Abstract
Introduction
Various treatment options exist to salvage stereotactic radiosurgery (SRS) failures for brain metastases, including repeat SRS and hypofractionated SRS (HSRS). Our objective was to report outcomes specific to salvage HSRS for brain metastases that failed prior HSRS/SRS.
Methods
Patients treated with HSRS to salvage local failures (LF) following initial HSRS/SRS, between July 2010 and April 2020, were retrospectively reviewed. The primary outcomes were the rates of LF, radiation necrosis (RN), and symptomatic radiation necrosis (SRN). Univariable (UVA) and multivariable (MVA) analyses using competing risk regression were performed to identify predictive factors for each endpoint.
Results
120 Metastases in 91 patients were identified. The median clinical follow up was 13.4 months (range 1.1–111.1), and the median interval between SRS courses was 13.1 months (range 3.0–56.5). 115 metastases were salvaged with 20–35 Gy in 5 fractions and the remaining five with a total dose ranging from 20 to 24 Gy in 3-fractions. 67 targets (56%) were postoperative cavities. The median re-treatment target volume and biological effective dose (BED10) was 9.5 cc and 37.5 Gy, respectively. The 6- and 12- month LF rates were 18.9% and 27.7%, for RN 13% and 15.6%, and for SRN were 6.1% and 7.0%, respectively. MVA identified larger re-irradiation volume (hazard ratio [HR] 1.02, p = 0.04) and shorter interval between radiosurgery courses (HR 0.93, p < 0.001) as predictors of LF. Treatment of an intact target was associated with a higher risk of RN (HR 2.29, p = 0.04).
Conclusion
Salvage HSRS results in high local control rates and toxicity rates that compare favorably to those single fraction SRS re-irradiation experiences reported in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stereotactic radiosurgery (SRS) is a standard treatment for limited brain metastases [1]. Randomized trials comparing SRS or surgery, with or without whole brain radiotherapy (WBRT), have confirmed worse neurocognitive outcomes with WBRT [2]. The current literature supports WBRT as a therapy of last resort and has led to the widespread adoption of SRS alone in patients with limited or multiple brain metastases [3–5]. Despite high local control rates with SRS, the risk of tumor recurrence remains around 10–30% with several factors such as tumor size, histologic subtype, and treatment dose predicting for local failure [3, 6, 7].
In the setting of local recurrence following SRS, optimal management varies depending on several patient and tumour related factors and requires a multi-disciplinary approach. Treatment options include salvage surgical resection, laser interstitial thermal therapy (LITT), systemic therapy, repeat single fraction SRS and hypofractionated SRS (HSRS) [8]. Although surgical resection may be preferred for symptom alleviation and pathological confirmation of recurrence, feasibility is dependent on tumor location, performance status and expected overall survival, and may be associated with morbidity and mortality [9–13]. LITT, which involves the placement of a fiber optic probe in a target lesion to create thermal tissue damage, is an emerging treatment as salvage therapy for recurrent brain metastases; however, is still an invasive procedure with technical limitations associated with size/volume, location, and experience [14–16]. Advances in systemic therapy have led to targeted therapy and immune check point inhibitors that cross the blood-brain barrier, and have shown efficacy in the treatment/salvage of brain metastases [17], however, most systemic therapy continues to have minimal response in the brain [18, 19]. For these reasons, re-irradiation may be the preferred or sometimes the only feasible option.
The efficacy of salvage re-irradiation with SRS has been reported in a few small institutional series. The median 1-year local control rates have ranged from 65 to 80% with radiation necrosis (RN) observed in approximately 25% of patients [20–23]. Factors that may affect local control and toxicity from repeat SRS are not well characterized; however, repeat single fraction SRS has been consistently observed as associated with high risk of adverse events [22, 23]. More recently, there has been increasing adoption of HSRS in the treatment of large metastases and those in eloquent areas, to achieve improved local control while reducing the risk of RN [24, 25]. A meta-analysis of 24 studies showed a 23.1% versus 7.3% incidence of RN for lesions 2-3 cm treated with SRS and HSRS, respectively [26]. We hypothesized that these same advantages may hold true in the re-irradiation setting and we adopted both 3 and 5 fraction HSRS schedules for the clinical indication of salvaging SRS failures.
The purpose of this study was to report outcomes specific to salvage HSRS as a treatment of locally recurrent brain metastases previously irradiated with SRS/HSRS.
Methods
Patient cohort
Patients treated with salvage HSRS for radiosurgical failures, between July 2010 and April 2020, were retrospectively reviewed (Institutional Research Board approval #267-2015). Baseline patient and treatment characteristics were recorded including age, sex, primary histology, lesion location, time interval between radiosurgical courses, exposure to concomitant systemic therapies, SRS dose, and target volume. Evidence of local recurrence as opposed to RN was based on serial MRI imaging and included perfusion and/or chemical exchange saturation transfer (CEST) sequences [27], and histological confirmation was available for those who underwent surgery. For non-resected targets, recurrence was suspected when serial MR showed growth of enhancing targets, particularly in the setting of clinical symptoms. Perfusion imaging showing increased cerebral blood volume, and/or CEST analysis were used as confirmatory tests when MR imaging was equivocal. A multi-disciplinary discussion was undertaken in all cases prior to repeat radiosurgery. The decision for repeat radiosurgery was typically made for patients with a high degree of suspicion for recurrence, not amenable to resection or other therapies. Salvage HSRS was given in the post-operative setting for some recurrent cases based on assessment of the risks versus benefits. All patients had at least one post-treatment clinical and imaging follow-up visit for study inclusion.
Treatment technique
Radiosurgical treatments were performed using either an image-guided, multi-leaf collimator based linear accelerator equipped with a six degree-of-freedom (6-DOF) couch top (Elekta AB, Stockholm Sweden) or Gamma Knife Icon (GKI, Elekta AB, Stockholm, Sweden) system. Patients were simulated with a 1 mm slice thickness computed tomography (CT) scan with the patient lying supine on a neck rest, and the head immobilized in a non-invasive mask. Volumetric T1-weighted post gadolinium enhanced, and T2 FLAIR magnetic resonance (MR) imaging sequences with 1–1.5 mm thick slices, were fused to the planning CT (or cone-beam CT in the case of GKI) for target volume delineation. For intact lesions, the gross tumor volume (GTV) was defined as the visible tumor on the T1-weighted post gadolinium sequence. For post-operative cavities, a clinical target volume (CTV) was generated according to the international consensus guidelines [13]. A planning target volume (PTV) margin was generated as a 2 mm isotropic expansion from the GTV or CTV for patients treated on LINAC. For patients treated on the GKI, the PTV expansion was 0–1 mm in cranio-caudal dimension and 0–0.5 mm radially. Treatments were typically prescribed to the 70–80% isodose line for linear accelerator based HSRS, and 50–60% when using the GKI.
Our institutional protocol is to treat de novo metastases with a single fraction using the GKI for metastases smaller than 1.5–2 cm in diameter [28]. Metastases greater than 1.5–2.0 cm, post-operative cavities, or those in eloquent locations such as the brainstem are typically treated with 3 or 5 fraction daily HSRS. In the retreatment setting, our initial institutional practice was to utilize HSRS with 25 Gy in 5 fractions. This de-escalated dose is based on our experience with treating brain metastases in the de novo setting, in which we found that the optimal dose is around 30–32.5 Gy in 5 fractions, and that the risk of adverse radiation effect was greater when > 10.5 cc of normal brain received 30 Gy [29, 30]. As our experience matured, we have escalated our prescription to 27.5 Gy for targets that were felt to be safe to do so. For targets near eloquent anatomy or at high risk of toxicity, we continue to use 25 Gy in 5 fractions.
Prescribed radiosurgery total doses were converted to an equivalent dose in 2 fractions (EQD210) with the formula: EQD210 = \(nd\left[\frac{d+ \alpha /\beta }{2+ \alpha /\beta }\right]\) and biologically effective dose (BED10) with the formula: BED10 = \(nd[1+ \frac{d}{\alpha /\beta }]\), where n represents the number of fractions and d the dose per fraction. The tumor α⁄β was assumed to be 10 Gy and that of normal brain tissue to be 2 Gy. Concomitant systemic therapy (chemotherapy, targeted therapy, or immunotherapy) use was defined as receipt of therapy within the 1-week preceding or after radiosurgery.
Follow up and endpoint definition
All patients were followed post-HSRS with a MRI and clinical assessment every 2–3 months, and all patients had a volumetric axial T1 post-gadolinium sequence to ensure comparability. The primary endpoints of this study were the rates of local failure (LF), radiation necrosis (RN), and symptomatic radiation necrosis (SRN) measured from the date of repeat HSRS until an event. LF was defined as tumor progression based on the Response Assessment in Neuro-Oncology Brain Metastases working group [31] with histopathological confirmation when feasible. RN was defined according to Sneed et al. in which there is radiographic lesion growth followed by stabilization or regression [7]. The subset of these patients who experienced treatment-related symptoms including the use of dexamethasone were classified as SRN. Pathological confirmation, perfusion MR and/or CEST [27] sequences were performed to best differentiate RN from tumour progression in cases where there was diagnostic uncertainty. Overall survival (OS) was defined from the date of repeat HSRS to the date of death from any cause or last follow-up.
Statistical analysis
Patient and lesion characteristics were summarized using counts and percentages for categorical variables, and measures of central tendency and dispersion for continuous variables. The Kaplan-Meier method was used to estimate the OS function for all patients. The cumulative incidence of LF and SRN were calculated using Fine and Gray’s competing risk method, with death from any cause as a competing event. Statistical difference between cumulative incidence curves were compared using Gray’s test. Univariable Cox regression was performed to identify predictors of survival. Univariable competing risk regression was performed to identify potential predictors of LF and SRN, with death from any cause as a competing event. Variables with a p-value ≤ 0.2 were selected for inclusion within the final multivariable analysis (MVA) models. All tests were two-sided, with a p-value < 0.05 deemed to be significant. All analyses were conducted using R version 4.0 (The R Foundation for Statistical Computing, Vienna, Austria, 2020).
Results
In total, 120 lesions in 91 patients were treated with a salvage HSRS course. Patient and metastases baseline characteristics are summarised in Table 1. The median follow-up for the whole cohort was 13.4 months (range, 1.1–111.1) with a median radiological follow up of 5.3 months (range 0.67–91.7). The median age at retreatment of 61.1 years (range, 25–91). The most common primary tumour histologies were lung (32.5%), breast (30.8%), and melanoma (21.7%). There was a nearly even division of intact lesions (44.2%) versus surgical cavities (55.8%). The median time between radiosurgery courses was 13.1 months (range 3.0–56.4). Seventy-eight metastases (65%) were initially treated with single fraction SRS ranging from 16 to 20 Gy, while the remainder were treated with HSRS ranging from 25 to 32.5 Gy in 5 fractions as well as one target treated with 24 Gy/3 fractions. Most patients did not receive any concomitant systemic therapy (51.7%) at the time of salvage HSRS. The median target volume was 9.5 cc (range, 0.05–111.79). The most common re-treatment prescriptions were 25 Gy (64.2%), 26.5–27.5 Gy (20.0%), and 30 Gy (6.7%) in 5 fractions. The median retreatment EQD210 was 31.3 Gy (range, 23.3–49.6). Eight lesions received a third course of repeat HSRS, with most receiving 25 or 27.5 Gy in 5 fractions (62.5%), and the remaining three treated with a longer 10 fraction course (Table S1).
Local control
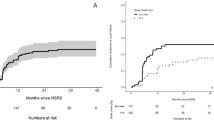
The 6-, 12-, and 24-month cumulative incidence of LF was 18.9% (95% CI 11.7–26.1), 27.7% (95% CI 19.5–35.9), and 29.6% (95% CI 21.2–38.0), respectively (Fig. 1A). MVA identified larger treatment volume (HR 1.02; p = 0.04) and shorter time between radiosurgery courses (HR 0.93; p = 0.001) to predict for a higher risk of LF (Table 2). For tumors > 9.5 cc, the 6-, 12-, and 24-month cumulative incidence of LF was 29.7% (95% CI 17.8–41.5), 38.8% (95% CI 26.0–51.5), 40.6% (95% CI 27.8–53.5), respectively, compared to 8.5% (95% CI 1.4–15.6), 17.0% (95% CI 7.4–26.6), and 18.9% (95% CI 8.8–28.9) for lesions < 9.5 cc, respectively (Fig. 1B). When stratified by the time interval between radiosurgery courses, intervals > 13.1 months had a 6-, 12-, and 24-month cumulative incidence of LF of 11.9% (95% CI 3.6–20.2), 18.8% (95% CI 8.8–28.9), and 20.7% (95% CI 10.2–31.1) compared to 26.1% (95% CI 14.7–37.4), 36.8% (95% CI 24.3–49.3), and 38.8% (95% CI 26.0–51.5) when < 13.1 months, respectively (Fig. 1C). There was no significant difference in local failure risk between cavity and intact targets.
A total of 34 local recurrences were observed after repeat HSRS (26.9%). Most received no further local therapy (50.0%) due to concerns regarding toxicity, poor patient performance status or the initiation of systemic therapy. Two recurrences (5.9%) were subsequently treated with WBRT, 4 (11.8%) with another course of radiosurgery, 7 (20.6%) with re-resection, and 4 (11.8%) with re-resection followed by adjuvant postoperative HSRS.
Radiographic and symptomatic radiation necrosis
Radiographic RN was identified in 23 lesions (19.2%). The 6-, 12-, and 24-month cumulative incidence of RN was 13% (95% CI 6.8–26.1), 15.6% (95% CI 8.9–35.9), and 20.5% (95% CI 12.9–38.0), respectively (Fig. 2A). On MVA, treatment of intact targets (HR 2.29; p = 0.04) was identified as a significant predictor of RN risk (Table 3). The 6-, 12-, and 24-month risk of RN was 15.7% (95% CI 5.7–25.8), 17.8% (95% CI 7.2–28.3), and 27.8% (95% CIL 14.6–41.0) for intact lesions, versus 10.8% (95% CI 3.2–18.4), 13.9% (95% CI 5.5–22.4), and 15.5% (95% CI 6.7–24.4) for postoperative cavities, respectively (Fig. 2B).
The crude incidence of SRN was 7.5% (9 of 120). The 6-, 12-, and 24-month cumulative incidence of SRN was 6.1% (95% CI: 1.7–10.4), 7.0% (95% CI: 2.3–11.6), and 7.9% (95% CI: 2.9–12.8), respectively (Fig. 3). UVA identified initial radiosurgery fractionation (single versus HSRS) and receipt of targeted or immunotherapy as potential predictive factors to be included on MVA. However, none of these factors were predictive of SRN on MVA.
Treatments for SRN included corticosteroids (78%), bevacizumab (11%), and surgical resection (11%). Most cases of SRN (89%) were successfully treated with resolution of symptoms. One patient who was treated with corticosteroids, transitioned to end of life care and passed away shortly thereafter.
Overall survival
The median OS was 15.9 months (95% CI: 11.8–20.8), and the 1-year and 2-year OS estimates were 58.6% (95% CI: 49.0–70.1) and 33.7% (95% CI: 24.7–46.1), respectively (Figure S1). UVA identified age and histology as potential predictors of survival; however, significance was not retained on MVA.
Discussion
Repeat SRS has been traditionally avoided because of perceived low rates of local control and high risk of toxicity [22]. Hypofractionation has been increasing in clinical practice as a strategy to limit toxicity and maintain local control in large brain metastases [24, 25]. Using a similar rationale, we adopted HSRS as salvage treatment of brain metastases after SRS failure. In this study, LF rates at 6- and 12-months were 18.9% and 27.7%, respectively, and compare well with larger de novo treated lesions [30]. Additionally, the 6- and 12-months risk of SRN were favorable at 6.1% and 7.0%, respectively, with the majority (89%) successfully managed with steroids, bevacizumab, or surgery [32].
Two factors were significant predictors on MVA of local failure after salvage HSRS. The first was target volume, with those greater than 9.5 cc (roughly 2.6 cm in diameter) having a nearly 38.8% actuarial risk of recurrence at 1-year, compared to just 17.0% for smaller lesions. This is consistent with previous observational series, in which smaller tumor volume was predictive of better local control [20, 23, 33, 34]. Kim et al. report on the outcomes of repeat single fraction SRS in 176 locally recurrent lesions. The median progression-free-survival (PFS) was 32.8 months for metastases ≤ 4 cc versus 13.0 months for those > 4 cc, and 17.2 months versus 9.4 months when the threshold is increased to 10 cc, respectively [33]. Koffer et al. also observed LF in 45.5% in metastases ≥ 4 cc and in none < 4 cc (p = 0.006) [34]. A multi-institutional retrospective series of 123 metastases treated with repeat single fraction SRS reported a very small metastases volume of > 1 cc as predictive of increased LF (HR 3.31, p = 0.01) [20]. Sneed et al. determined the quadratic mean diameter (QMD) to be predictive of freedom-from-progression (FFP) in 229 metastases retreated with single fraction SRS. Patients with a QMD < 0.75 cm, 0.75–2 cm, and 2.1–3 cm had a 1-year FFP of 86%, 82%, and 65%, respectively [23]. A notable difference between these results of these studies and ours is the use of HSRS vs. repeat single fraction SRS. Despite the median target volume in our series being larger (median 9.5 cc, ~ 2.6 cm diameter), our crude local control rate compares favourably to these reference series (83% versus 61–82%) [20, 23, 34]. Most (84%) of our repeat SRS courses were treated with 25 or 27.5 Gy in 5 fractions (BED10 = 37.5 and 42.6 Gy, respectively), and over half of the retreat targets were post-operative cavities (55.8%) which tended to have larger target volumes (Table 1).
The second factor that impacted local control for repeat SRS was the time interval between treatments. The 1-year LF rate was 18.8% (95% CI: 75.5–93.2) in those with an interval > 13.1 months versus 36.8% for those retreated within 13.1 months. Sneed et al. also observed a similar association between interval between SRS treatments and FFP risk. Patients with intervals of < 6 months, 6–11.9 months, 1–1.9 years, and > 2 years had 1-year FFP probabilities of 60%, 76%, 82%, and 95%, respectively (p < 0.0005) [23]. These observations likely reflect a more aggressive biology for early progressors and relative radioresistance resulting in lower rates of LC.
RN, and in particular SRN, is the most critical dose limiting toxicity of SRS, and a concern that is amplified in the re-irradiation setting. In the current study, the crude incidence of SRN was 7.5%, with a 1-year and 2-year actuarial risk of 6.1% and 7.0%, respectively. The crude incidence of SRN was 24% in a 46-lesion series by McKay et al., using a repeat single fraction SRS prescription dose of 20 Gy [22]. Kowalchuck et al. reported a crude incidence of 7% in their multi-institutional analysis, with an observed 2-year freedom from SRN of 90% given a median repeat single fraction SRS prescription dose of 18 Gy [20]. Our target volumes were generally larger compared to other series [20, 23, 33], and all patients treated with HSRS. Our result compares favorably to a series of 47 lesions retreated with 3-fraction hypofractionated SRS, with a crude incidence of SRN of 13% although their median target PTV was quite large at 16 cc [21].
Accounting for radiographic RN, our overall crude incidence was 19.2% which is similar to a meta-analysis of eight retrospective studies that reported a rate of 16.1% [8]. Notably, we found a greater than 2-fold increased risk of RN for intact targets versus postoperative cavities on MVA, adjusted for volume. This has been observed in our de novo SRS experience as well. Our previous institutional experience reported 3.7 greater odds of SRN for intact lesions compared to cavities treated with HSRS [32]. Similarly, Andruska et al. observed a trend towards higher SRN risk (HR 2.5; p = 0.17) for intact lesions in a series of 117 metastases treated in the de novo setting. As they summarize, the lack of observed statistical significance may be due to insufficient study power [35]. Rana et al. observed a trend towards lower RN risk for postoperative cavities in a series of 32 metastases undergoing salvage SRS [36]. These observations suggest that RN may be driven by a multifactorial mechanism, including factors beyond simply the target volume. In the reirradiation setting, surgical resection may remove adjacent tissue that has been exposed to previous high dose irradiation and may in part explain the lower rates of RN. Conversely, radiobiological mechanisms at the tumor-brain interface inherent in the treatment of intact metastases may also influence RN risk. No significant predictors of SRN on MVA were found in our series. It should be noted that we did not find an association between dose and RN or SRN risk, suggesting that careful dose escalation may be appropriate for suitable targets at risk of re-irradiation failure.
Despite the encouraging outcomes reported in the current series, our institution is investigating new strategies of repeat radiosurgery. Recently, we have begun implementing staged radiosurgery for these lesions in which we deliver 3 total fractions separated by 2 weeks between fractions. This strategy has been employed in the treatment of large brain metastases in the de novo setting with good local control and acceptable toxicity [37, 38]. This approach allows for potential volume reduction with subsequent fractions, and therefore maintain higher rates of local control while mitigating rates of radiation necrosis.
The current study has several strengths. First, all patients had consistent contrast-enhanced dedicated volumetric MRI follow up every 2–3 months adhering to strict institutional protocols, and interpretation was by dedicated neuro-radiologists. Secondly, we carefully characterized all cases of LF or RN with multi-disciplinary evaluation utilizing advanced diagnostic techniques including perfusion and CEST MR, and in some cases pathological confirmation [27, 39].
We acknowledge limitations of the current study. The retrospective nature of the data risks inherent biases and inaccuracies in data capture. Although radiosurgery prescriptions were recorded, detailed dosimetric data such as volumetric normal brain parameters were unavailable given that many of the lesions were retreated across different platforms. Normal brain dosimetry is an established predictor of RN in de novo radiosurgery, and various volumetric predictors have been identified in other re-irradiation series [20–22]. Lastly, there is heterogeneity in the repeat radiosurgery dose fractionations used in the current study, which we address however, by converting to BED and EQD2 doses.
In conclusion, HSRS for the salvage of local recurrence post SRS is an effective and feasible option with good rates of control and toxicity rates that compare favorably to the single fraction re-irradiation. Larger target volume and a shorter interval between radiosurgery courses were predictive of lower rates of local control. Re-irradiation of intact metastases vs. cavities was associated with an increased risk of RN. Despite the favourable outcomes of HSRS in this series, further research is required to establish the optimal dose fractionation for HSRS in the subset of patients that are at the highest risk of local failure or toxicity.
References
Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. https://doi.org/10.1016/j.prro.2022.02.003
Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17(5):279–299. https://doi.org/10.1038/s41571-019-0320-3
Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M et al (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91(4):710–717. https://doi.org/10.1016/j.ijrobp.2014.10.024
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D et al (2022) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40(5):492–516. https://doi.org/10.1200/jco.21.02314
Sahgal A, Ruschin M, Ma L, Verbakel W, Larson D, Brown PD (2017) Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neurooncology 19(suppl2):ii2–ii15. https://doi.org/10.1093/neuonc/nox001
Vogelbaum MA, Angelov L, Lee S-Y, Li L, Barnett GH, Suh JH (2006) Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg 104(6):907–912. https://doi.org/10.3171/jns.2006.104.6.907
Sneed PK, Mendez J, Vemer-Van Den Hoek JGM, Seymour ZA, Ma L, Molinaro AM et al (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 123(2):373–386. https://doi.org/10.3171/2014.10.jns141610
Singh R, Didwania P, Lehrer EJ, Palmer JD, Trifiletti DM, Sheehan JP (2022) Repeat stereotactic radiosurgery for locally recurrent brain metastases previously treated with stereotactic radiosurgery: a systematic review and meta-analysis of efficacy and safety. J Radiosurg SBRT 8(1):1–10
Heßler N, Jünger ST, Meissner A-K, Kocher M, Goldbrunner R, Grau S (2022) Recurrent brain metastases: the role of resection of in a comprehensive multidisciplinary treatment setting. BMC Cancer. https://doi.org/10.1186/s12885-022-09317-6
Vogelbaum MA, Suh JH (2006) Resectable brain metastases. J Clin Oncol 24(8):1289–1294. https://doi.org/10.1200/jco.2005.04.6235
Nguyen TK, Sahgal A, Detsky J, Atenafu EG, Myrehaug S, Tseng C-L et al (2020) Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neurooncology 22(1):84–93. https://doi.org/10.1093/neuonc/noz144
Cagney DN, Lamba N, Sinha S, Catalano PJ, Bi WL, Alexander BM et al (2019) Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol 5(5):703. https://doi.org/10.1001/jamaoncol.2018.7204
Soliman H, Ruschin M, Angelov L, Brown PD, Chiang VLS, Kirkpatrick JP et al (2018) Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 100(2):436–442. https://doi.org/10.1016/j.ijrobp.2017.09.047
Luther E, Mansour S, Echeverry N, McCarthy D, Eichberg DG, Shah A et al (2020) Laser ablation for cerebral metastases. Neurosurg Clin N Am 31(4):537–547. https://doi.org/10.1016/j.nec.2020.06.004
Srinivasan ES, Grabowski MM, Nahed BV, Barnett GH, Fecci PE (2021) Laser interstitial thermal therapy for brain metastases. Neuro-Oncol Adv 3(Supplement5):v16–v25. https://doi.org/10.1093/noajnl/vdab128
Shao J, Radakovich NR, Grabowski M, Borghei-Razavi H, Knusel K, Joshi KC et al (2020) Lessons learned in using laser interstitial thermal therapy for treatment of brain tumors: a case series of 238 patients from a single Institution. World Neurosurg 139:e345–e54. https://doi.org/10.1016/j.wneu.2020.03.213
Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S et al (2022) Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 28(9):1840–1847. https://doi.org/10.1038/s41591-022-01935-8
Alvarez-Breckenridge C, Remon J, Piña Y, Nieblas-Bedolla E, Forsyth P, Hendriks L et al (2022) Emerging systemic treatment perspectives on brain metastases: moving toward a better outlook for patients. Am Soc Clin Oncol Educational Book. https://doi.org/10.1200/edbk_352320
Franchino F, Ruda R, Soffietti R (2018) Mechanisms and therapy for Cancer metastasis to the brain. Front Oncol 8:161. https://doi.org/10.3389/fonc.2018.00161
Kowalchuk RO, Niranjan A, Lee CC, Yang HC, Liscak R, Guseynova K et al (2022) Reirradiation with stereotactic radiosurgery after local or marginal recurrence of brain metastases from previous radiosurgery. Int J Radiat Oncol Biol Phys 112(3):726–734. https://doi.org/10.1016/j.ijrobp.2021.10.008
Minniti G, Scaringi C, Paolini S, Clarke E, Cicone F, Esposito V et al (2016) Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol 126(1):91–97. https://doi.org/10.1007/s11060-015-1937-4
Mckay WH, Mctyre ER, Okoukoni C, Alphonse-Sullivan NK, Ruiz J, Munley MT et al (2017) Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J Neurosurg 127(1):148–156. https://doi.org/10.3171/2016.5.jns153051
Sneed PK, Chan JW, Ma L, Braunstein SE, Theodosopoulos PV, Fogh SE et al (2022) Adverse radiation effect and freedom from progression following repeat stereotactic radiosurgery for brain metastases. J Neurosurg. https://doi.org/10.3171/2022.4.jns212597
Yan M, Holden L, Wang M, Soliman H, Myrehaug S, Tseng C-L et al (2022) Gamma knife icon based hypofractionated stereotactic radiosurgery (GKI-HSRS) for brain metastases: impact of dose and volume. J Neurooncol 159(3):705–712. https://doi.org/10.1007/s11060-022-04115-3
Gutschenritter T, Venur VA, Combs SE, Vellayappan B, Patel AP, Foote M et al (2020) The judicious use of stereotactic radiosurgery and hypofractionated stereotactic radiotherapy in the management of large brain metastases. Cancers 13(1):70. https://doi.org/10.3390/cancers13010070
Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103(3):618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ (2017) Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res 23(14):3667–3675. https://doi.org/10.1158/1078-0432.ccr-16-2265
Mouraviev A, Detsky J, Sahgal A, Ruschin M, Lee YK, Karam I et al (2020) Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neurooncology 22(6):797–805. https://doi.org/10.1093/neuonc/noaa007
Soliman H, Myrehaug S, Tseng C-L, Ruschin M, Hashmi A, Mainprize T et al (2019) Image-guided, linac-based, surgical cavity-hypofractionated stereotactic radiotherapy in 5 daily fractions for brain metastases. Neurosurgery 85(5):E860–E869. https://doi.org/10.1093/neuros/nyz162
Myrehaug S, Hudson J, Soliman H, Ruschin M, Tseng CL, Detsky J et al (2021) Hypofractionated stereotactic radiation therapy for intact brain metastases in 5 daily fractions: effect of dose on treatment response. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2021.09.003
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270–e278. https://doi.org/10.1016/s1470-2045(15)70057-4
Faruqi S, Ruschin M, Soliman H, Myrehaug S, Zeng KL, Husain Z et al (2020) Adverse radiation effect after hypofractionated stereotactic radiosurgery in 5 daily fractions for surgical cavities and intact brain metastases. Int J Radiat Oncol Biol Phys 106(4):772–779. https://doi.org/10.1016/j.ijrobp.2019.12.002
Kim I-Y, Jung S, Jung T-Y, Moon K-S, Jang W-Y, Park J-Y et al (2018) Repeat stereotactic radiosurgery for recurred metastatic brain tumors. J Korean Neurosurg Soc 61(5):633–639. https://doi.org/10.3340/jkns.2017.0238
Koffer P, Chan J, Rava P, Gorovets D, Ebner D, Savir G et al (2017) Repeat stereotactic radiosurgery for locally recurrent brain metastases. World Neurosurg 104:589–593. https://doi.org/10.1016/j.wneu.2017.04.103
Andruska N, Kennedy WR, Bonestroo L, Anderson R, Huang Y, Robinson CG et al (2021) Dosimetric predictors of symptomatic radiation necrosis after five-fraction radiosurgery for brain metastases. Radiother Oncol 156:181–187. https://doi.org/10.1016/j.radonc.2020.12.011
Rana N, Pendyala P, Cleary RK, Luo G, Zhao Z, Chambless LB et al (2017) Long-term outcomes after salvage stereotactic radiosurgery (SRS) following in-field failure of initial SRS for brain metastases. Front Oncol 7:279. https://doi.org/10.3389/fonc.2017.00279
Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST et al (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129(2):366–382. https://doi.org/10.3171/2017.3.jns162532
Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M et al (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74(5):1543–1548. https://doi.org/10.1016/j.ijrobp.2008.10.035
Detsky JS, Keith J, Conklin J, Symons S, Myrehaug S, Sahgal A et al (2017) Differentiating radiation necrosis from tumor progression in brain metastases treated with stereotactic radiotherapy: utility of intravoxel incoherent motion perfusion MRI and correlation with histopathology. J Neurooncol 134(2):433–441. https://doi.org/10.1007/s11060-017-2545-2
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MY, AS, and HS developed study concept. MY, ML, and HS obtained study data. MY was responsible for statistical analysis. MY and HS wrote the initial manuscript draft. MY and LH prepared all figures and tables. All authors reviewed manuscript and final approval.
Corresponding author
Ethics declarations
Conflict of interest
HS reports travel and education grants from Elekta. SD reports research grants from Alkermes Medical and consultant fees from Medexus. SM reports research support and honoraria from AAA/Novartis and Ipsen. CLT reports honoraria from Elekta and serves on the advisory board for Sanofi. AS reports consulting fees and grants from Varian and Elekta, as well as honoraria from Varian, BrainLab, and Elekta. MR owns intellectual property related to the image-guidance component on the Elekta Gamma Knife system. All other authors have no disclosures.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, M., Lee, M., Myrehaug, S. et al. Hypofractionated stereotactic radiosurgery (HSRS) as a salvage treatment for brain metastases failing prior stereotactic radiosurgery (SRS). J Neurooncol 162, 119–128 (2023). https://doi.org/10.1007/s11060-023-04265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04265-y