping at the N-terminus of NF-κB of human origin (1:200; Santa Cruz Biotechnology) overnight at 4 °C. Immunoreactivity was detected with biotinylated anti-goat rabbit secondary antibodies and the avidin-biotin-peroxidase complex. Immunoreactive signal was developed using diaminobenzidine as a substrate for 2 min. Photomicrographs were taken with a Meiji microscope enabled with lumenera camera. The images were analyzed with lumenera analyze 3 software [41]. All immunohistochemical samples were analyzed in a blinded fashion. For the quantification of the protein expression semi automatically, Image J 1.49 software was used to estimate the volume fraction of immune-reactive cells within the tissue sample. The range of pixel intensities of images was in between 0 and 250. Value 0 and 250 indicates the darkest and lightest shade of the image colour respectively [42].
Statistical Analysis
Results were expressed as the mean ± standard error of mean (SEM). The statistical significance of difference between groups was determined using one-way analysis of variance (ANOVA) followed by Tukey’s test. P value < 0.05 was considered statistically significant. Error bars represent the SEM. All statistical tests were performed using the Prism software package (version 4, GraphPad, San Diego, CA).
Results
Molecular Docking Analysis
Docking studies were carried out by using ligand docking to study the binding mode of rosuvastatin with the active site of NF-κB p50 using Schrodinger programme Maestro 10.5. For the validation, the protein was docked with the standard piracetam. The NF-kB p50/p65 heterodimer is the classical member of the Rel family of transcription factors that regulate diverse cellular functions such as immune response, cell growth, and development. Secondary structures of the subunits are equivalent, apart from a 32-aminoacid insert in the N-terminal domain of p50 that adds a second α-helix [43].
The docking scores, binding energy and interaction with amino acids of the rosuvastatin and piracetam with the active site of NF-κB p50 are summarized in Table 2. Rosuvastatin is well fitted in the active sites of NF-κB p50. Three hydrogen bond was observed between –SO2NH of rosuvastatin with Gly365, Hie364 and Arg356 similar to the standard piracetam (C=O of amide portion). These interactions underscore the importance of sulphonamide portion of the rosuvastatin interacting with the same amino acid as amide portion of standard drug piracetam (Fig. 2). Additional hydrogen bond interactions were also observed with pyrimidine ring of rosuvastatin and Ser363, hydroxyl group and Lys414, Cys416, Gly413, carboxylic acid group with Gly419, Asp418 and Gly413. This may be attributed for the slight increase in the docking score and higher binding energy of rosuvastatin as compared to piracetam. Residues within 2.8 Å areas of piracetam and rosuvastatin are shown in Fig. 3. These results along with the experimental study outcome further confirmed that rosuvastatin could ameliorate neuroinflammation and cognitive impairment.
Representation of 2D ligand interaction of rosuvastatin and piracetam. a 2D ligand interaction representation of rosuvastatin showing hydrogen bond interaction with purple colour arrow line and pi–pi stacking with green line in the binding site of NF-κB p50. b 2D ligand interaction representation of piracetam showing hydrogen bond interaction with purple colour arrow line and pi–pi stacking with green line in the binding site of NF-κB p50. (Color figure online)
Representation of docked pose ligand interaction of rosuvastatin and piracetam. a Docked pose of rosuvastatin represented as stick in the binding site of NF-κB p50 showing hydrogen bond interaction and pi–pi stacking. b Docked pose of piracetam represented as stick in the binding site of NF-κB p50 showing hydrogen bond interaction and pi–pi stacking
MM-GBSA Binding Free Energy Estimation
The rosuvastatin, piracetam and protein were prepared from the described methods and all the water molecules were deleted prior to the running of MM-GBSA prime. The binding free energy calculation was carried out using model Prime MMGBSA DG binds from Maestro 10.5. The free energy results from the Table 2 showed that the ligand rosuvastatin and piracetam fit into the protein receptor NF-κB p50 (PDB ID: 1VKX), the best one is the rosuvastatin having more favorable conformation and showed highest DG binding (Kcal/mol). The binding free energy of the standard drug piracetam was found to be −25.06 Kcal/mol which is lower than the potent test compound rosuvastatin (−37.63 Kcal/mol).
Morris Water Maze Task
The ability of the rats to learn and process spatial information was tested by the Morris water maze. As Fig. 4a shows, the mean escape latency to find the platform declined progressively during the 5 days of training. TC group rats had significant (### p < 0.001) impairment in spatial learning ability during the 5-day place navigation training indicated by the longer escape latency relative to the NC group, while treatment with R15, P200 and R10 + P200 group rats significantly (***p < 0.001) shortened the escape latency. R10 group rats also showed a significant (1st, 2nd, 4th day; **p < 0.001 and 3rd, 5th day ***p < 0.001) reduction in the time to reach platform. A non-significant (p > 0.05) difference in R5 group was observed in comparison to TC group rats.
Effect of rosuvastatin, piracetam and their combination on spatial learning and memory ability of rats, based on the Morris water maze task. a Escape latencies to find a hidden platform in the water maze during the five consecutive days of training. b Time spent in the target quadrant within 120 s in the probe task. c Swim path length in the target quadrant within 120 s in the probe task. Data are presented as mean ± SEM for six rats in each group. ### p < 0.001 vs. TC group. ***p < 0.001, **p < 0.01, *p < 0.05 vs. TC group. $$$ p < 0.001, $$ p < 0.01, $ p < 0.05 vs. R5 group
To assess the spatial memory more directly, the rats were subjected to the probe trial in which the target platform was removed on the next day after the end of navigation training. For the TC group, time in target quadrant was significantly less (### p < 0.001) when compared to the NC group. Rosuvastatin administration significantly (R10, *p < 0.05 and R15, **p < 0.001) increases the time in target quadrant in comparison to TC group. A non-significant (p > 0.05) increase in time in target quadrant of R5 group rats was observed in comparison to TC group. The combination of both R10 + P200 also showed a significant (***p < 0.001) as well as more marked increase time in the target quadrant (Fig. 4b). In addition, rats in the treatment groups were found significantly to have longer (R5, *p < 0.05, R10, R15, P200, R10 + P200, ***p < 0.001) path length in the target quadrant in comparison with TC group (Fig. 4c).
Effect on Brain Nitrite Level
A significant increase (### p < 0.001) in nitrite level in TC group was observed when compared with NC group. This effect was significantly reversed by rosuvastatin (R5, R10, R15) administration in dose dependent manner (*p < 0.05, **p < 0.01, ***p < 0.001, respectively). This effect was also significantly (***p < 0.001) reversed by P200 administration. The combination of both drugs (R10 + P200) showed better reduction in the nitrite level in the brain when the drugs were given alone (Fig. 5).
Effect on Pro-inflammatory and Anti-inflammatory Cytokine Levels
Activated microglia are known to express several pro- and anti-inflammatory cytokines, while statins are able to reduce the inflammatory effect in the vicinity of atherosclerotic plaques. We therefore expected rosuvastatin to regulate the amount of cytokines released by the microglia. There was significant increase (### p < 0.001) in TNF-α level in TC group animals when compared to NC group animals (Fig. 6a). This effect was significantly reversed by rosuvastatin (R5, R10, R15, respectively) administration in dose dependent manner. P200 group showed a significant reduction in the TNF-α level (***p < 0.001) but the diminution was less than that observed in the R15 group. The combination of R10 + P200 also showed a significant reduction in the TNF-α level (***p < 0.001). Moreover, rosuvastatin affected the production of IL-10, an anti-inflammatory cytokine (Fig. 6b). Rosuvastatin-treated levels of IL-10 in the HSCD fed rats were not significantly different, although elevated IL-10 production was noted after rosuvastatin treatment. Interestingly, HSCD decreased the IL-10 protein expression significantly (### p < 0.001) when compared with NC group. Solitary administration of rosuvastatin, and piracetam decreased IL-10 levels, but a significant effect was not observed. When R10 was co-administered with P200, it boosted the IL-10 protein expression significantly (***p < 0.001), indicating the very strong anti-inflammatory action of rosuvastatin.
Effect of rosuvastatin, piracetam and their combination on cytokines level. a TNF-α concentration (pg/ml). b IL-10 concentration (pg/ml). Data are presented as mean ± SEM for six rats in each group. ### p < 0.001 vs. TC group. ***p < 0.001, **p < 0.01, *p < 0.05 vs. TC group. $$$ p < 0.001 vs. R5 group
Effect on Brain Immunohistochemistry Analysis of NF-κB Protein by Fluorescent Microscope
Further assessing the inflammatory effects of HSCD on the brain of rat, we examined the expression of nuclear trans-localized NF-κB in CA1 region of hippocampus. HSCD fed rats demonstrated increased expression of NF-κB as compared with NC group animals. Rosuvastatin treatment decreased the nuclear expression of NF-κB in HSCD fed rats (Fig. 7).
Immunohistochemistry analysis of NF-κB protein by fluorescent microscope in coronal brain sections at the level of CA1 region of hippocampus. The profound expression of nuclear NF-κB were observed in TC group as compared to NC group, treatment groups of rosuvastatin, piracetam and their combination have shown effect on staining of NF-κB. Black arrows are showing the positively stained cells at 10× magnification
In the CA1 region of hippocampus, densitometry results confirmed a significant increase (### p < 0.001) expression of NF-κB in TC group was observed when compared with NC group. This effect was significantly reversed by rosuvastatin (R10, R15) administration in dose dependent manner (**p < 0.01, ***p < 0.001, respectively). This effect was also significantly (***p < 0.001) reversed by P200 administration. However, no significant effect was observed in case of the R5 group. The combination of both drugs (R10 + P200) showed better reduction in expression of NF-κB (***p < 0.001) in the CA1 region of hippocampus when compared to the drugs were given alone (Fig. 8).
Effect of rosuvastatin, piracetam and their combination on the level reciprocal intensity of NF-κB in the CA1 region of hippocampus. Data are presented as mean ± SEM for six rats in each group. ### p < 0.001 vs. TC group. ***p < 0.001, **p < 0.01, *p < 0.05 vs. TC group. $$ p < 0.01, $ p < 0.05 vs. R5 group
Discussion
The present study demonstrated that a HSCD was significantly associated with neuroinflammation and cognitive impairment in rats, which is supported by previous studies [5, 11]. Cognitive impairment is one of the strongest determinants of quality of life in elderly persons [44]. HSCD-induced neuroinflammation is associated with cognitive impairment in rat [15]. Rosuvastatin showed anti-inflammatory and memory-recovering effect in HSCD induced neuroinflammation and cognitive impairment [32].
In the current study, our findings are consistent with previous findings that HSCD impaired the cognitive performance in rats determined by the MWM task [44]. Learning and memory skills of HSCD fed rats were weakened, as evidenced by increase in escape latency during training days in the MWM task (Fig. 4a). In the probe trials, the swimming path length and time in the target quadrant that had held the hidden platform was used to estimate cognitive performance [45]. The swimming path length distance and time in the target quadrant was longer in groups of rats treated with HSCD plus rosuvastatin than the group of rats treated with HSCD alone (Fig. 4b, c). Significant improvements were observed in the R10, R15 and R10 + PCT200 group, whereas there were non-significant improvements in the R5 group. Piracetam also showed a significant effect, but less than higher dose of rosuvastatin. These observations indicate that rosuvastatin has the potential to alleviate HSCD induced cognitive impairment.
Neuroinflammation is known to be associated with the pathology of neurodegenerative diseases including AD [46]. It is well recognized that activation of astrocyte is accompanied by increased production of potentially neurotoxic factors including cytokines, NO and reactive oxygen species [47]. In neuroinflammatory process, redox-sensitive transcription factor such as NF-κB may be initiated by the NO and reactive oxygen species [48]. The transcription factor, NF-κB regulates the transcription of several proinflammatory cytokines that play an important role in neuroinflammation mediated neurodegeneration. Several studies have confirmed that, over activation of proinflammatory genes were observed in AD patients as well as in animal model of AD [49]. In the present study, HSCD increased NF-κB protein (Fig. 8) expression in CA1 region of the hippocampus as compared to NC group animals. NF-κB protein expression was significantly attenuated by rosuvastatin in a dose dependent manner. But, the effect on the R5 group was insignificant when compared TC group. Similar results were obtained from the densitometric analysis. These immunohistochemistry results are consistent with previous findings and support the role of rosuvastatin in inhibiting NF-κB expression.
Cytokines, which are important immunomodulators in the normal functioning of the central nervous system, can be released, among others, by the microglial cells [50]. Cytokines can also be harmful: previous studies have shown that neurodegeneration originating through neuroinflammation is often elicited by activated microglia through the release of different pro-inflammatory cytokines and chemokines [51]. High levels of TNF-α and IL-1β could be observed, for instance, in the vicinity of amyloid plaques of AD patients, where activated microglia accumulate [52]. As expected, pro-inflammatory cytokines (TNF-α) were increased significantly in TC group rats as compared to the NC group rats. Rosuvastatin strongly inhibited levels of the pro-inflammatory cytokines TNF-α when tested in HSCD fed rats. Additionally, the levels of IL-10 were significantly reduced in the TC group and administration of rosuvastatin led to a dose-dependent surge in anti-inflammatory IL-10 levels. Thus, rosuvastatin showed a marked decrease in pro-inflammatory cytokines as well as significantly increased the anti-inflammatory cytokines which could be responsible for its potent anti-inflammatory action. Previous studies also suggested that statins reduce levels of some of the pro-inflammatory cytokines and increase the anti-inflammatory cytokine IL-10 [53, 54].
Nitrite estimation in the biological material is gradually being used as a marker for NO production [55]. NO levels were increased in HSCD fed rat brain as earlier reported. Increased level of NO is implicated in neurological and aging-related disorders [56]. Hence, the regulation of NO release is helpful to alleviate inflammatory damage [57]. Results of the present study suggest that rosuvastatin exerts its anti-inflammatory effects by inhibiting NF-κB nuclear translocation and the subsequent release of cytokines and activation of NO in HSCD fed rats. Such endogenously defensive mechanism further broadens the way for rosuvastatin to exhibit its neuroprotective effects.
Our present study showed that rosuvastatin ameliorated HSCD-induced DNA- binding activities of NF-κB, interrupting p50 translocation. NF-κB p50 has a critical regulatory role in both non-specific and specific functional immune responses [58]. These results demonstrated that binding of rosuvastatin to NF-κB p50 results in the inhibitory effect of rosuvastatin on NF-κB, and inhibition of NF-κB could be significant for the anti-inflammatory effect of rosuvastatin [59]. According to the docking results, rosuvastatin showed a high docking score, indicating high affinity with the NF-kB and tight binding capacity to the active site of the p50. Rosuvastatin form ten hydrogen bond with DNA-binding region (DBR), but piracetam makes five hydrogen bonds with DBR. Our present study confirmed that rosuvastatin binds to p50 of the NF-kB subunit through the docking model. Rosuvastatin displayed a higher binding free energy (dG bind) for p50 which indicates that rosuvastatin may have higher selectivity towards NF-kB. The binding energy of piracetam was found to be −25.06 Kcal/mol and less than the rosuvastatin (−37.63 Kcal/mol). This result justify that the rosuvastatin have high affinity for NF-kB than piracetam. The way to inhibit NF-kB by rosuvastatin is important for the basic principle for inhibition of NF-kB. In this regard, it is noteworthy that rosuvastatin binds to protein of p50, subunit of NF-kB evidenced by docking model. Rosuvastatin has shown that direct binding to NF-kB subunit could result in inhibition of NF-kB activities. The molecular docking study of the rosuvastatin was performed for the better understanding of drug-receptor interactions, which supports for the in vivo as well as behavioral results of rosuvastatin.
Conclusion
In conclusion, our present findings indicate that rosuvastatin could be effective for the treatment of HSCD induced neroinflammation and cognitive impairment, through anti- inflammatory responses by inhibiting NF-kB pathway.
References
Medina-RemÓn A, Kirwan R, Lamuela-Raventós RM, Estruch R (2016) Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and mental health problems. Crit Rev Food Sci Nutr. doi:10.1080/10408398
Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H (2009) The Japanese society of hypertension guidelines for the management of hypertension. Hypertens Res 32:3–107
Popa-Wagner A, Buga A-M, Popescu B, Muresanu D (2015) Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm 122:47–54
Sobesky JL, Barrientos RM, Henning S, Thompson BM, Weber MD, Watkins LR, Maier SF (2014) High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun 42:22–32
Mogi M, Tsukuda K, Li J-M, Iwanami J, Min L-J, Sakata A, Fujita T, Iwai M, Horiuchi M (2007) Inhibition of cognitive decline in mice fed a high-salt and cholesterol diet by the angiotensin receptor blocker, olmesartan. Neuropharmacology 53:899–905
Bhat NR, Thirumangalakudi L (2013) Increased tau phosphorylation and impaired brain insulin/IGF signaling in mice fed a high fat/high cholesterol diet. J Alzheimers Dis 36:781–789
Chugh G, Asghar M, Patki G, Bohat R, Jafri F, Allam F, Dao AT, Mowrey C, Alkadhi K, Salim S (2013) A high-salt diet further impairs age-associated declines in cognitive, behavioral, and cardiovascular functions in male Fischer brown Norway rats. J Nutr 143:1406–1413
Rubio-Perez JM, Morillas-Ruiz JM (2012) A review: inflammatory process in Alzheimer’s disease, role of cytokines. Sci World J. doi:10.1100/2012/756357
Karunaweera N, Raju R, Gyengesi E, Münch G (2015) Plant polyphenols as inhibitors of NF-κB induced cytokine production—a potential anti-inflammatory treatment for Alzheimer’s disease? Front Mol Neurosci 16:8–24
Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE (2005) Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol Aging 26:995–1000
Zhang X, Dong F, Ren J, Driscoll MJ, Culver B (2005) High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol 191:318–325
Cole SL, Vassar R (2007) The Alzheimer’s disease β-secretase enzyme, BACE1. Mol Neurodegener 15:2–22
Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH (2002) Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci 22:6331–6335
Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JNP, Perry VH (2009) Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 65:304–312
Zhu S, Shi R, Li V, Wang J, Zhang R, Tempier A, He J, Kong J, Wang J-F, Li X-M (2015) Quetiapine attenuates glial activation and proinflammatory cytokines in APP/PS1 transgenic mice via inhibition of nuclear factor-κB pathway. Int J Neuropsychopharmacol. doi:10.1093/ijnp/pyu022
Lu J, Wu D-m, Zheng Y-l, Hu B, Cheng W, Zhang Z-f, Shan Q (2011) Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun 25:1658–1667
Gu SM, Park MH, Hwang CJ, Song HS, Lee US, Han SB, Oh KW, Ham YW, Song MJ, Son DJ (2015) Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J Neuroinflammation 12:124–136
Huang X, Chen Y, Zhang H, Ma Q, Zhang Y-w, Xu H (2012) Salubrinal attenuates β-amyloid-induced neuronal death and microglial activation by inhibition of the NF-κB pathway. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.10.007
Camandola S, Mattson MP (2007) NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets 11:123–132
Kang EB, Koo JH, Jang YC, Yang CH, Lee Y, Cosio-Lima LM, Cho JY (2016) Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J Neuroendocrinol. doi:10.1111/jne.12385
Craft JM, Watterson DM, Van Eldik LJ (2005) Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets 9:887–900
Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov 9:387–398
Flicker L, Grimley Evans J (2004) Piracetam for dementia or cognitive impairment. Cochrane Libr. doi:10.1002/14651858
Rinwa P, Kumar A (2013) Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuro-inflammation associated with mouse model of chronic unpredictable stress. Arch Pharmacal Res. doi:10.1007/s12272-013-0205-4
Minter MR, Taylor JM, Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem 136:457–474
Zhou Q, Liao JK (2010) Pleiotropic effects of statins: basic research and clinical perspectives. Circ J 74:818–844
Reiss AB, Wirkowski E (2009) Statins in neurological disorders: mechanisms and therapeutic value. Sci World J 9:1242–1259
Schachter M (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19:117–125
Laks T, Keba E, Leiner M, Merilind E, Petersen M, Reinmets S, Väli S, Sööt T, Otter K (2008) Achieving lipid goals with rosuvastatin compared with simvastatin in high risk patients in real clinical practice: a randomized, open-label, parallel-group, multi-center study: the DISCOVERY-beta study. Vasc Health Risk Manag 4:1407–1416
Uekawa K, Hasegawa Y, Ma M, Nakagawa T, Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Kawano T (2014) Rosuvastatin ameliorates early brain injury after subarachnoid hemorrhage via suppression of superoxide formation and nuclear factor-kappa B activation in rats. J Stroke Cerebrovasc Dis 23:1429–1439
Wang Q, Yan J, Chen X, Li J, Yang Y, Weng J, Deng C, Yenari MA (2011) Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol 230:27–34
Ghaisas MM, Dandawate PR, Zawar SA, Ahire YS, Gandhi SP (2010) Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology 18:169–177
Hsieh M-T, Tsai F-H, Lin Y-C, Wang W-H, Wu C-R (2002) Effects of ferulic acid on the impairment of inhibitory avoidance performance in rats. Planta Medica 68:754–756
Liu RY, Gu R, Qi XL, Zhang T, Zhao Y, He Y, Pei JJ, Guan ZZ (2008) Decreased nicotinic receptors and cognitive deficit in rats intracerebroventricularly injected with beta-amyloid peptide (1-42) and fed a high-cholesterol diet. J Neurosci Res 86:183–193
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Siddiqui N, Alam MS, Ali R, Yar MS, Alam O (2016) Synthesis of new benzimidazole and phenylhydrazinecarbothiomide hybrids and their anticonvulsant activity. J Med Chem 25:1390–1402
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Kamat PK, Tota S, Shukla R, Ali S, Najmi AK, Nath C (2011) Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacol Biochem Behav 100:311–319
Ahmad M, Najmi A, Mujeeb M, Akhtar M (2016) Protective effect of guggulipid in high fat diet and middle cerebral artery occlusion (MCAO) induced ischemic cerebral injury in rats. Drug Res 66:407–421
Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F (2015) Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0003321
Raza S, Khan M, Ahmad A, Ashafaq M, Islam F, Wagner A, Safhi M (2013) Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 230:157–171
Nguyen D, Zhou T, Shu J, Mao J (2013) Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes. doi:10.1038/protex.2013.097
Chen FE, Huang D-B, Chen Y-Q, Ghosh G (1998) Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 391:410–413
Gillette-Guyonnet S, Van Kan GA, Andrieu S, Barberger-Gateau P (2007) IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging 11:132–153
Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL (2004) Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res 55:498–506
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35:419–432
Tilleux S, Hermans E (2007) Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85:2059–2070
Hsieh H-L, Yang C-M (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res Int. doi:10.1155/2013/484613
Pannu R, Singh I (2006) Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 49:170–182
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97–107
Merrill JE, Benveniste EN (1996) Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 19:331–338
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 10:136–159
Kata D, Földesi I, Feher L, Hackler L, Puskas L, Gulya K (2016) Rosuvastatin enhances anti-inflammatory and inhibits pro-inflammatory functions in cultured microglial cells. Neuroscience 314:47–63
Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4:977–987
Moshage H, Kok B, Huizenga JR, Jansen P (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41:892–896
Li W-C, Zou Z-J, Zhou M-G, Chen L, Zhou L, Zheng Y-K, He Z-J (2015) Effects of simvastatin on the expression of inducible NOS in acute lung injury in septic rats. Int J Clin Exp Pathol 8:15106–15111
Nussler AK, Billiar TR (1993) Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol 54:171–178
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a001651
Kim YS, Ahn Y, Hong MH, Kim KH, Park HW, Hong YJ, Kim JH, Kim W, Jeong MH, Cho JG (2007) Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and nuclear factor-κB in endothelial cells. J Cardiovasc Pharmacol Ther 49:376–383
Acknowledgements
This research work was supported by the grant funded by the Department of Science and Technology (IF130014), Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husain, I., Akhtar, M., Vohora, D. et al. Rosuvastatin Attenuates High-Salt and Cholesterol Diet Induced Neuroinflammation and Cognitive Impairment via Preventing Nuclear Factor KappaB Pathway. Neurochem Res 42, 2404–2416 (2017). https://doi.org/10.1007/s11064-017-2264-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2264-2