Abstract
Introduction
In RPA V-VI glioblastoma patients both hypofractionated radiotherapy and exclusive temozolomide can be used; the purpose of this trial is to compare these treatment regimens in terms of survival and quality of life.
Methods
Patients with histologic diagnosis of glioblastoma were randomized to hypofractionated radiotherapy (RT–30 Gy in 6 fractions) and exclusive chemotherapy (CHT–emozolomide 200 mg/m2/day 5 days every 28 days). Overall (OS) and progression free survival (PFS) were evaluated with Kaplan Maier curves and correlated with prognostic factors. Quality- adjusted survival (QaS) was evaluated according to the Murray model (Neurological Sign and Symptoms–NSS)
Results
From 2010 to 2015, 31 pts were enrolled (CHT: 17 pts; RT: 14pts). Four pts were excluded from the analysis. RPA VI (p = 0.048) and absence of MGMT methylation (p = 0.001) worsened OS significantly. Biopsy (p = 0.048), RPA class VI (p = 0.04) and chemotherapy (p = 0.007) worsened PFS. In the two arms the initial NSS scores were overlapping (CHT: 12.23 and RT: 12.30) and progressively decreased in both group and became significantly worse after 5 months in CHT arm (p = 0.05). Median QaS was 104 days and was significantly better in RT arm (p = 0.01).
Conclusions
The data obtained are limited by the poor accrual. Both treatments were well tolerated. Patients in RT arm have a better PFS and QaS, without significant differences in OS. The deterioration of the NSS score would seem an important parameter and coincide with disease progression rather than with the toxicity of the treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current standard treatment of glioblastoma is radical surgery followed by high dose radiation therapy and concomitant and adjuvant chemotherapy with Temozolomide [1]. The prognosis of these patients is strongly influenced by age, performance status and the extension of surgery; the patients who benefit from this integrated radio-chemotherapy treatment are young, with good general and neurological performance status and undergoing radical surgery (median overall survival 16–18 months) [2,3,4,5].
The best treatment for “poor prognosis” patients (RPA V and VI classes) is still debated, since the median survival reported in the literature varies between 4.5 and 7.5 months with conventional radiotherapy, with a limited advantage when compared to support therapy [1, 6]. Numerous studies have addressed the results of hypofractionated radiotherapy, that seems to be able to obtain survival results comparable to conventionally fractionated treatments, without an undue excess of toxicity [7,8,9,10]. Mono-chemotherapy with temozolomide (150–200 mg/m2) as first line treatment in elderly patients and in those with a poor performance status produced similar survival results, and improvements of neurological status [11].
The preservation -as long as possible- of the quality of life (QoL) is a primary objective for these patients, due to the limited impact of the treatment on prognosis; it is therefore acceptable to consider supportive therapy alone, to avoid a possible treatment related deterioration in QoL [12, 13].
However, continuing clinical research efforts are devoted to ameliorate treatment results also in this very unfavorably selected subset of glioma patients [14]; and the issue of withholding active or more aggressive cancer treatments is always accompanied by relevant clinical, social and ethical issues [15, 16].
We therefore decided to compare, with a prospective, multicentric, randomized Phase II trial, survival and QoL results of poor prognosis glioblastoma patients following hypofractionated radiotherapy alone or temozolomide chemotherapy alone.
Patients and methods
Design of the study and ethics statements
Eligibility criteria included: histologically confirmed diagnosis of glioblastoma grade IV (WHO), RPA class V or VI [2] and compliance to treatment and follow up. Exclusion criteria included: age < 50 years, pregnancy or lactation status, previous brain irradiation, absence of histologically confirmed diagnosis of high-grade glioma and unrelated malignancy with the exception of intraepithelial cervical carcinoma.
The ethics committee of the promoter center and of every other participating centers approved the study design and statistical methods, before the start of enrolment. The ethics committee of the promoter center also requested 6-months interim reports from the start up to the end of the trial. Each patient has received informative material for himself and for the family physician, in order to have time to decide whether to participate. Afterwards, for each eligible patient, the informed consent was written down, often in the presence of a caregiver. They were subsequently randomly assigned to receive hypofractionated radiotherapy or Temozolomide chemotherapy.
Staging at baseline and follow-up examinations
Patients were examined at baseline at least with contrast-enhanced CT scan no more than 3 weeks before the randomization to evaluate the size and initial characteristics of the brain disease; however, contrast-enhanced MRI with/without perfusion was considered advisable. The same imaging procedures were repeated during follow-up: 30 days after the end of radiotherapy and then every 2 months (radiotherapy group); 45 days after the beginning of treatment and then every 2 months (chemotherapy group). Response evaluation was performed according to the Macdonald criteria [17], since the RANO criteria were published after the submission of this trial to the ethics committee (5 December 2009).
At baseline and at each evaluation a general and neurological examination, and the evaluation of the general and neurological status according to the KPS index and the MRC-NPS scale has been done; the tests (Neurological signs and symptoms—NSS. - according to Murray’s model) for the measurement of the quality adjusted survival were also performed [18].
Treatment
Radiotherapy
The treatment of the disease site evidenced at imaging, including the whole contrast enhanced lesion with adequate margin (CTV = GTV + 1 cm isotropic) had to start within 6 weeks from the diagnosis. The total prescribed dose was 30 Gy in 6 fractions (5 Gy/day) on alternate days in 2 weeks. Immobilization of the patient with a thermoplastic mask was recommended.
Chemotherapy
Mono-chemotherapy with Temozolomide 200 mg/m2/day for 5 days every 28 days was prescribed until disease progression or to a maximum of six cycles. Chemotherapy had to be administered in association with supportive therapies (anti-emetic prophylaxis). Dosage modifications and discontinuations were defined in the study protocol on the basis of hematologic toxicities.
Study design, end point definition and statistical considerations
This was a phase II, multicenter, randomized trial to evaluate two palliative therapeutic options in patients affected by poor prognosis glioblastoma in terms of survival and quality of life. The primary end point of the trial was progression-free survival (PFS), calculated from the date of histological diagnosis to the date of progression on images studies, and the secondary end- points were overall survival (OS), calculated from the histological diagnosis and death or the last follow up, and quality adjusted survival (QaS), according to the Murray method [18].
The sample-size calculation was based on PFS rate. At the time of study design, the 6-months PFS in patients treated with Temozolomide and radiotherapy was reported to be 44% and 21%, respectively [11, 19]. Therefore, we calculated that 60 patients per treatment arm would provide the study with a 80% power, or β, to detect a 20% difference in compliance, by using a two-sided χ2 test with a 5% significance level, or α. The randomization procedure, which was done with a 1:1 ratio, was centralized, computerized and managed by the scientific coordinator.
Continuous data values were described by using median, minimum and maximum values or the median and 25th to 75th interquartile range. The interarm differences were assessed using a Pearson’s χ2 test for categorical variables or by means of an analysis of variance for continuous variables. Kaplan–Meier survival analysis was used to estimate survival end points, and the log-rank test was used to compare differences between curves. Cox regression analysis (CI 95%) was used for the multivariate analysis: in consideration of the small number of enrolled patients, despite the high number of events (27/27), we correlated 3 variables for each analysis, the significant ones in the univariate, with the aim of maintaining a better statistical quality. All tests are two-sided, and a p < 0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed with the SPSS software (version 17.0; SPSS, Chicago, IL).
The maximum diameter of the tumor, intended as the enhanced area in the T1-weighted sequences, was considered in the statistical analysis just to understand if larger tumors could negatively influence the quality of life and the success of the treatment, in consideration of the hypofractionation at 5 Gy/fraction, despite this cut off (5 cm) does not have a rationale in the literature.
Results
Between May 2010 and June 2015, 31 patients were enrolled across six sites in Italy, 17 patients in the chemotherapy arm and 14 in radiotherapy one. The trial was closed early after 5 years, because of the slow accrual. Table 1 delineates patients, tumour and treatment characteristics at baseline. Patients were well balanced between the treatment arms. Figure 1 illustrates the CONSORT flow diagram of the study.
MRI is available at diagnosis for all the patients enrolled, MRI was available for follow up in 21/27 patients; 6/27 were followed with CT alone because of clinical deterioration.
The methylation state of the MGMT gene promoter was not required at randomization, due to the interest for this information, we however retrospectively obtained the data of 26/27 patients (96%). The mutation status of IDH knowledge was not necessary for randomization: the data was however available for 10/27 patients (37%), all IDH1-wild type. Considering the exiguity of the data, IDH status was not used for the statistical analysis.
Toxicity
In the radiotherapy arm, 10 pts of patients (77%) had mild headache (CTCAE 4.1 grade I and II), regressed with prescription or dose adjustment of steroid therapy; only one patient had spatial and temporal disorientation during radiotherapy, regressed with steroids.
In the chemotherapy arm, three patients (21%) had to reduce the dose of temozolomide for grade II thrombocytopenia. One patient (7%) had pancytopenia and E-Coli sepsis and discontinued Temozolomide after 1 cycle.
Survival
Median PFS was 114 days (range 97–131 days).
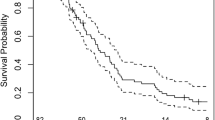
At univariate analysis RPA class VI (p = 0.04), biopsy only (p = 0.048) and treatment with chemotherapy (p = 0.007–6 month-PFS 53% vs 15%) are related with a significantly worse PFS (Fig. 2) (Table 2).
In the subgroup analysis according to methylation status, non-methylated patients had a significant PFS advantage if treated with radiotherapy (p = 0.02); no difference in PFS was found in methylated patients, between the two treatment arms.
At multivariate analysis the methylation of MGMT gene promoter (p = 0.041) and radiotherapy treatment (p = 0.003) persist as positive prognostic factor (Table 3).
Median OS was 188 days (range 73–302 days).
At univariate analysis RPA VI class (p = 0.048) and absence of promoter of the MGMT gene methylation (p = 0.001) are significantly related with a worse OS; age, extension of surgery, performance status and tumor dimensions did not influence survival. No statistically significant overall survival differences were observed at univariate analysis between the two treatment arms, although a trend in favor of radiotherapy was evident (p = 0.08; 1 year-OS 28% vs 12%). (Table 2) MGMT gene.
promoter methylation resulted to be a favorable prognostic factor in both treatment arms (p = 0.01) and was the sole positive prognostic factor at multivariate analysis (Table 3).
In a subgroup analysis according to methylation status, type of treatment (radiotherapy vs chemotherapy) did not impact on overall survival (p = NS).
Quality adjusted-survival
In the two arms the initial NSS scores were overlapping: 12.23 and 12.30 in the chemotherapy and radiotherapy arm, respectively. Figure 3 show how NSS score progressively decreased in both groups but became significantly worse after 5 months in the CHT arm (p = 0.05–Anova test). Median QaS was 104 days (range 90–117 days) and was significantly better in the radiotherapy arm (p = 0.01; 6 months QaS, 54% vs 7%, Fig. 4).
Discussion
The standard therapy for patients with glioblastoma in the RPA prognostic classes V and VI is still debated, as the minimum therapeutic benefit often compromises the already inadequate quality of life of these fragile patients.
The prognosis of this category of patients is conditioned by the fact that in most cases it is not possible to obtain a macroscopically complete removal of the lesion, not only because of the volume or the site of the disease, but also because of the other clinical features at presentation. In fact, although it is has been clearly shown that macroscopically complete removal of the tumor positively impacts on the patient's survival, it is also known that age, a poor performance status, the presence of multiple comorbidities, the use of anticoagulants and neurological symptomatology (i.e. confusion, disorientation, lethargy) may increase the risk of intra-operative and post-surgical complications, thus greatly reducing the survival benefit due to a more aggressive surgery [4, 5, 14, 15, 20]. When total or subtotal macroscopic disease resection is not achievable, standard conventionally fractionated adjuvant radio-chemotherapy is less frequently adopted, particularly in frail, prognostically unfavored patients.
Therefore, for this subset of patients, the interest for shorter, better tolerated treatments, aiming at preserving quality of life, is high. However, at the time this clinical trial was planned, no published randomized trials comparing hypo-fractionated radiotherapy and chemotherapy alone were available. Multiple prospective or retrospective case studies on hypo-fractionated radiotherapy alone, with various fractionation schedules [7,8,9, 21, 21,22,23] or temozolomide chemotherapy alone [10, 24, 25], were available, although not planned considering also the methylation status of the MGMT promoter. According to these studies, both treatments were safe and well tolerated, with overlapping global survival but benefit in terms of PFS in favor of chemotherapy alone. Chemotherapy resulted also better tolerated, with a reduced impact on quality of life. The used of this hypofractionation (30 Gy in 6 fractions in 2 weeks) was selected, considering the palliative intent of the treatment, because it was the shorter and safe fractionation used in a relatively large series of poor prognosis patients, at the time of the drafting of our protocol trial [7].
The NORDIC trial [25] compared 3 treatment arms in elderly patients: standard radiotherapy (60 Gy in 30 fractions), hypofractionated radiotherapy (34 Gy in 10 fractions) and Temozolomide alone (200 mg/m2-5 days every 28). Median survival was longer in the hypofractionated group and the most of patients completed the treatment without interruptions: short-course radiotherapy should be better tolerated, with equivalent survival outcomes. Roa et al. [8], instead, compared standard radiotherapy (60 Gy in 30 fractions) to hypofractionated course (40 Gy in 15 fractions); in this randomized trial, the standard course was not superior to the hypofractionated one; no significant differences in overall survival between the two groups were found. Recently Guedes de Castro et al. [26] published the data from a randomized phase 3 trial, involving elderly ( ≥ 65 years) and/or frail patients (KPS 50–70), suggesting the equivalence in terms of PFS and OS of a short-course radiotherapy (25 Gy in 5 fractions in 1 week) compared to hypofractionation in 15 fractions (40 Gy in 3 weeks). The fractionation proposed by this author and the characteristics of enrolled patients, seem to be very similar to our series, with superimposable results in median OS (6.2 months) and median PFS (3.2 months).
For elderly patients, there is some evidence to support the use of TMZ alone, especially in patients with MGMT promoter methylation [27]: two randomized trials, NORDIC [25] and NOA-8 [28], included a temozolomide monotherapy arm. In the first trial elderly patients ( > 70 years) had longer overall survival in the temozolomide arm. In the NOA-8 trial, median overall survival was not different between TMZ (1 week on, 1 week off schedule) and long-course radiotherapy (60 Gy in 30 fractions).
MGMT promoter methylation was a predictive biomarker of response to TMZ, confirmed in the recent NCIC CE.6/EORTC 26062 trial [29]. In fact, randomized evidence from this last trial demonstrates a survival benefit for elderly patients ( > 65 yeas) with concurrent TMZ during short-course radiation therapy (40 Gy in 15 fractions) [28], without differences in health- related quality of life measures between the groups. In our cases the tolerance to temozolomide was good and superimposable to data reported in literature, with predictable and acceptable adverse events (only one case of interruption for sepsis from severe pancytopenia). The patients’ compliance with temozolomide was excellent, the interruption was always determined by tumour progression with deterioration of the general clinical conditions. With a median PFS of 3.5 months in the group treated with chemotherapy, the average number of cycles administered was equal to 2 (range 1–6).
The NCIC CE.6/EORTC trial, unlike the present one, enrolled patients in good clinical conditions and fit for a combined treatment; hence, the results are not comparable in terms of expected benefits.
The results of the recent trials, described above, caused a shift in the recommendations of international guidelines [30, 31] in favor of the addition of concomitant chemotherapy to short-course radiotherapy in elderly patients in good clinical conditions (ECOG 0–1).
Exclusive hypofractionated radiotherapy or chemo-therapeutic treatment with temozolomide alone are strategies to be proposed to frail elderly or poor prognosis patients, not eligible for combined treatments. The choice of which treatment to prefer is conditioned by the methylation of the MGMT gene promoter: the methylated patients could therefore be candidates for chemotherapy alone. None of the clinical trials previously described performed a correlation with quality of life assessment.
The present clinical trial has shown that the tolerance to both the chosen hypofractionated radiotherapy and temozolomide chemotherapy was good, with a low incidence of high-grade toxicity in both the treatment arms and no difference in overall survival. An increase in PFS in the radiotherapy arm was observed, along with better maintenance of cognitive functions and a better quality of life. There is a scarcity of studies in the literature evaluating the quality of life of patients in these very poor prognostic classes, and the few available compared supportive therapy and palliative treatments only [6, 32]. To the best of our knowledge, there are no randomized trials comparing temozolomide with radiotherapy in terms of patients' quality of life in this clinical subgroup. From the present study emerges that the decrease of the quality of life, parallels the decrease in PFS and is significantly greater in the chemotherapy arm 5 months after treatment.
The main limit of this randomized clinical trial is the poor and slow accrual of patients (31 patients enrolled/120 designed patients). Despite a multicentric trials, the difficulties of accrual emerged from the beginning and were multifactorial. For the first two years the accrual was reserved to elderly and frail patients (age > 65 in RPA classes V and VI); in September 2012 the protocol was emended, including patients aged > 50 years, as per the RPA classification. Moreover, many candidates were not subjected to lesion biopsy, due to the refusal of patients or neurosurgeons, who considered the biopsy too risky and invasive, compared to the benefit of a treatment in this poor prognosis category of patients. In addition, in some Centres the devices and software for stereotactic biopsies have been temporarily not available and not replaced immediately, diverting the choice of performing the few necessary biopsies only on patients with better prognosis. The final consideration is that a lot of patients eligible for this trial were not submitted at all to invasive procedures, neither, as already said, biopsy nor any kind of specific treatment. All these problems led to a low and slow accrual of a few patients, in a longer period of time than expected.
Conclusions
The data obtained from this multicentre randomized phase II clinical trial are limited by the poor accrual. Both treatments were well tolerated by patients without evidence of severe toxicities. Patients treated with hypofractionated radiotherapy have a better PFS and QaS, compared to patients in the CHT group, without significant differences in OS. In this series, the deterioration of the NSS score would seem an important parameter and coincide with disease progression rather than with the toxicity of the treatment given.
References
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Curran WJ Jr, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85(9):704–710
Scott CB, Scarantino C, Urtasun R et al (1998) Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90–06. Int J Radiat Oncol Biol Phys 40(1):51–55
Scoccianti S, Magrini SM, Ricardi U et al (2010) Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the Central Nervous System Study Group of Airo (italian Association of Radiation Oncology). Neurosurgery 67(2):446–458
Magrini SM, Ricardi U, Santoni R et al (2006) Patterns of practice and survival in a retrospective analysis of 1722 adult astrocytoma patients treated between 1985 and 2001 in 12 Italian radiation oncology centers. Int J Radiat Oncol Biol Phys 65(3):788–799
Medical Research Council (1990) Prognostic factors for high-grade malignant glioma. Prognostic factors for high-grade malignant glioma development of a prognostic index. A Report of the Medical Research Council Brain Tumour Working Party. J Neuroncol 9:47–55
Thomas R, James N, Guerrero D et al (1994) Hypofractionated radiotherapy as palliative treatment in poor prognosis patients with high grade glioma. Radiother Oncol 33(2):113–116
Roa W, Brasher PM, Bauman G et al (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22(9):1583–1588
Hoegler DB, Davey P (1997) A prospective study of short course radiotherapy in elderly patients with malignant glioma. J Neurooncol 33(3):201–204
Buglione M, Spiazzi L, Saiani F et al (2014) Three-dimensional conformal radiotherapy, static intensity-modulated and helical intensity-modulated radiotherapy in glioblastoma. Dosimetric comparison in patients with overlap between target volumes and organs at risk. Tumori. 100(3):272–277
Glantz M, Chamberlain M, Liu Q et al (2003) Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer 97(9):2262–2266
Taphoorn MJ, Stupp R, Coens C et al (2005) Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol 6(12):937–944
Sizoo EM, Pasman HRW, Buttolo J et al (2012) Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer 4(8):226–232
Buglione M, Borghetti P, Pedretti S et al (2015) Post-surgical therapeutic approaches to glioblastoma patients submitted to biopsy (BA) or "partial" resection (PR): the possibilities to treat also them without renunciations. Study from the Brescia Neuro-Oncology Group. Radiol Med 120(10):975–981
Mathiesen T (2013) To operate or not–the impact of a lecture on radical glioblastoma surgery and different treatment options on decision-making for oneself and patients. Acta Neurochir (Wien) 155(8):1425–1429
Kesselheim JC, Norden AD, Wen PY, Joffe S (2011) Discontinuing bevacizumab in patients with glioblastoma: an ethical analysis. Oncologist 16(10):1435–1439
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Murray KJ, Nelson DF, Scott C et al (1995) Quality-adjusted survival analysis of malignant glioma. Patients treated with twice-daily radiation (RT) and carmustine: A report of radiation therapy oncology group (RTOG) 83–02. Int J Radiat Oncol Biol Phys 31(3):453–459
Chinot L, Barrie M, Frauger E et al (2004) Phase II Study of Temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly population. Cancer 100(10):2208–2214
Karsy M, Yoon N, Boettcher L et al (2018) Surgical treatment of glioblastoma in the elderly: the impact of complications. J Neurooncol 138(1):123–132
McAleese JJ, Stenning SP, Ashley S et al (2003) Hypofractionated radiotherapy for poor prognosis malignant glioma: matched pair survival analysis with MRC controls. Radiother Oncol 67:177–182
Slotman BJ, Kralendonk JH, van Alphen HA et al (1996) Hypofractionated radiation therapy in patients with glioblastoma multiforme: results of treatment and impact of prognostic factors. Int J Radiat Oncol Biol Phys 34:895–898
Phillips C, Guiney M, Smith J et al (2003) A randomized trial comparing 35 Gy in ten fractions with 60Gy in 30 fractions of cerebral irradiation for glioblastoma multiforme and older patients with anaplastic astrocytoma. Radiother Oncol 68:23–26
Chibbaro S, Benvenuti L, Caprio A et al (2004) Temozolamide as first line agent in treating high-grade gliomas: phase II study. J Neurooncol 67(1–2):77–81
Malmström A, Grønberg BH, Marosi C et al (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13(9):916–926
Guedes de Castro D, Matiello J, Roa W et al (2017) Survival outcomes with short-course radiation therapy in elderly patients with glioblastoma: data from a randomized phase 3 trial. Int J Radiat Oncol Biol Phys 98(4):931–938
Reifenberger G, Hentschel B, Felsberg J et al (2012) Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 131(6):1342–1350
Wick W, Platten M, Meisner C et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13(7):707–715
Perry JR, Laperriere N, O'Callaghan CJ et al (2017) Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 376(11):1027–1037
Sulman EP, Ismaila N, Armstrong TS et al (2017) Radiation therapy for glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. J Clin Oncol 35(3):361–369
Weller M, van den Bent M, Hopkins K et al (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15(9):e395–403
Song K, Amatya B, Voutier C, Khan F (2016) Advance care planning in patients with primary malignant brain tumors: a systematic review. Front Oncol 6:223
Acknowledgements
On behalf of Brain Study Group of the Italian Association of Radiation Oncology (AIRO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Al the authors declared that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pedretti, S., Masini, L., Turco, E. et al. Hypofractionated radiation therapy versus chemotherapy with temozolomide in patients affected by RPA class V and VI glioblastoma: a randomized phase II trial. J Neurooncol 143, 447–455 (2019). https://doi.org/10.1007/s11060-019-03175-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03175-2