Abstract
Background and objective
The ideal delivery of radiation to the surgical cavity of brain metastases (BMs) remains the subject of debate. Risks of local failure (LF) and radiation necrosis (RN) have prompted a reappraisal of the timing and/or modality of this critical component of BM management. IORT delivered at the time of resection for BMs requiring surgery offers the potential for improved local control (LC) afforded by the elimination of delay in time to initiation of radiation following surgery, decreased uncertainty in target delineation, and the possibility of dose escalation beyond that seen in stereotactic radiosurgery (SRS). This study provides a retrospective analysis with identification of potential predictors of outcomes.
Methods
Retrospective data was collected on patients treated with IORT immediately following surgical resection of BMs at three institutions according to the approval of individual IRBs. All patients were treated with 50kV portable linear accelerator using spherical applicators ranging from 1.5 to 4.0 cm. Statistical analyses were performed using IBM SPSS with endpoints of LC, DBC, incidence of RN, and overall survival (OS) and p < 0.05 considered significant.
Results
54 patients were treated with IORT with a median age of 64 years. The most common primary diagnosis was non-small cell lung cancer (40%) with the most common location in the frontal lobe (38%). Median follow-up was 7.2 months and 1-year LC, DBC, and OS were 88%, 58%, and 73%, respectively. LMD was identified in 2 patients (3%) and RN present in 4 patients (7%). The only predictor of LC was extent of resection with 1-year LC of 94% for GTR versus 62% for STR (p = 0.049).
Conclusions
IORT is a safe and effective means of delivering adjuvant radiation to the BM resection cavities with high rates of LC and low incidence of RN. Further studies are warranted directly comparing LC outcomes to SRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of adjuvant cranial radiation in patients requiring surgical resection for large brain metastases (BMs) remains a standard of care in most regions. Concerns related to the potential neurocognitive impact of whole brain radiation (WBRT) have resulted in the adoption of stereotactic radiosurgery (SRS) approaches for use in treatment of metastatic lesions with or without surgical resection [4, 19]. Unfortunately, local control rates and the risks of leptomeningeal dissemination (LMD) in post-operative SRS treated patients have failed to surpass rates achieved via post-operative WBRT [3]. Accordingly, proposals have hypothesized that the use of pre-operative SRS could decrease the risks of LMD and local failure, while others have developed dedicated SRS targeting guidelines which are still being validated [15, 20].
Advances in radiation delivery techniques have led to a re-emergence of intra-operative radiotherapy (IORT) as a viable mechanism for delivery of adjuvant treatment following tumor resection. The safety of IORT in the setting of glioblastoma has been established in conjunction with the addition of external beam radiotherapy (EBRT) on the Stupp protocol and its efficacy is being evaluated in Phase III clinical trial [6]. The published data regarding the use of IORT for surgically resected BMs is limited to single institutional studies [9, 24, 25]. Moreover, recent evidence suggests that the dose delivered locally to the resection cavity may be escalated beyond that safely achievable with traditional SRS techniques, eliminating delay in time to initiation of radiation treatment following surgery and avoiding the complexity of target delineation in post-operative period [24].
The current study represents a retrospective analysis of multi-institutional data from centers in the United States and Europe designed to provide insight into the ability of IORT to provide improvement in local control and reduced toxicity compared to historical data from SRS.
Methods
Following institutional review board approval, an international multi-institutional data set was retrospectively formulated between three participating centers. Data was shared in de-identified format using a comprehensive data set formulated by all study investigators. Inclusion criteria included patients treated with IORT in conjunction with resection of brain metastases. The decision to proceed with surgical resection was made by the primary treating neurosurgeon and followed the guidelines of being a lesion not amenable to SRS based on size or requiring surgical removal for mass effect and/or need for tissue diagnosis. Patients with primary brain tumors were excluded. Patients who underwent surgery and IORT followed by SRS for additional untreated lesions were included in the analysis. Although the goal of surgical treatment was to achieve a gross total resection, patients with post-operative radiographic evidence of a subtotal resection was also included in the analysis.
All patients were treated with IORT using the Intrabeam device (Carl Zeiss Meditec AG, Oberkochen, Germany) with spherical applicators ranging from 1.5 to 4.0 cm in diameter using a low-energy 50 kV X-ray portable linear accelerator. IORT prescription doses were specified to the spherical applicator surface and delivered in a single fraction following resection of the tumors. Intra-operative navigation was utilized to determine the distance to critical organs, i.e., brainstem and optic apparatus. Following from the INTRAGO II trial guidelines for glioblastoma, dose reduction or abandonment of the IORT procedure were reserved for cases in which the dose to critical organs exceeded tolerances. BMs in the posterior fossa where excluded from IORT treatment based on proximity to the brain stem. Follow-up imaging was performed at the discretion of each institution’s practice, but generally included gadolinium enhanced magnetic resonance imaging (MRI) with perfusion imaging or positron emission tomography (PET) for evaluation of radiation necrosis versus disease progression. Radiation necrosis was determined by the presence of contrast enhancement in the absence of increased perfusion or FDG uptake. Imaging was performed at a minimum interval of every 3 months for the first two years following IORT dependent on overall survival. Radiographic progression was determined based on RANO criteria, with the definition of distant lesions being those not in continuity with the post-operative resection cavity not previously identified prior to IORT.
Statistical analyses were completed using IBM SPSS version 24 (Armonk, NY). Local failure (LF, defined as radiographic progression at the site surgery and IORT), distant brain control (DBC, defined as the absence lesions at other intracranial sites independent of surgery and IORT), and overall survival (OS) were calculated using Kaplan–Meier method. Comparisons between groups were made using the log-rank method. Variables examined included: gender, age, primary histology, IORT dose, applicator size, lesion location, eloquent versus non-eloquent location, and extent of resection based on post-operative imaging. Continuous variables were categorized around the median for comparisons. Due to a low number of events relative to the number of potential covariates, a valid multivariate model could not be generated. Variables potentially impacting the development of radiation necrosis were compared using a two-sided Chi square test. A p < 0.05 was considered significant for all analyses.
Results
Baseline patient and treatment characteristics for the included 54 patients are detailed in Table 1. Briefly the median age was 64 years, the most common histology was non-small cell lung cancer (40%, n = 23), the most common location was frontal lobe (38%, n = 22), and the majority were in non-eloquent regions of the brain (72%, n = 42). Post-operative imaging verified gross total resection was achieved in 81% (n = 44). The median prescription dose for IORT was 30Gy to the applicator surface [interquartile range (IQR) 20–30Gy]. The median applicator size was 2.0 cm (IQR 2.0–2.5), with a median treatment time of 16.8 min (IQR 12.1–22.3).
The median follow-up was 7.2 months (IQR 3.4–15.3).
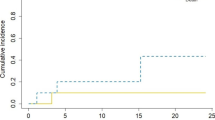
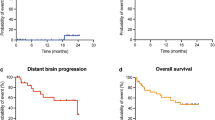
The 1-year local control (LC), distant brain control (DBC), and overall survival were 88% (95% CI 76–100%), 58% (95% CI 41–75%), and 73% (95% CI 59–88%), respectively (Fig. 1). During the entire study period, 6 patients (11%) developed local disease progression. Treatment of these local failures included GammaKnife® SRS (n = 1), repeat surgical resection (n = 3), whole brain radiotherapy (n = 1), and/or hypofractionated stereotactic radiotherapy (HSRT). Two patients developed leptomeningeal failure (3%). The only significant predictor of local control following surgery and IORT was extent of resection [1-year local control 94% (95% CI 83–100%) for gross total resection vs. 62% (26–97%) for subtotal resection, p = 0.049, Fig. 2]. There were no significant predictors of distant brain failure based on IORT parameters.
Kaplan–Meier estimations following IORT. Local control (LC) at 1-year was 88% (top panel); Distant Brain Control (DBC) was 58% at 1-year (middle panel); Overall survival (OS) was 73% percent at 1-year post IORT treatment (bottom panel). Numbers of patients at risk are listed at the bottom of each graph with 54 patients for LC and DBC and 51 patients in OS, accounting for 3 patients lacking survival status data at the time of collection
Dedicated perfusion imaging was available for 28 patients (52%). The remaining patients were imaged with positron emission tomography (PET)-CT. Radiation necrosis was noted in 4 patients (7% of the total cohort). Table 2 outlines comparative rates of radiation necrosis between groups. There were no significant differences in the development of radiation necrosis by IORT dose, applicator size, IORT treatment time, extent of resection or eloquent brain location. Only one patient developed symptomatic radiation necrosis that was treated with steroid administration. This patient has received two previous external beam radiation treatments, receiving 40 Gy (2 Gy, 20 fractions) and 20 Gy (4 Gy, 5 fractions) prior to salvage treatment with IORT for an in-field recurrence. Two additional patients in the analysis also received WBRT prior to IORT without subsequent evidence of radiation necrosis.
Discussion
The optimal technique for the delivery of adjuvant radiation in surgically resected brain metastases remains the subject of considerable debate. Factors such as timing, modality, and targeting technique serve as the basis for a multitude of ‘best practice” approaches, each offering a unique advantage with regard to disease control. In comparison to EBRT from a linear accelerator, IORT provides a low-energy (50 kV) system for delivery a higher linear energy transfer (LET), thereby producing a relatively higher proportion of lethal DNA lesions, such as double-stranded breaks (DSBs) and a higher radiobiological effect [7, 8]. Moreover, our previous work has demonstrated that the steep dose drop off inherent in IORT provides the advantage of decreased dose exposure to organs at risk, such as the brainstem and optic apparatus [24].
The data presented in this study supports a promising local control rate of 88% at 1-year and a minimal associated toxicity with radiation necrosis incidence of 7%. Comparatively, the 1-year local control rate in historical trials that treated brain metastases with surgery alone was 40–50% with the addition of WBRT post-operatively increasing the 1-year local control to 70–80% [12, 16]. Similarly, Weil et al. (2015) and reported local control rates of 70% in a cohort of brain metastases patients receiving and IORT dose of 14 Gy at 2 mm, significantly lower than the 30 Gy at the applicator surface described here [25]. Single institutional studies using SRS following surgical resection demonstrate 1-year LC at 70–80% while a recent report by Traylor et al. placed 1-year LC at 85% in patients treated with a fractionated stereotactic radiotherapy protocol [14, 23].
The recent randomized clinical trial data from N107C/RTOG1270 comparing WBRT to SRS for the treatment of post-operative resection cavities demonstrated superiority in local control in the WBRT arm at the expense of worse cognition [3]. In development of guidelines for post-operative SRS for brain metastases, Soliman et al. hypothesized that the inferior LC in N107C was due to poor target delineation in the post-operative tumor cavity [20]. Remodeling of the tumor cavity along with the development of post-operative gadolinium enhancement not due to tumor growth can be significant obstacles in developing an accurate SRS target following surgery [1]. To overcome this issue, several groups have proposed the addition of a margin beyond the resection cavity [21]. Although measuring up to 2 mm in most studies, Susko et al. have proposed extending this margin up to 10mm for metastatic tumors with a dural attachment [22]. In an SRS targeting paradigm where submillimeter accuracy is the goal, utilization of centimeter margins seems suboptimal. With the direct placement of the spherical applicator within the resection cavity following tumor removal, the interpretation of post-operative imaging artifact and associated uncertainty are excluded. By insuring direct apposition of the applicator surface with the cavity wall, treatment dose delivery is made simple and highly accurate. Pre-operative planning and applicator selection can also be facilitated using IORT planning software. Comparisons with potential SRS plans are also possible to determine the dosimetric differences between the two treatment modalities (Fig. 3). One clear distinction that would need to be made in such a comparison would involve the phenomenon of post-operative cavity remodeling. The traditional delay between surgical resection and adjuvant radiation typically results in change in cavity size [1, 11]. Predictive capacities for the percent size reduction are more difficult to ascertain, but would need to be factored into any dose comparison analysis.
Dosimetric representation of IORT versus SRS plan in a sample patient. The pre-operative navigation CT scan was used for dose map development for an IORT plan using a 2.0 cm applicator and a surface prescription dose of 30 Gy (left panel) while the same images were used for the creation of a potential SRS plan using GammaPlan® with a prescription dose of 16 Gy at the margin (middle panel). Both plans were imported into independent dose calculation software with the generation of the dose map according the scale shown (right panel)
In addition to complex nature of targeting, the delivery of adjuvant radiation to the surgical resection cavity of large brain metastases is further complicated by the potential for local regrowth during the interval between surgery and initial of radiation treatment. While the majority of post-operative adjuvant radiation literature is concerned with complications in surgical wound integrity, few studies have been aimed at determining an optimal time to initiation (TTI) between surgery for intracranial metastatic disease and radiation [17]. Yusuf et al. have provided such analysis, indicating that a TTI greater than 30 days post-resection was associated with a higher rate of local failure in patients treated with CyberKnife [26]. Similarly, Iorio-Morin et al. have described an increase in LF rate in post-operative cavities treated with SRS using the Gamma Knife beyond 3 weeks after surgery [10]. In the present study, the IORT dose was administered immediately after the tumor resection, affording the patients the complete elimination of delay in TTI.
Although more widely studied in the context of breast cancer, there is mounting evidence to support the importance of changes in the tumor microenvironment following IORT and the ability to provide superior control of local disease. Wound fluids (WF) collected from the post-operative cavities in the days following IORT and breast conserving surgery have the ability to exhibit inhibitory activity on the epithelial-mesenchymal transition for several breast cancer cell lines in vitro. Conversely, the WF from non-IORT treated cases was found to increase migratory capacity and expression of mesenchymal markers, such as N-cadherin [2, 13]. The durability of this effect and its uniqueness in response to the high dose of radiation involved in IORT is underscored by the ability of such WF extracts to exert radiation induced bystander effects (RIBE) on additional cells without additional radiation delivery [18]. Such a prolonged effect, possibly mediated through the activity caspases, could provide further insight into the ability of single fraction IORT to provide superior local tumor control. In the same context, such remote effects following IORT may also contribute the low incidence of LMD identified in our current patient cohort. Further studies directed at the brain tumor microenvironment will be needed to address this potential anti-tumor activity beyond traditional radiation-induced cell death pathways.
From a technique standpoint, the impact of extent of resection was found to be the only significant predictor of local control following IORT treatment of the resection cavity in our patient population. While the most (54%) of patients treated received a prescription dose of 30 Gy to the applicator surface and the majority (80%) received a single dose great than 20 Gy, the ability to detect a dose–response effect was hindered. Conversely, the extent of resection effect on 1-year LC could be seen as a proxy indicator of dose effect. With the 1-year LC being on 62% in the STR group versus 94% in the GTR cohort (p = 0.049), we could propose that the STR patient may be better served by increasing the prescription dose to account for the residual tumor at the time of IORT treatment. This would require more precise measurement of the residual tumor and underscores the continued importance of maximizing extent of resection. The use of intra-operative MRI for dose determination could be performed with the use of an intra-cavity trial spherical applicator, also confirming the appropriateness of applicator size in addition to the pre-operative and intra-operative measurements.
Finally, the use of IORT in surgically resected brain metastases has a potential system impact on the delivery of care to cancer patients. The majority of patients treated with IORT in the current study were from rural/suburban areas of the United States or Germany. Several studies have confirmed the presence of significant disparities in healthcare afforded to patients in rural areas, based on the relative lack of providers and/or limited services available [5]. By allowing for consolidation of care episodes at an academic cancer facility, the socio-economic burden to patients and their care-takers is substantially reduced compared with standard surgery followed by multi-visit adjuvant radiation treatments while providing a level of care not normally available close to the patients’ residences. Further studies of the value that is care provides for patients will be required to establish the presence or absence of benefit beyond oncologic control.
The presented analysis is limited by the inherent constraints imposed by its retrospective design including potential selection and indication biases. The incorporation of multi-institutional international data helps to mitigate this flaw. Our ability to predict the safety of IORT with respect to the development of radiation necrosis are further constrained by the small number of patients actually developing RN along with the lack of follow-up in all patients until expiration. Future development of a patient registry to follow outcomes could also assist in providing a more reliable metric of the utility of IORT in patients undergoing indicated surgical resection. Prospective studies will allow for measurement of impact on neuro-cognition, cost-analysis, and overall value in comparison to SRS and traditional WBRT approaches.
Conclusions
Overall, the current study establishes IORT as a safe and effective means to delivery relatively high dose radiation to the resection cavity of surgically removed brain metastases. The high 1-year local control rate and low incidence of complication are equivalent or exceed current standard techniques with the further benefits of consolidation of care. Future studies will be needed to evaluate the value that such treatment brings to patients with surgically indicated brain metastases.
References
Atalar B, Choi CY, Harsh GR et al (2013) Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 72(suppl. 2):180–5; (discussion 185)
Belletti B, Vaidya JS, D'Andrea S et al (2008) Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. 14(suppl. 5):1325–1332
Brown PD, Ballman KV, Cerhan JH, et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol;18(suppl. 8):1049–1060
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(suppl. 11):1037–1044
Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P (2015) Challenges of rural cancer care in the united states. Oncology (Williston Park) 29(suppl. 9):633–640
Giordano FA, Brehmer S, Murle B et al (2019) Intraoperative radiotherapy in newly diagnosed glioblastoma (INTRAGO): an open-label, dose-escalation phase I/II trial. Neurosurgery 84(suppl. 1):41–49
Goodhead DT, Thacker J, Cox R (1993) Weiss lecture. effects of radiations of different qualities on cells: molecular mechanisms of damage and repair. Int J Radiat Biol ;63(suppl. 5):543–556
Herskind C, Wenz F (2014) Radiobiological aspects of intraoperative tumour-bed irradiation with low-energy X-rays (LEX-IORT). Trans Cancer Res;3(suppl. 1):3-17
Hsieh J, Elson P, Otvos B et al (2015) Tumor progression in patients receiving adjuvant whole-brain radiotherapy vs localized radiotherapy after surgical resection of brain metastases. Neurosurgery 76(suppl. 4):411–420
Iorio-Morin C, Masson-Cote L, Ezahr Y, Blanchard J, Ebacher A, Mathieu D (2014) Early gamma knife stereotactic radiosurgery to the tumor bed of resected brain metastasis for improved local control. J Neurosurg 121(Suppl):69–74
Jarvis LA, Simmons NE, Bellerive M et al (2012) Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys 84(suppl. 4):943–948
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29(suppl. 2):134–141
Kulcenty K, Piotrowski I, Zaleska K, et al (2019) Wound fluids collected postoperatively from patients with breast cancer induce epithelial to mesenchymal transition but intraoperative radiotherapy impairs this effect by activating the radiation-induced bystander effect. Sci Rep; 9(suppl. 1):7891
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 18(suppl. 8):1040–1048
Marchan EM, Peterson J, Sio TT et al (2018) Postoperative cavity stereotactic radiosurgery for brain metastases. Front Oncol 8:342
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(suppl. 8):494–500
Patel DM, Agarwal N, Tomei KL, Hansberry DR, Goldstein IM (2015) Optimal timing of whole-brain radiation therapy following craniotomy for cerebral malignancies. World Neurosurg 84(suppl. 2):412–419
Piotrowski I, Kulcenty K, Murawa D, Suchorska W (2018) Surgical wound fluids from patients treated with intraoperative radiotherapy induce radiobiological response in breast cancer cells. Med Oncol; 36(suppl. 2):14
Soffietti R, Kocher M, Abacioglu UM et al (2013) A european organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31(suppl. 1):65–72
Soliman H, Ruschin M, Angelov L et al (2018) Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 100(suppl. 2):436–442
Soltys SG, Adler JR, Lipani JD et al (2008) Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 70(suppl. 1):187–193
Susko M, Yu Y, Ma L et al (2019) Preoperative dural contact and recurrence risk after surgical cavity stereotactic radiosurgery for brain metastases: new evidence in support of consensus guidelines. Adv Radiat Oncol 4(suppl. 3):458–465
Traylor JI, Habib A, Patel R, et al (2019) Fractionated stereotactic radiotherapy for local control of resected brain metastases. J Neurooncol; 144(2):343-350
Vargo JA, Sparks KM, Singh R, Jacobson GM, Hack JD, Cifarelli CP (2018) Feasibility of dose escalation using intraoperative radiotherapy following resection of large brain metastases compared to post-operative stereotactic radiosurgery. J Neurooncol 140(suppl. 2):413–420
Weil RJ, Mavinkurve GG, Chao ST et al (2015) Intraoperative radiotherapy to treat newly diagnosed solitary brain metastasis: initial experience and long-term outcomes. J Neurosurg 122(suppl. 4):825–832
Yusuf MB, Amsbaugh MJ, Burton E et al (2018) Increasing time to postoperative stereotactic radiation therapy for patients with resected brain metastases: Investigating clinical outcomes and identifying predictors associated with time to initiation. J Neurooncol 136(suppl. 3):545–553
Funding
Funding support was provided to CPC by the NIH/NIGMS via P20GM121322-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Each of the authors performed the following tasks in submission of this manuscript: CPC—Study design, Data Collection, Statistical Analysis, Manuscript Drafting, Review/Revision of Manuscript. SB—Data Collection, Review/Revision of Manuscript. JAV—Study design, Data Collection, Statistical Analysis, Manuscript Drafting, Review/Revision of Manuscript. JDH—Data Collection, Review/Revision of Manuscript. KHK—Data Collection, Review/Revision of Manuscript. GSV—Data Collection, Review/Revision of Manuscript. FAG—Data Collection, Review/Revision of Manuscript
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kahl reports personal fees from ELEKTA AB, personal fees from Varian, outside of the submitted work. Dr. Sarria reports grants and personal fees from Carl Zeiss Meditec AG, outside of the submitted work. Dr. Giordano reports grants and personal fees from Carl Zeiss Meditec AG, during the conduct of the study; grants and personal fees from NOXXON Pharma AG, grants and personal fees from ELEKTA AB, personal fees from Bristol-Myers Squibb, personal fees from Roche Pharma AG, personal fees from MSD Sharp and Dohme GmbH, personal fees from AstraZeneca GmbH, other from Implacit GmbH, non-financial support from Oncare GmbH, outside the submitted work; In addition, Dr. Giordano has a patent Radiation Therapy with Immune Response Monitoring (US 62/435405) pending to Carl Zeiss Meditec AG. All other authors have no disclosures relevant to the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cifarelli, C.P., Brehmer, S., Vargo, J.A. et al. Intraoperative radiotherapy (IORT) for surgically resected brain metastases: outcome analysis of an international cooperative study. J Neurooncol 145, 391–397 (2019). https://doi.org/10.1007/s11060-019-03309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03309-6