Abstract
Preliminary studies conducted in our laboratory have confirmed that Bacopaside I (BS-I), a saponin compound isolated from Bacopa monnieri, displayed antidepressant-like activity in the mouse behavioral despair model. The present investigation aimed to verify the antidepressant-like action of BS-I using a mouse model of behavioral deficits induced by chronic unpredictable mild stress (CUMS) and further probe its underlying mechanism of action. Mice were exposed to CUMS for a period of 5 consecutive weeks to induce depression-like behavior. Then, oral gavage administrations with vehicle (model group), fluoxetine (12 mg/kg, positive group) or BS-I (5, 15, 45 mg/kg, treated group) once daily were started during the last two weeks of CUMS procedure. The results showed that BS-I significantly ameliorated CUMS-induced depression-like behaviors in mice, as characterized by an elevated sucrose consumption in the sucrose preference test and reduced immobility time without affecting spontaneous locomotor activity in the forced swimming test, tail suspension test and open field test. It was also found that BS-I treatment reversed the increased level of plasma corticosterone and decreased mRNA and protein expressions of glucocorticoid receptor induced by CUMS exposure, indicating that hypothalamic–pituitary–adrenal (HPA) axis hyperactivity of CUMS-exposed mice was restored by BS-I treatment. Furthermore, chronic administration of BS-I elevated expression levels of brain-derived neurotrophic factor (BDNF) (mRNA and protein) and activated the phosphorylation of extracellular signal-regulated kinase and cAMP response element-binding protein in the hippocampus and prefrontal cortex in mice subjected to CUMS procedure. Taken together, these results indicated that BS-I exhibited an obvious antidepressant-like effect in mouse model of CUMS-induced depression that was mediated, at least in part, by modulating HPA hyperactivity and activating BDNF signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is one of the most frequently-occurring, debilitating and life-threatening mental disorders worldwide and brings a serious health burden to the families and society [1, 2]. It is clinically featured by a loss of interest, guilty conscience, appetite changes, difficulties in sleeping or concentrating, and suicidal tendencies [3]. Available data from the World Health Organization revealed that by the year 2020, depressive disorder would become the second leading cause of disabling disease, only after heart disease [4]. Currently, pharmacological therapies treating depressive disease include the tricyclic antidepressants, selective neurotransmitters reuptake inhibitors and monoamine oxidase inhibitors [2, 5]. However, not all people with depression respond to them, and most clinical antidepressants exhibited a low efficacy and even undesirable adverse effects, such as fatigue, insomnia, dryness of the mouth, sedation, and sexual dysfunction [6, 7]. Therefore, higher effective and better tolerated antidepressants that could further ameliorate pharmacotherapy of depression should be sought.
Clinical studies have shown that stressful life events are one of most great pathogenic conditions in psychiatric disorders such as depression, and play a key role in the onset and evolution of depressive disease in human [8]. In rodents, chronic unpredictable mild stress (CUMS) induced depressive behaviors and neurobiological changes resemble those observed in depressive patients [9, 10]. Moreover, the animal model of CUMS has been commonly employed in preclinical antidepressant assessment for probing the pathophysiology of depressive disorder and the beneficial therapeutic effect [11].
Recently, sufficient evidence has suggested that pathophysiology of neurological disorders and stress responses are related to the hypothalamic–pituitary–adrenal (HPA) axis hyperactivity [12, 13]. Preclinical and clinical investigations have confirmed that chronic repeated stress leaded to depression-like behaviors accompanied by hyperactivation of HPA axis, characterized by elevated levels of circulating glucocorticoids [14] and decreased expressions of glucocorticoid receptor (GR) mRNA and protein [15]. However, chronic stress resulted in depression-like behaviors and the HPA axis dysregulation are significantly restored by antidepressant drugs [16]. Brain-derived neurotrophic factor (BDNF), a kind of trophic factor, is closely linked with neuronal cell survival and neurogenesis and plays a vital role in the pathological mechanisms of depressive disease. Clinical evidence has shown a decreased levels of BDNF in brain tissues from depressive patients in the postmortem analyses [17], while in rodent experiments an antidepressant-like effect was observed by brain infusion of BDNF [18]. It has been reported that a reduced BDNF level was also found in patients suffering from depression compared to the control subjects [19], and chronic administration of antidepressants was revealed to normalize the BDNF levels [20]. These studies implied that stimulation of BDNF signaling pathway could provide a novel strategy for depression treatment.
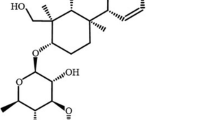
Bacopa monnieri (L.) Wettst. (Scrophulariaceae), an ancient Ayurvedic medicinal herb, has been applied for thousands of years as a neural tonic and memory enhancer [21]. In recent years, pharmacological studies have shown that standardized extracts of B. monnieri possess a variety of functions, such as anti-oxidant, anticonvulsant, antiepileptic, anti-inflammatory functions, antidepressant-like [22], as well as promote memory and intellect [23]. Bacopaside I (BS-I) (Fig. 1), one saponin compound isolated from B. monnieri. [24], has been studied because of its antidepressant-like effect in rodent models [25]. Earlier investigations have verified that oral gavage administration with BS-I leaded to an obvious decrease of immobility duration in both tail suspension test (TST) and forced swim test (FST) in our laboratory, which might be related to the activation of antioxidant and the noradrenergic system [26]. Previous studies have also shown that BS-I exhibited neuroprotective effects in rats subjected to cerebral ischemia injury [27]. However, the potential mechanism for antidepressant-like activity of BS-I whether involved in the HPA axis activation or BDNF signaling pathway remains unknown.
Considering the above, the current study was therefore carried out to explore the protective effect of BS-I on CUMS-induced depressant mice using various behavioral experiments, including the sucrose preference test (SPT), open field test (OFT), TST and FST. Furthermore, we then explored whether BS-I possesses the ability to down-regulate HPA hyperactivity or activate BDNF signaling pathway in mice exposed to CUMS.
Materials and Methods
Drugs and Chemicals
BS-I was obtained and identified by our laboratory (purity >98%, as shown in supporting information). Fluoxetine was purchased from Changzhou Siyao Pharmaceuticals Co., Ltd (Changzhou, China). BS-I and fluoxetine were respectively dissolved in saline. The volume for oral gavage administration was 0.1 mL/10 g body weight.
Animals
All experimental protocols carried out in our study were conducted in keeping with the Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Second Military Medical University, Shanghai, China. Male C57BL/6J mice (20–22 g; 7–8 weeks old) were provided by the Experiment Animal Center of the Second Military Medical University. The mice were housed (6–10 per cage) under standard laboratory conditions (room temperature 22–24 °C and humidity 50 ± 10%) with a 12-h light/dark cycle. All mice were allowed to acclimatize for 7 days with ad libitum access to food pellets and water before the beginning of the experiments.
Chronic Unpredictable Mild Stress Procedure
The CUMS regimen was conducted as recorded earlier [28] with minor modifications. During the experiment, the animals from the control (non-stressed) group were undisturbed in the cages except for ordinary daily care such as cage cleaning, whereas each mouse in CUMS-exposed groups was kept in a single cage and subjected to the following stimuli once per day for 5 consecutive weeks. The stressors included cage tilting at 45° for 24 h, restraint in a plastic tube for 6 h, cage shaking at 200 rpm for 40 min, wet bedding for 20 h, water deprivation for 24 h, overnight illumination and food deprivation for 24 h. And the stressor of overnight illumination was performed twice per week with an interval of 3–4 days. These various stressors were applied in random sequence over a period of 1 week and repeated with an unpredictable order during the following 4 weeks (as shown in Table 1).
Experimental Design
The doses and route of BS-I and fluoxetine administration adopted in this experiment were selected on the basis of previous studies [26]. The mice were randomly assigned to six different groups (10 individuals in each group), including a non-stressed group (Control + vehicle), a CUMS group (CUMS + vehicle), a fluoxetine-treated group (CUMS + 12 mg/kg Flu) and 3 BS-I treated groups (CUMS + 5, 15, 45 mg/kg BS-I, respectively). Animals were orally administered with BS-I, fluoxetine or vehicle once a day from 9:00 a.m.–10:00 a.m. during the last two weeks along with chronic stress. Mice from the control group were also orally treated with saline solution of equal volume. The schedule of the experimental design is given in Fig. 2.
The schedule of the experimental design for the CUMS procedure, drug administration and behavioral tests. Blood samples and brain tissues (hippocampus and prefrontal cortex) were collected and kept at −80 °C for subsequent determination. CUMS chronic unpredictable mild stress, FST forced swimming test, OFT open field test, SPT sucrose preference test, TST tail suspension test
Behavioral Evaluation
Sucrose Preference Test (SPT)
The SPT was conducted as reported previously [29] with slight modifications. Briefly, before the examination, each mouse was separately housed and simultaneously provided with two bottles, one filling with 1% sucrose solution (w/v) and the other tap water. After 24 h, one bottle of sucrose solution was taken the place of tap water for another 24 h. Then, 24 h of water and food deprivation was managed after the adaptation process. To prevent possible position preference, the distance of the two bottles to the mouse in the cage was equal and their position was interchanged every 12 h. The SPT was conducted during the dark phase (7:00 p.m. to 9:00 p.m.), in which mice were presented with two bottles filling with sucrose solution (1%, w/v) and tap water respectively. The consumptions of the two solutions were measured respectively after 2 h of exposure. The baseline SPT was performed before stress, and then carried out at the end of 3- and 5-week CUMS procedure. Sucrose preference (SP) was calculated via the sucrose intake rate by the following formula: SP = (sucrose intake (g)/(sucrose intake (g) + water intake (g))) × 100%.
Open Field Test (OFT)
The OFT was employed to assess spontaneous locomotor activity in animals, and the experimental method was based on those reported earlier [30]. The instrument applied in the test was made of a black square wooden arena (40 × 60 × 50 cm) with 12 equal squares on the floor of arena and equipped with a red bulb above the arena. Each animal was softly placed in the center of the arena for 30 s of acclimation, and freely permitted exploring the zone for 5 min. Total behaviors including the total running distance and time spent in the center zone were recorded by ANY-maze software. After each trial, the floor was wiped thoroughly with a 75% alcohol solution to remove possible clues from previous animal. The technical observers were blinded to the whole experiment.
Tail Suspension Test (TST)
The immobility duration in the TST was detected by the conventional method documented previously [31]. Briefly, the single mouse was suspended by an adhesive tape for 6 min and hung 50 cm above the floor. The total time of immobility during the last 4 min of each trial was recorded by ANY-maze software. Animals were defined to be immobile when they passively hung and did not show any body movement during the trials. The trained person was ignorant of the animals groups.
Forced Swimming Test (FST)
The FST was conducted as reported earlier after 5-week CUMS exposure [32]. Briefly, the animal was separately placed in a glass cylinder (20 cm diameter × 40 cm height) contained approximately 30 cm high water (25 ± 1 °C) and compelled to swim for 6 min. The water for each trial needed to be changed. The immobility duration during the final 4 min was recorded. The total time of immobility was judged the time spent by the animal floating passively in an upright posture without struggling, and making only slight motion to maintain the head above the water. The technical observers were blinded to the animals groups.
Determination of Corticosterone Concentrations in Plasma
Mice were anesthetized 1 day after the behavioral assessments. Blood from each mouse was obtained through the retro-orbital plexus into a centrifuge tube containing 10 µl of heparin solution (6250 IU/ml). Plasma was collected by centrifugation at 3000×g for 15 min at 4 °C and then kept at −80 °C until examination. The levels of corticosterone were analyzed using a commercially available ELISA kit (Enzo Life Sciences, USA) on the basis of the manufacturer’s protocol.
Determination of Glucocorticoid Receptor and Brain-Derived Neurotrophic Factor mRNA Expressions by Real-Time Quantitative PCR Assay
After blood collection, the brain tissue was quickly removed from each mouse by dissection and rinsed with ice-cold saline. The tissues of hippocampus and prefrontal cortex were obtained on a cold plate, then rapidly frozen in liquid nitrogen and kept at −80 °C until assay. Total RNA was extracted from these various brain regions by Trizol reagent (Invitrogen, San Diego, CA, USA) on the basis of the manufacturer’s manual. Isolated RNA samples were reverse transcribed by a PrimeScript™ RT Master Mix kit (Takara Bio, Inc., JP). The primers were applied in this study as follows: BDNF (forward 5′-TTATTTCATACTTCGGTTGC-3′; reverse 5′-TGTCAGCCAGTGATGTCG-3′; product size, 165 bp), GR (forward 5′-AGCTCCCCCTGGTAGAGAC-3′; reverse 5′-GGTGAAGACGCAGAAACCTT-3′; product size, 120 bp), and β-actin (forward 5′-GGCTGTATTCCCCTCCATCG-3′; reverse 5′-CCAGTTGGTAACAATGCCATGT; product size, 154 bp). Real-time quantitative PCR was conducted with a StepOnePlus™ real-time PCR system (Applied Biosystems, Inc., Foster City, CA). The amplified conditions was used as follows: 30 s hold at 95 °C, followed by 40 cycles of 5 s at 95 °C, 45 s at 57 °C, and 45 s at 72 °C. The relative levels of detected mRNAs were analyzed by the 2−ΔΔCt method with β-actin expressions as an internal control.
Western Blot Analysis
The frozen hippocampus and prefrontal cortex from the mice were homogenized in radio-immunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, US), and then centrifuged at 12,000×g for 30 min at 4 °C. The supernatants were harvested, and the concentrations of protein were detected by a bicinchoninic acid protein analysis kit (Thermo Fisher Scientific, US). The same quantity of protein was separated by 15% SDS-PAGE, and subsequently transferred to polyvinylidene difluoride membranes. After blocking with 5% skimmed milk for 1 h at room temperature, the transferred membranes were incubated overnight at 4 °C with primary antibody anti-BDNF (1:1000; Santa Cruz Biotechnology, CA, USA), anti-GR (1:1000; abcam, UK), anti-extracellular signal-regulated kinase (ERK, 1:1000; Cell Signaling Technology, MA, USA), anti-phosphor-ERK (1:5000; Cell Signaling Technology, MA, USA,) anti-cAMP response element-binding protein (CREB, 1:1000; Cell Signaling Technology, MA, USA), anti-phosphor-CREB (1:1000; abcam, UK) and anti-β-actin (1:2000; Cell Signaling Technology, MA, USA). The antigen–antibody complexes were then treated with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology, CA, USA) at room temperature for 1 h. The immunoreactive bands were visualized with a Tanon™ 4200 Chemiluminescent Imaging System and analyzed using the Gel Image System Software (Tanon™ Version 4.2).
Statistical Analysis
The GraphPad Prism software (Version 5.0; San Diego, CA, USA) was utilized to perform the calculation and prepare graphical data. Significant differences were performed using a one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test between groups. All the data are presented as the mean ± standard error of the mean (SEM). Statistical significance was determined as P < 0.05.
Results
BS-I Treatment Reversed the Chronic Unpredictable Mild Stress-Induced Anhedonia
Anhedonia, as an important characteristic of depressive disease, can be reflected by the decreased percentages of sucrose consumption in depressed animals. Figure 3a–c showed the effects of BS-I treatment on sucrose preference in mice exposed to CUMS procedure. Before the CUMS regimen, No obvious difference was found in sucrose consumption among any of the experimental groups (P > 0.05). However, after 3 weeks of CUMS exposure, the sucrose preference was evidently reduced in stressed mice relative to the control mice (P < 0.01). BS-I administration at doses of 5, 15 and 45 mg/kg for 2 weeks distinctly reversed CUMS-induced decrease in sucrose consumption relative to the CUMS-vehicle group (P < 0.05, P < 0.01 and P < 0.01, respectively). The sucrose consumption was increased by 65 ± 10.91%, 71 ± 11.18 and 67 ± 9.15 respectively. Similarly, treatment with the positive control fluoxetine (12 mg/kg) dramatically elevated the levels of the percentages of sucrose solution intake in animals exposed to CUMS procedure (P < 0.01).
BS-I treatment reversed the depression-like behaviors of mice induced by CUMS procedure. a–c BS-I treatment elevated the percentages of sucrose consumption of mice subjected to CUMS in the SPT. The SPT was conducted before CUMS (a), 3 weeks after CUMS (b) and 5 weeks after CUMS (c). In the OFT, BS-I treatment displayed no evident changes on spontaneous locomotor activity in mice. Each column represented mean d total running distance and e time spent in the center zone. f BS-I treatment reduced the immobility duration of mice exposed to CUMS in the TST. g BS-I treatment decreased the immobility time of mice exposed to CUMS in the FST. Data are expressed as the mean ± SEM. ## P < 0.01, compared with the control group. * P < 0.05 and ** P < 0.01, compared with CUMS-vehicle group. CUMS chronic unpredictable mild stress, FST forced swimming test, OFT open field test, SPT sucrose preference test, TST tail suspension test
BS-I Treatment Displayed No Changes on Spontaneous Locomotor Activity of Mice
The OFT was used to probe the effect of BS-I administration on spontaneous locomotor activity in animals. As shown in Fig. 3d–e, after 5 consecutive weeks of CUMS exposure, no significant changes in the total running distance and time spent in the center zone were observed between the stressed mice and non-stressed mice (P > 0.05). Moreover, animals treated with BS-I (5, 15 and 45 mg/kg) or fluoxetine (12 mg/kg) did not result in significant changes in above tested motivational behaviors (P > 0.05).
BS-I Treatment Reversed the Increased Immobility Time of Chronic Unpredictable Mild Stress Mice
The effect of BS-I administration on immobility duration in the TST was presented in Fig. 3f. Mice exposed to CUMS displayed a distinct elevation in immobility time relative to the control mice (P < 0.01). Chronic administrations of BS-I at the three selected doses all distinctly reduced the immobility duration in CUMS-exposed mice relative to the model group (P < 0.01, P < 0.05 and P < 0.01, respectively). Besides, fluoxetine treatment at dose of 12 mg/kg evidently decreased the immobility duration relative to the stressed mice treated with saline (P < 0.01).
The similar results obtained from the FST also indicated the antidepressant-like effect of BS-I (Fig. 3g). In CUMS-exposed animals, there was an obvious elevation in immobility time relative to the control mice in the FST. BS-I treatment at doses of 5, 15 or 45 mg/kg (P < 0.01, P < 0.05 and P < 0.01, respectively) reversed CUMS-induced elevation of immobility duration relative to the model animals (CUMS + vehicle). The immobility duration of mice subjected to the CUMS procedure was also significantly reduced by the treatment of fluoxetine at dose of 12 mg/kg after 2-week administration (P < 0.01).
BS-I Treatment Reduced the Chronic Unpredictable Mild Stress-Induced Elevation of Plasma Concentration
As presented in Fig. 4, after 5 weeks of CUMS exposure, the levels of plasma corticosterone were dramatically elevated in the CUMS group relative to those in the control mice. However, repeated administration with BS-I (5, 15 and 45 mg/kg) for 2 weeks distinctly decreased the CUMS-induced elevation of the plasma corticosterone levels relative to the CUMS-vehicle group (P < 0.05, P < 0.05 and P < 0.01, respectively). Fluoxetine (12 mg/kg) also produced an obvious decrease in the corticosterone concentrations in plasma of mice subjected to CUMS paradigm compared with the model (CUMS + vehicle) group (P < 0.01).
BS-I Treatment Up-regulated Glucocorticoid Receptor and Brain-Derived Neurotrophic Factor mRNA Expressions in the Hippocampus and Prefrontal Cortex of Chronic Unpredictable Mild Stress Mice
The findings revealed that the levels of GR mRNA in the hippocampus and prefrontal cortex from the model animals were both dramatically decreased when compared with that of the control animals (P < 0.01). However, after administration of BS-I for 2 consecutive weeks, the expressions of GR mRNA were elevated in the hippocampus and prefrontal cortex, especially at high dose (P < 0.01). Administration with fluoxetine at dose of 12 mg/kg also obviously reversed CUMS-induced reduction in GR mRNA expressions (Fig. 5a, b).
BS-I administration elevated the CUMS-induced reduction of GR and BDNF mRNA levels. a, b The levels of GR mRNA in the hippocampus and prefrontal cortex. c, d The levels of BDNF mRNA in the hippocampus and prefrontal cortex. Data are expressed as the mean ± SEM. ## P < 0.01, compared with the control group. * P < 0.05 and ** P < 0.01, compared with CUMS-vehicle group. CUMS chronic unpredictable mild stress, GR glucocorticoid receptor, BDNF brain-derived neurotrophic factor
A similar trend was found in BDNF mRNA levels. Significant reductions of BDNF mRNA expressions were observed in the hippocampus and prefrontal cortex in mice subjected to CUMS paradigm relative to the control mice (P < 0.01). However, the levels of BDNF mRNA were significantly increased by chronic administration of BS-I at the doses of 5, 15 or 45 mg/kg in the hippocampus (P < 0.05, P < 0.01 and P < 0.01, respectively), similar to that in prefrontal cortex (P < 0.01, P < 0.05 and P < 0.01, respectively) (Fig. 5c, d). This effect was also observed in the positive control group.
BS-I Treatment Up-regulated Protein Expressions of Glucocorticoid Receptor, Brain-Derived Neurotrophic Factor, Phosphorylations of ERK and CREB in the Hippocampus and Prefrontal Cortex of Chronic Unpredictable Mild Stress Mice
As presented in Fig. 6, western blot results revealed that the expressions of GR, BDNF, pERK and pCREB were reduced in both the hippocampus and prefrontal cortex in CUMS-vehicle mice relative to that in the control mice treated with saline (P < 0.01). By contrast, after treatment of graded doses of BS-I, the expressions of GR were significantly reversed in the hippocampus (P < 0.05, P < 0.01 and P < 0.01, respectively) and prefrontal cortex (P < 0.05, P < 0.05 and P < 0.01, respectively). Similar phenomenon about BDNF protein expressions was also observed in the hippocampus and prefrontal cortex. In addition, compared with the CUMS-vehicle group, BS-I markedly elevated pERK (P < 0.05, P < 0.05 and P < 0.01) and pCREB (P < 0.05, P < 0.01 and P < 0.01) at doses of 5, 15 or 45 mg/kg in the hippocampus and prefrontal cortex. Similarly, fluoxetine administration (12 mg/kg) restored the pERK and pCREB levels to the control level. By contrast, the levels of ERK and CREB protein were not altered among all experimental groups.
BS-I administration reversed the CUMS-induced reductions in GR, BDNF, pERK and pCREB expressions in the hippocampus (a–e) and prefrontal cortex (f–j). a and f Representative bands of GR, BDNF, pERK and pCREB expressions analyzed by western blotting in the hippocampus. b–e Densitometric analysis for the protein expressions of GR, BDNF, pERK and pCREB in the hippocampus. g–j Densitometric analysis for the protein expressions of GR, BDNF, pERK and pCREB in the prefrontal cortex. Data are expressed as the mean ± SEM. ## P < 0.01, compared with the control group. * P < 0.05 and ** P < 0.01, compared with CUMS-vehicle group. CUMS chronic unpredictable mild stress, GR glucocorticoid receptor, BDNF brain-derived neurotrophic factor, ERK extracellular signal-regulated kinase, CREB cAMP response element-binding protein
Discussion
In the current study, mice subjected to CUMS process for 5 consecutive weeks exhibited an obvious depressive state, as characterized by a decreased sucrose preference, and elevated immobility durations in the FST and TST, suggesting that the animal depression model was successfully established. Anhedonia, a prominent characteristic of human depressive disease, is mostly applied to assess the depression-like behaviors in preclinical investigations [33]. Sucrose preference is widely utilized to represent the alteration of anhedonia-like behavior in CUMS depression mode, indicating a loss of interest or pleasure [33, 34]. The present investigation confirmed that CUMS animals displayed a reduction of sucrose preference compared with the control animals, which was consistent with previous findings [28, 30]. Chronic administration of BS-I significantly ameliorated the behavioral change, indicating the antidepressant-like activity of BS-I in depression mice induced by CUMS.
The FST and TST, also known as the behavior despair paradigms, are most frequently used to assess antidepressant drug effects in experimental studies and determine underlying mechanisms involved in antidepressant responses, due to their ease of use, high predictive validity, and good reliability [35, 36]. Numerous investigations have demonstrated that mice exposed to repeated stress displayed an obvious extension of immobility duration in the FST and TST [36, 37]. Consistently, the present investigation found that increased total time of immobility in both FST and TST was also observed in mice subjected to CUMS paradigm compared with that in unstressed mice. The data showed that BS-I administration for 2 weeks dramatically reduced the immobility duration of CUMS-treated mice, similar to the established antidepressant effect of fluoxetine. Additionally, to avoid the possibility that the decrease in the immobility duration caused by the compound might be due to an enhancement in spontaneous activity, the OFT was performed to examine locomotor and exploratory behaviors in experimental mice. The data from the current investigation showed that there were no alterations observed in time spent in the center zone and total running distance among any of the experimental group. This finding suggested that BS-I had no direct psycho-stimulation or unspecific responses. Taken together, these results from behavioral experiments suggested that BS-I exhibited a robust antidepressant-like efficacy in mouse model of depressive disorder caused by CUMS exposure.
After the behavioral evaluation, we then focused on the influence on the HPA axis by detecting the corticosterone level in the plasma. As we all known, the activation of HPA system is associated with various stressful stimuli and plays a critical role in the pathological mechanisms of stress-induced mental diseases such as depressive illness [38, 39]. Previous study data indicated that elevated blood glucocorticoid levels resulting from HPA axis feedback dysfunction observed in rodents (corticosterone) or human depressive disorder (cortisol) [16]. In accord with several previous results, we observed that mice from CUMS-vehicle group displayed higher plasma corticosterone concentrations when compared with the control mice, and all BS-I treatment groups attenuated the abnormal alterations, especially at high dose. Two different receptor including GR and mineralocorticoid receptor (MR) are associated with glucocorticoid feedback regulation [15]. It’s generally believed that MR is involved in the adjustment of circadian fluctuations while GR regulates stress responses [16]. Glucocorticoid suppresses stress-related injure and the secretion of corticotrophin releasing hormone through GR activation, which conversely reduces corticosterone production [36]. However, once subjected to chronic stress, excessive glucocorticoid secretion results in the impaired HPA axis negative feedback and decreased GR expression [40]. It has been documented that the GR expressions (mRNA and protein) in hippocampus were reduced in animal model of depression, and that long-term antidepressants treatment could reverse this changes by regulating GR expression [16, 36]. Consistent with this, present data revealed that mice subjected to repeated stress exhibited lower levels of GR mRNA and protein in the hippocampus and prefrontal cortex, while chronic treatment with either BS-I or fluoxetine significantly restored the down-regulated expressions of GR mRNA and protein induced by CUMS exposure.
To further elucidate the pharmacological mechanism of BS-I, the current investigation probed the effects of BS-I on BDNF signaling pathway. A large number of previous studies suggested that chronic stress could result in neuronal atrophy and stress-induced inhibition of neurogenesis in the brain tissues, which are involved in the pathogenesis of depressive illness [30, 41]. Trophic factors, especially BDNF are thought to be associated with the development of depressive disease and responsible for antidepressants activity in preclinical and clinical studies. It has been suggested that various stress procedures like CUMS caused an evident reduction of BDNF expressions (mRNA and protein) in the hippocampus and prefrontal cortex, while chronic treatment with antidepressants obviously elevated BDNF levels in those regions [42]. Consistently, in this investigation, Exposure of CUMS paradigm was observed to reduce levels of BDNF mRNA and protein in the hippocampus and prefrontal cortex of mice, and BS-I administration inhibited the decrease of the expressions of BDNF mRNA and protein induced by CUMS paradigm, which provided evidence that elevation of BDNF expression may be contributed to the antidepressant property of BS-I. Then, we detected the changes in ERK and CREB activity. Increasing evidence showed that phosphorylations of ERK and CREB are involved in affecting neurotrophic activity and neurogenesis via activating intracellular signaling cascades [43, 44]. More interestingly, activation of pERK and pCREB was also observed in the hippocampus and prefrontal cortex of animals administrated with BS-I. As a result, chronic administration of BS-I for 2 consecutive weeks alleviated the CUMS-induced inhibition of ERK and CREB phosphorylations in those regions. Our results suggested that the antidepressant-like property of BS-I might be related to elevation of BDNF mediated by activation of pERK/pCREB/BDNF signaling.
In conclusion, our studies demonstrated that chronic administration of BS-I exhibited significant antidepressant-like properties in CUMS-induced depressed mice. And this beneficial effect is mediated, at least in part, by modulating HPA hyperactivity and activating BDNF signaling pathway in the hippocampus and prefrontal cortex. Hence, our investigation provided new insights into the potential of BS-I in therapeutic interventions for depressive disorder. Further studies are need to explore whether BDNF signaling is essential mediator of the antidepressant-like effects as well as the side effects of BS-I. It remains unclear that whether BS-I modulate other pathways such as P13K-Akt signaling pathways as well as neurotransmitters.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- BS-I:
-
Bacopaside I
- CUMS:
-
Chronic unpredictable mild stress
- FST:
-
Forced swimming test
- GR:
-
Glucocorticoid receptor
- HPA:
-
Hypothalamic–pituitary–adrenal
- MR:
-
Mineralocorticoid receptor
- OFT:
-
Open field test
- Pcreb:
-
Phosphorylated cAMP response element-binding protein
- pERK1/2:
-
Phosphorylated extracellular signal-regulated kinase ½
- SPT:
-
Sucrose preference test
- TST:
-
Tail suspension test
References
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Nemeroff CB (2007) The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res 41:189–206
Shelton RC (2008) The molecular neurobiology of depression. Nature 455:894–902
Peng YL, Liu YN, Lei L, Xia W, Jiang CL, Wang YX (2012) Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflamm 9:1–12
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, Mcgrath PJ (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40
Kennedy SH (2006) A review of antidepressant treatments today. Eur Neuropsychopharmacol 16:S619–S623
Hill MN, Hellemans KGC, Verma P, Gorzalka BB, Weinberg J (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117
Henn FA, Vollmayr B (2005) Stress models of depression: Forming genetically vulnerable strains. Neurosci Biobehav Rev 29:799–804
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4: 775–790
Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 33:450–455
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501.
Barden N (2004) Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci 29:185–193
Jindal A, Mahesh R, Bhatt S (2013) Etazolate rescues behavioral deficits in chronic unpredictable mild stress model: modulation of hypothalamic-pituitary-adrenal axis activity and brain-derived neurotrophic factor level. Neurochem Int 63:465–475
Medina A, Seasholtz AF, Sharma V, Burke S, Jr BW, Myers RM, Schatzberg A, Akil H, Watson SJ (2013) Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res 47:307–314
Mao QQ, Ip SP, Ko KM, Tsai SH, Che CT (2009) Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry 33:1211–1216
Castrén E, Võikar V, Rantamäki T (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7:18–21
Hoshaw BA, Malberg JE, Lucki I (2005) Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 1037:204–208
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada SI (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54:70–75
Aguiar S, Borowski T (2013) Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res 16:313–326
Banerjee R, Hazra S, Ghosh AK, Mondal AC (2014) Chronic administration of bacopa monniera increases BDNF protein and mRNA expressions: a study in chronic unpredictable stress induced animal model of depression. Psychiatry Investig 11:297–306
Mathur D, Goyal K, Koul V, Anand A (2016) The molecular links of re-emerging therapy: a review of evidence of Brahmi (Bacopa monniera). Front Pharmacol 7:44
Chakravarty AK, Sarkar T, Masuda K, Shiojima K, Nakane T, Kawahara N (2002) Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry 33:553–556
Zhou Y, Shen YH, Zhang C, Su J, Liu RH, Zhang WD (2007) Triterpene saponins from Bacopa monnieri and their antidepressant effects in two mice models. J Nat Prod 70:652–655
Liu X, Liu F, Yue R, Li Y, Zhang J, Wang S, Zhang S, Wang R, Lei S, Zhang W (2013) The antidepressant-like effect of bacopaside I: possible involvement of the oxidative stress system and the noradrenergic system. Pharmacol Biochem Behav 110:224–230
Liu X, Yue R, Zhang J, Shan L, Wang R, Zhang W (2013) Neuroprotective effects of bacopaside I in ischemic brain injury. Restor Neurol Neurosci 31:109–123
Tang J, Xue W, Xia B, Li R, Tao W, Chen C, Zhang H, Wu R, Wang Q, Wu H (2015) Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep 5:338–341
Pothion S, Bizot JF, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155:135–146
Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL, Wang F, Chen JG (2012) Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 166:1872–1887
Cheng M (2005) The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology 228:157–166
Kurhe Y, Radhakrishnan M, Gupta D (2014) Ondansetron attenuates depression co-morbid with obesity in obese mice subjected to chronic unpredictable mild stress; an approach using behavioral battery tests. Metab Brain Dis 29:701–710
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. NeuropsychoBiology 52:90–110
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Li M, Fu Q, Li Y, Li S, Xue J, Ma S (2014) Emodin opposes chronic unpredictable mild stress induced depressive-like behavior in mice by upregulating the levels of hippocampal glucocorticoid receptor and brain-derived neurotrophic factor. Fitoterapia 98:1–10
Tao W, Dong Y, Su Q, Wang H, Chen Y, Xue W, Chen C, Xia B, Duan J, Chen G (2016) Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav Brain Res 308:177–186
Mcewen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185
Ge JF, Gao WC, Cheng WM, Lu WL, Tang J, Peng L, Li N, Chen FH (2013) Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur Neuropsychopharmacol 24:172–180
Taksande BG, Faldu DS, Dixit MP, Sakaria JN, Aglawe MM, Umekar MJ, Kotagale NR (2013) Agmatine attenuates chronic unpredictable mild stress induced behavioral alteration in mice. Eur J Pharmacol 720:115–120
Fuchs E, Czéh B, Kole MH, Michaelis T, Lucassen PJ (2004) Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol 14(Suppl 5):S481–S490
Yi LT, Li J, Geng D, Liu BB, Fu Y, Tu JQ, Liu Y, Weng LJ (2013) Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in mice. J Ethnopharmacol 147:245–253
Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H (2013) Brain-derived neurotrophic factor and glucocorticoids: Reciprocal influence on the central nervous system. Neuroscience 239:157–172
Tardito D, Musazzi L, Tiraboschi E, Mallei A, Racagni G, Popoli M (2009) Early induction of CREB activation and CREB-regulating signalling by antidepressants. Int J Neuropsychopharmacol 12:1367–1381
Acknowledgements
This work was supported by the National Major Project of China (2011ZX09307-002-03), the National Natural Science Foundation of China (81230090, 81520108030, 81573318, 81373301 and 1302658), the Scientific Foundation of Shanghai China (13401900103, 13401900101 and 12401900801).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared that there is no potential conflict of interest.
Additional information
Xianpeng Zu and Mingjian Zhang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zu, X., Zhang, M., Li, W. et al. Antidepressant-like Effect of Bacopaside I in Mice Exposed to Chronic Unpredictable Mild Stress by Modulating the Hypothalamic–Pituitary–Adrenal Axis Function and Activating BDNF Signaling Pathway. Neurochem Res 42, 3233–3244 (2017). https://doi.org/10.1007/s11064-017-2360-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2360-3