Abstract
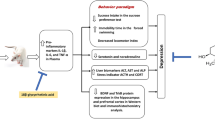
The dried roots of Rehmannia glutinosa Libosch. (Scrophulariaceae) are of both medicinal and nutritional importance. Our previous study has found that the 80% ethanol extract of R. glutinosa (RGEE) produced antidepressant-like activities in mouse behavioral despair depression models. However, its mechanisms are still unclear. The present study aimed to observe the antidepressant-like mechanisms of RGEE on a rat chronic unpredictable mild stress (CUMS) model by involving monoaminergic neurotransmitters and brain-derived neurotrophic factor (BDNF). CUMS-stressed rats were orally given RGEE daily (150, 300, and 600 mg/kg) or fluoxetine hydrochloride (FH) for 3 weeks after starting the CUMS procedure. Sucrose preference test was carried out to observe depression-like behavior, and serum and brain tissues were used for neurochemical and fluorescent quantitative reverse transcription PCR analysis. Results demonstrated that CUMS induced depression-like behavior, whereas RGEE and FH administration inhibited this symptom. Furthermore, CUMS caused excessively elevated levels of serum corticosterone (CORT), an index of hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, in a manner attenuated by RGEE and FH administration. RGEE administration also further elevated monoamine neurotransmitters and BDNF levels, up-regulated the mRNA expression of BDNF and tropomyosin-related kinase B (TrkB) in hippocampus of rats suffering CUMS. Together, our findings suggest that RGEE can improve CUMS-evoked depression-like behavior, and indicate its mechanisms may partially be associated with restoring HPA axis dysfunctions, enhancing monoamineergic nervous systems, and up-regulating BDNF and TrkB expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is very common but serious threat to human life. According to a statistics from the World Health Organization (WHO), depression has been the world’s 4th largest disease with the global incidence about 11%, and will be up to the 2nd largest disease in 2020 following the heart disease and gradually become the main killer of the human in the twenty-first century (Kowalska et al. 2013). In recent years, the development of new antidepressants from natural herbal medicine has become one of the important research hotspots (Mou et al. 2017; Ren et al. 2017; Ye et al. 2017).
The dried roots of Rehmannia glutinosa Libosch. (Scrophulariaceae) are firstly recorded in the “Shen Nong’s Materia Medica” as listed one of “top grades” in Han Dynasty of China with over 2000 years of medicinal history (Zhang et al. 2016a, b). R. glutinosa is traditionally thought as a tonic brain medicine, and commonly used in the prevention and treatment of some central nervous system (CNS)-related diseases such as depression, cognitive impairment, ischemic stroke, anti-anxiety, etc. (Cui et al. 2013; Dong et al. 2016; Lee et al. 2011; Zhang et al. 2016a, b). Among them, especially in the treatment of depression is the most common (Zhang et al. 2016a, b). In clinic, RG and RG-contained traditional prescriptions including Baihe Dihuang decoction, Yiguan Decoction, Xuefu Zhuyu Decoction, etc., have been used in the treatment of depression (Zhang et al. 2016a, b). However, as for its antidepressant mechanisms, it is unclear until now.
Monoamine deficiency and hypothalamic–pituitary–adrenal (HPA) axis hyperactivity are thought involving the pathogenesis of depression (Freitas et al. 2010; Pariante and Lightman 2008). Growing evidence demonstrates that depression in patients with central brain-derived neurotrophic factor (BDNF) and its cognate receptor tropomycin-related kinase B (TrkB) expression decreased, while given antidepressant drug treatment, can significantly up-regulate BDNF and TrkB expression, suggesting BDNF and TrkB also participate in the pathophysiology of depression (Dwivedi 2009; Haghighi et al. 2013; Ladea and Bran 2013; Ray et al. 2014).
Our previous study confirmed that the 80% ethanol extract of R. glutinosa (RGEE) shortened the immobility time during forced swim and tail suspension tests in mice (Wang et al. 2014), suggesting that RGEE exhibits an antidepressant-like effect on mouse behavioral despair depression models. However, the therapeutic effects of RGEE on the chronic unpredictable mild stress (CUMS) model of depression and whether the antidepressant-like action of RGEE involves monoamine, HPA axis and BDNF remain unknown. Therefore, the purpose of this study was to explore the antidepressant-like effect of RGEE by involving monoamine, HPA axis and BDNF on a rat model of depression evoked by CUMS.
Materials and methods
Experimental animals
Sprague Dawley (SD) male rats weighted from 180 g to 220 g were provided by Shandong Lukang Pharmaceutical Co., Ltd. (Jining, China). Rats were given rodent laboratory chow and water ad libitum and kept under controlled conditions with a temperature of 22 ± 1 °C, relative humidity of 60% ± 10%, and a 12/12 h light/dark cycle (lights on at 7:00 A.M.). All the procedures were in strict accordance with the P.R. China legislation on the use and care of laboratory animals and guidelines formulated by the Institute for Experimental Animals of Henan University of Chinese Medicine. The procedures were approved by the university committee for animal experiments.
Reagents and drugs
R. glutinosa roots were collected from Wuzhi county in Jiaozuo City of Henan province (Wuzhi, China) and identified by Prof. Cheng-Ming Dong, at the Department of Pharmacognosy, College of Pharmacy, Henan University of Chinese Medicine (Zhengzhou, China). RGEE was prepared with 80% ethanol by conventional reflux extraction according to our previous report (Wang et al. 2014). The content of catalpol in RRGE was 33.48 mg/g as assayed by high-performance liquid chromatography (HPLC) (Wang et al. 2014). Fluoxetine hydrochloride (FH) was provided by Changzhou Siyao Pharmaceuticals Co., Ltd. (Changzhou, China). Rat corticosterone (CORT), norepinephrine (NE), dopamine (DA), serotonin (5-HT), 5-hydroxy-indoleacetic acid (5-HIAA) and BDNF enzyme-linked immunosorbent assay (ELISA) kits were provided by R&D Systems China Co., Ltd. (Shanghai, China).
CUMS procedure
CUMS procedure was slightly modified in terms of a previous report from Willner et al. (Willner et al. 1987). The weekly stress included deprivation of drinking water and eating, stroboscopic illumination, white noise, light/dark succession every 2 h, overnight illumination, 45° cage tilt, soiled cage, and pair housing. All the stress is done separately and continuously. Rats in the control group (non-CUMS-evoked) were placed in a separate room without contact with the CUMS-evoked animals, and only received solvent containing 0.5% sodium carboxymethyl cellulose (CMC-Na) rather than any pressure or medication.
Sucrose preference and drug administrations
All rats were given 1% sucrose solution for 24 h before the start of CUMS. The rats then drank sugar and fresh water for another 24 h. After deprivation of drinking for 23 h, rats were given 1% sucrose solution and fresh water for another 1 h. After such a sucrose consumption training field, animals were randomly divided into 6 groups (n = 8): control, Vehicle (CUMS), CUMS-FH (positive control, 10 mg/kg), and CUMS-RGEE at doses of 150, 300, and 600 mg/kg. These groups, except the control, underwent CUMS procedure for 3 weeks. All drugs were suspended in 0.5% CMC-Na and administered by by intragastric administration (ig) once a day at 11:00 A.M. for 3 weeks. Through the period of CUMS and treatment, sucrose preference test was conducted following an 18 h food and water deprivation at 11:00 A.M. every Thursday. Sucrose preference was calculated as sucrose preference (%) = sucrose intake (mL)/[sucrose intake (mL) + water intake (mL)] × 100%.
Collection of blood and brain tissue samples
At 1 h after completing the last stress and administration, all rats were killed between 11:00 A.M. and 1:00 P.M. to avoid fluctuations in hormone levels. After immediately collecting blood samples, the hippocampus of each hemisphere was independently separated on an ice plate and stored at −80 °C until analysis.
Assay for serum CORT and brain BDNF
Serum CORT and brain BDNF levels were measured by the commercial ELISA kits (R&D Systems China Co. Ltd., China) according to the manufacturer’s protocols.
Fluorescent quantitative reverse transcription PCR (FQ-RT-PCR)
Hippocampus total RNA was extracted by using TRIzol reagent following the manufacturer’s instructions. RT was performed according to the manufacturer’s instructions using the cDNA synthesis kit. The gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as an internal control. The PCR primer sequences were as follows: BDNF forward 5’-CAGGGGCATAGACAAAAG-3′, reverse 5’-CTTCCCCTTTTAATGGTC-3′ (153 bp product); TrkB forward 5’-TTTCCGCCACCTTGACTTG-3′, reverse 5’-ACAGGAACACGTGAACGGATT-3′ (61 bp product); and GAPDH forward 5’-CAAGGTCATCCATGACAACTTTG-3′, reverse 5’-GGGCCATCCACAGTCTTCTG-3′ (90 bp product). FQ-RT-PCR was performed using Hot Start Fluorescent PCR Core Reagent Kits (SYBR Green I) on a real-time PCR instrument (ABI StepOnePlus, Applied Biosystems). The PCR thermal cycling parameters were as follows: denaturing step at 94 °C for 4 min, followed by 40 cycles of annealing step at 94, 60, and 72 °C for 30, 30, and 30 s, respectively. All amplifications and detections were performed in a MicroAmp optical 96-well reaction plate with optical adhesive covers. The relative expression of mRNA (%) was obtained as follows: 2-ΔCT(1–2) × 100%, where CT represents the threshold cycle, ΔCT1 = CT(BDNF) − CT(GAPDH), and ΔCT2 = CT(TrkB) − CT(GAPDH).
Statistical analysis
All experimental data were expressed as mean ± standard deviation of the means. The treatment effect was determined through one-way ANOVA followed by least significant difference (P < 0.05) using the Statistics Package for Social Science program version 17.0. A value of P < 0.05 was considered statistically significant.
Results
Effects of RGEE on sucrose preference and body weight in CUMS rats
As shown in Fig. 1a, there was no significant difference in the sucrose preference among groups from the beginning of the CMUS procedure. CUMS caused obvious decrease in sucrose preference beginning from week 1 and maintaining in the following two weeks (P < 0.01). After 3 weeks of administration, RGEE (150, 300 and 600) and FH (10 mg/kg) all prevented the CUMS-caused decrease of sucrose preference (P < 0.05, P < 0.05, P < 0.01, and P < 0.01).
As shown in Fig. 1b, there was no significant difference in body weight per group of rats before the CUMS procedure was performed. After 3 weeks, the body weight of rats in the CUMS group was significantly reduced compared to that in the control (non-CUMS) group. There was no significant change in body weight in each administration group compared with CUMS group.
RGEE decreased serum CORT levels in CUMS-exposed rats
As shown in Fig. 2, compared with the control group, CUMS procedure significantly elevated the serum CORT levels of rats (P < 0.01). Compared with the CUMS group, chronic FH (10 mg/kg) and RGEE (150, 300 and 600 mg/kg) administration all significantly reduced the excessively high CORT levels in the serum of rats (P < 0.01, P < 0.05, P < 0.01, P < 0.01). Furthermore, no significance was found in serum CORT levels of rats among the three groups of FH (10 mg/kg) and RGEE (300 and 600 mg/kg).
RGEE enhanced central monoamine neurotransmitter levels
The levels of central monoamine neurotransmitters including 5-HT, 5-HIAA, NE and DA in hippocampus were assessed to investigate whether monoamineergic nervous system involves CUMS-evoked depression. As shown in Fig. 3, CUMS evoked significant reduction in the levels of 5-HT, 5-HIAA, NE and DA compared with the control group (P < 0.01, P < 0.01, P < 0.05 and P < 0.05). Administration with RGEE (150, 300 and 600 mg/kg) or FH (10 mg/kg) all significantly elevated the levels of 5-HT (P < 0.05, P < 0.05, P < 0.01 and P < 0.01) and 5-HIAA (P < 0.05, P < 0.01, P < 0.01 and P < 0.01). Moreover, RGEE at the dose of 600 mg/kg prevented the CUMS-induced decrease of the levels of NE (P < 0.05) and DA (P < 0.05) but FH at the dose of 10 mg/kg did not.
RGEE increased BDNF levels, and upregulated BDNF and TrkB mRNA expression
The level of BDNF, and the mRNA expression of BDNF and its cognate receptor TrkB in hippocampus were evaluated to observe whether BDNF participates in CUMS-evoked depression. As shown in Fig. 4, the BDNF level was significantly decreased in CUMS rats compared with those in the control group (P < 0.01) but were increased by administering 150, 300 and 600 mg/kg of RGEE (P < 0.05, P < 0.01 and P < 0.01) and 10 mg/kg of FH (P < 0.01). As shown in Fig. 5, BDNF and TrkB mRNA expression was significantly down-regulated in CUMS rats compared with that in the control group (both P < 0.01) and was reversed when 150, 300 and 600 mg/kg of RGEE (P < 0.05, P < 0.01 and P < 0.01) and 10 mg/kg of FH (both P < 0.01) were administered.
Discussion
Chronic stress is a common cause of depression, and some of the symptoms induced by it are similar to those of depression (Mou et al. 2017; Ren et al. 2017; Workman et al. 2016; Ye et al. 2017). CUMS is one of the most commonly used animal models of depression. It is not only widely used in the screening of antidepressants, but also more suitable for the study of depression and antidepressant mechanisms than acute stress models (Farhan et al. 2014; Ye et al. 2017). During the CUMS process, animals suffered a variety of mild stress, thus mimicking the daily life events of chronic stress in human depression and resulting in a core symptom of human depression: loss of pleasure (Farhan et al. 2014; Ye et al. 2017). As a result, CUMS induces a decrease in the sugar preference, which can be restored by the use of potent antidepressants (Ye et al. 2017). Thus, in this study, CUMS-evoked decrease in sucrose preference was obviously reversed by chronic RGEE administration at the dosages of 150, 300 and 600 mg/kg with obvious dose-dependent manners, further suggesting the antidepressant-like effects of RGEE.
There are many “hypotheses” about the pathogenesis of depression, including the “monoamine deficiency hypothesis” (Wang et al. 2017), “HPA axis hyperactivity hypothesis” (Aihara et al. 2007; Mou et al. 2017; Pariante and Lightman 2008), “BDNF deficiency hypothesis” (Dwivedi 2009; Haghighi et al. 2013; Ladea and Bran 2013; Ray et al. 2014; Ye et al. 2017) and so on.
Firstly, as for the monoamine deficiency hypothesis of depression, which has been widely recognized and concerned, it is commonly believed that depression in the central monoamine neurotransmitters mainly including 5-HT, 5-HIAA, NE, and (or) DA levels are lower, but after treatment with antidepressant drugs can be improved (Colla et al. 2014; de Sousa et al. 2014; Nutt 2006; Tian et al. 2006; Yu et al. 2015; Wang et al. 2017). In this study, CUMS induced the monoamine deficiency through evidenced by the reduction of hippocampus levels of monoamines including 5-HT, 5-HIAA, DA and NE, and RGEE (600 mg/kg) administration significantly reversed these reduction (P < 0.01, P < 0.01, P < 0.05 and P < 0.05, respectively), suggesting that promoting monoamineergic nervous systems may play key roles in the antidepressant-like mechanisms of RGEE, and to a certain extent, RRGE is more sensitive to 5-HT and its metabolite 5-HIAA than DA and NE. Moreover, it’s interesting to note that positive control FH at 10 mg/kg has no effect on hippocampal NE and DA but RGEE at 600 mg/kg shows a beneficial effect. Why would such phenomenon happen? We speculate that this may be related to the lower dose of FH and the higher dose of RRGE, and may also be related to the experimental environment, experimental period, experimental animals and other factors, and even different mechanisms.
Secondly, as for the HPA axis hyperactivity hypothesis of depression, which has been to some extent recognized and concerned, it is commonly believed that depression may be in the HPA axis hyperactivity state, but after treatment with antidepressant drugs can be improved (Aihara et al. 2007; Garza et al. 2012; Mou et al. 2017; Nguyen et al. 2017; Pariante and Lightman 2008). In this study, CUMS at least partially induced the HPA axis hyperactivity through evidenced by the excessive elevation of serum CORT level, and RGEE (150, 300 and 600 mg/kg) administration all significantly reversed such elevation, suggesting that inhibiting HPA axis hyperactivity may be at least partially involved in the antidepressant-like mechanisms of RGEE. In this regard, similar results to RRGE occurred following FH administration.
Thirdly, as for the BDNF deficiency hypothesis of depression, which has been widely recognized and concerned, it is commonly believed that depression in the central BDNF and its cognate receptor TrkB expression levels are down-regulated, but after treatment with antidepressant drugs can be improved (Dwivedi 2009; Haghighi et al. 2013; Ladea and Bran 2013; Ray et al. 2014; Ye et al. 2017). In this study, CUMS induced the BDNF deficiency through evidenced by the down-regulation of hippocampus mRNA expression of BDNF and its cognate receptor TrkB, and RGEE (150, 300 and 600 mg/kg) administration all significantly reversed such reduction. Actually, previous studies had reported the BDNF-up-regulated activities from both the R. glutinosa extract and it-contained compound catalpol (Liu et al. 2006; Wang et al. 2009; Zhang et al. 2014). These results indicate that RGEE partially exhibits antidepressant effects by inhibiting or reversing CUMS-evoked abnormalities in the BDNF expression. In this regard, similar results to RRGE occurred following FH administration.
In fact, hyperactivity of the HPA axis may damage hippocampal monoaminergic neurons, leading to a decrease in monoamine neurotransmitters. Antidepressants may protect monoaminergic neurons from damage by inhibiting HPA axis hyperactivity. Moreover, monoamine neurotransmitters can activate BDNF and its cognate receptor TrkB through some signal pathways. Taken together, based on the experimental results, we speculate that RRGE may enhance the monoamines by inhibiting the hyperactivity of HPA axis or enhance the BDNF-TrkB signal by increasing the monoamines, thereby generating antidepressant effects.
References
Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M (2007) HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res 155(3):245–256
Colla AR, Oliveira A, Pazini FL, Rosa JM, Manosso LM, Cunha MP, Rodrigues AL (2014) Serotonergic and noradrenergic systems are implicated in the antidepressant-like effect of ursolic acid in mice. Pharmacol Biochem Behav 124:108–116
Cui Y, Rong C, Wang J, Cui C, Wang L, Feng Z, Feng J, Niu B (2013) Mechanism-based anti-anxiety effects of polysaccharides extracted from shudihuang (radix rehmanniae preparata) by two-dimensional electrophoresis analysis in rat hippocampus proteins. J Tradit Chin Med 33(4):524–530
Dong W, Xian Y, Yuan W, Huifeng Z, Tao W, Zhiqiang L, Shan F, Ya F, Hongli W, Jinghuan W, Lei Q, Li Z, Hongyi Q (2016) Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats' stroke model. J Ethnopharmacol 191:169–179
Dwivedi Y (2009) Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat 5:433–449
Farhan M, Ikram H, Kanwal S, Haleem DJ (2014) Unpredictable chronic mild stress induced behavioral deficits: a comparative study in male and female rats. Pak J Pharm Sci 27(4):879–884
Freitas AE, Budni J, Lobato KR, Binfaré RW, Machado DG, Jacinto J, Veronezi PO, Pizzolatti MG, Rodrigues AL (2010) Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: evidence for the involvement of the monoaminergic system. Prog Neuro-Psychopharmacol Biol Psychiatry 34(2):335–343
Garza JC, Guo M, Zhang W, Lu XY (2012) Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signaling. Mol Psychiatry 17(8):790–808
Haghighi M, Salehi I, Erfani P, Jahangard L, Bajoghli H, Holsboer-Trachsler E, Brand S (2013) Additional ECT increases BDNF-levels in patients suffering from major depressive disorders compared to patients treated with citalopram only. J Psychiatr Res 47(7):908–915
Kowalska J, Rymaszewska J, Szczepańska-Gieracha J (2013) Occurrence of cognitive impairment and depressive symptoms among the elderly in a nursing home facility. Adv Clin Exp Med 22(1):111–117
Ladea M, Bran M (2013) Brain derived neurotrophic factor (BDNF) levels in depressed women treated with open-label escitalopram. Psychiatr Danub 25(2):128–132
Lee B, Shim I, Lee H, Hahm DH (2011) Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol 21(8):874–883
Liu J, He QJ, Zou W, Wang HX, Bao YM, Liu YX, An LJ (2006) Catalpol increases hippocampal neuroplasticity and up-regulates PKC and BDNF in the aged rats. Brain Res 1123(1):68–79
Mou Z, Huang Q, Chu SF, Zhang MJ, Hu JF, Chen NH, Zhang JT (2017) Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed Pharmacother 92:962–971
Nguyen ET, Streicher J, Berman S, Caldwell JL, Ghisays V, Estrada CM, Wulsin AC, Solomon MB (2017) A mixed glucocorticoid/mineralocorticoid receptor modulator dampens endocrine and hippocampal stress responsivity in male rats. Physiol Behav 178:82–92
Nutt DJ (2006) The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry 67(S6):3–8
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31(9):464–468
Ray MT, Shannon Weickert C, Webster MJ (2014) Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry 4:e389
Ren Y, Wang JL, Zhang X, Wang H, Ye Y, Song L, Wang YJ, Tu MJ, Wang WW, Yang L, Jiang B (2017) Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res Bull 134:211–219
de Sousa FC, Oliveira IC, Silva MI, de Melo CT, Santiago VR, de Castro Chaves R, Fernandes ML, Gutierrez SJ, Vasconcelos SM, Macêdo DS, Barbosa Filho JM (2014) Involvement of monoaminergic system in the antidepressant-like effect of riparin I from Aniba Riparia (Nees) Mez (Lauraceae) in mice. Fundam Clin Pharmacol 28(1):95–103
Tian YY, An LJ, Jiang L, Duan YL, Chen J, Jiang B (2006) Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci 80(3):193–199
Wang Z, Liu Q, Zhang R, Liu S, Xia Z, Hu Y (2009) Catalpol ameliorates beta amyloid-induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors. Neuroscience 163(4):1363–1372
Wang JM, Feng WS, Cui Y, Zhang YY, Wang GF, Zhou G, Wang XX (2014) Antidepressant-like effect of extracts from dried root of Rehmannia glutinosa (Dihuang). Chin. Pharm J 49(23):2073–2076
Wang GL, He ZM, Zhu HY, Gao YG, Zhao Y, Yang H, Zhang LX (2017) Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax Ginseng C.A. Meyer. J Ethnopharmacol 204:118–124
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93(3):358–364
Workman JL, Gobinath AR, Kitay NF, Chow C, Brummelte S, Galea LA (2016) Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis. Neuropharmacology 105:443–453
Ye YL, Zhong K, Liu DD, Xu J, Pan BB, Li X, Yu YP, Zhang Q (2017) Huanglian-Jie-du-tang extract ameliorates depression-like behaviors through BDNF-TrkB-CREB pathway in rats with chronic unpredictable stress. Evid Based Complement Alternat Med 2017:7903918
Yu HL, Sun LP, Li MM, Quan ZS (2015) Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neurosci Lett 593:24–28
Zhang LN, Jin GQ, Zhang XL, Gong ZB, Gu CY (2014) Effects of 5-hydroxymethyl furfural extracted from Rehmannia glutinosa Libosch on the expression of signaling molecules relevant to learning and memory among hippocampal neurons exposed to high concentration of corticosterone. Chin J Integr Med 20(11):844–849
Zhang Y, Chen YX, Huang SJ (2016a) Application status of the dry root of Rehmannia glutinosa. World J Integr Trad Western Med 11(2):275–277
Zhang K, Yang J, Wang F, Pan X, Liu J, Wang L, Su G, Ma J, Dong Y, Xiong Z, Wu C (2016b) Antidepressant-like effects of Xiaochaihutang in a neuroendocrine mouse model of anxiety/depression. J Ethnopharmacol 194:674–683
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81773928), the National Science & Technology Pillar Program of China during the 12th Five-Year Plan Period (2011BAI06B02), the Funding Scheme for Young Key Teachers of Colleges and Universities in Henan Province (2014GGJS-072), and the Science and Technology Project of Zhengzhou (20150309).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Wang, JM., Pei, LX., Zhang, YY. et al. Ethanol extract of Rehmannia glutinosa exerts antidepressant-like effects on a rat chronic unpredictable mild stress model by involving monoamines and BDNF. Metab Brain Dis 33, 885–892 (2018). https://doi.org/10.1007/s11011-018-0202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0202-x