Abstract
Most high-grade gliomas (HGG) recur after initial multimodal therapy and re-irradiation (Re-RT) has been shown to be a valuable re-treatment option in selected patients. We evaluated the prognostic value of dynamic time-to-peak analysis and early static summation images in O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) PET for patients treated with Re-RT ± concomitant bevacizumab. We retrospectively analyzed 72 patients suffering from recurrent HGG with 18F-FET PET prior to Re-RT. PET analysis revealed the maximal tumor-to-background-ratio (TBRmax), the biological tumor volume, the number of PET-foci and pattern of time-activity-curves (TACs; increasing vs. decreasing). Furthermore, the novel PET parameters early TBRmax (at 5–15 min post-injection) and minimal time-to-peak (TTPmin) were evaluated. Additional analysis was performed for gender, age, KPS, O6-methylguanine-DNA methyltransferase methylation status, isocitrate dehydrogenase 1 mutational status, WHO grade and concomitant bevacizumab therapy. The influence of PET and clinical parameters on post-recurrence survival (PRS) was investigated. Shorter TTPmin was related to shorter PRS after Re-RT with 6 months for TTPmin < 12.5 min, 7 months for TTPmin 12.5–25 min and 11 months for TTPmin >25 min (p = 0.027). TTPmin had a significant impact on PRS both on univariate (p = 0.027; continuous) and multivariate analysis (p = 0.011; continuous). Other factors significantly related to PRS on multivariate analysis were increasing vs. decreasing TACs (p = 0.008) and Karnofsky Performance Score (p = 0.015; <70 vs. ≥70). Early TBRmax as well as the other conventional PET parameters were not significantly related to PRS on univariate analysis. Dynamic 18F-FET PET with TTPmin provides a high prognostic value for recurrent HGG prior to Re-RT, whereas early TBRmax does not. Dynamic 18F-FET PET using TTPmin might help to personalize Re-RT treatment regimens in future through voxelwise TTPmin analysis for dose painting purposes and PET-guided dose escalation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although there is ongoing research concerning the treatment of high-grade glioma (HGG), the prognosis remains poor, with e.g. a median survival of 12–14 months for patients with glioblastoma receiving standard multidisciplinary treatment [1, 2]. Besides surgical resection and chemotherapy, radiotherapy plays a key role in the therapeutic workup of patients suffering from primary and recurrent HGG. Despite those therapeutic efforts, tumor recurrence is the main issue in the following workup [3], hence, there is a clear need of highly effective therapeutic options concerning recurrent HGG [4]. One promising and very effective treatment option for recurrent high-grade glioma is represented by re-irradiation (Re-RT) with concurrent administration of bevacizumab in highly selected patients [5–7].
Several molecular biomarkers, such as the O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, mutations in the cytosolic isocitrate dehydrogenase (IDH) 1 and 2 gene and the loss of heterozygosity in the chromosomes 1p and 19q have been shown to be prognostic during primary treatment of HGG patients [8–10], which just have a minor importance in case of tumor recurrence. In this scenario, recent studies indicated additional clinical value of PET for response assessment and prognostication [11–13] besides magnetic resonance imaging (MRI) [14–16].
Regarding PET in neuro-oncology, dynamic O-(2-18F-fluoroethyl)-l-tyrosine positron emission tomography (18F-FET PET) has shown clinical value in planning of surgery, biopsy guidance and radiotherapy in previous studies [17–20]. Therefore, amino acid tracers such as 18F-FET have recently been recommended by the Response Assessment in Neuro-Oncology working group and the European Association for Neuro-Oncology providing a framework for the use of PET to assist in the management of patients with gliomas [21].
In a former study, 18F-FET PET prior to Re-RT showed clinical value in the treatment of HGG recurrence [22]; increasing tumoral 18F-FET uptake during the 40-min scan, reflected by increasing time-activity-curves (TACs) in the dynamic analysis, correlated significantly with longer survival after re-irradiation with 12 months for patients with increasing TACs, 10 months for patients with mixed TACs and only 7 months with decreasing TACs.
Additionally, dynamic 18F-FET PET has recently shown remarkably high prognostic value in both newly diagnosed astrocytic HGG and low-grade glioma (LGG) [23, 24], especially using the newly established parameter minimal time to peak (TTPmin). In particular TTPmin was highly prognostic and may allow more explicit identification of aggressive tumor tissue than the mere qualitative classification of increasing vs. decreasing TACs [23].
Besides the value of static 18F-FET PET for antiangiogenic therapy response assessment [25], TTPmin has predictive value for the response assessment in patients with recurrent HGG undergoing bevacizumab therapy, where an early TTPmin besides reduction of the biological tumor volume was predictive for a treatment failure [26].
Additionally, a recent study reported that early static 18F-FET PET scans have a higher accuracy than the conventionally acquired scans at 20–40 min for the differentiation of HGG and LGG at the initial diagnosis, when comparing the early maximal tumor-to-background ratio (early TBRmax) [21]. However, these newly established PET parameters (i.e. TTPmin, early TBRmax) were not yet evaluated regarding their prognostic value prior to Re-RT of recurrent HGG with concomitant antiangiogenic therapy using bevacizumab.
Thus, the aim of this study was to determine the additional prognostic value of the newly derived TTPmin and the novel assessment of the early summation images from 5 to 15 min post-injection in patients with recurrent HGG prior to Re-RT with bevacizumab.
Methods
Patients
Patients undergoing dynamic 18F-FET PET examination prior to Re-RT were identified by searching the clinical database of the Department of Radiation Oncology, University hospital of Munich. Re-RT was performed at least 6 months after the initial radiotherapy in patients with recurrent HGG as diagnosed by MRI or proven histologically by stereotactic biopsy [27]. 18F-FET PET with dynamic acquisitions performed in the routine management was used to confirm disease progression and to exclude radiation necrosis in cases of suspicious findings in the MRI scan. For all patients gadolinium-enhanced brain MRI, 18F-FET PET as well as physical and neurological examination were performed prior to Re-RT. Treatment follow-up was evaluated by clinical examination and gadolinium-enhanced brain MRI, followed by 18F-FET PET examination in the case of abnormal brain MRI findings.
18F-FET PET acquisition and evaluation of kinetic parameters
40 min dynamic 18F-FET PET scans were performed with an ECAT EXACT HR+ scanner (Siemens) according to standard protocols as described previously [28, 29]. Static and dynamic 18F-FET PET parameters were evaluated on a Hermes workstation (Hermes Medical Solutions, Sweden) [28]. The consecutive semiquantitative evaluation consisted of the calculation of the maximal tumor-to-background-ratio (TBRmax) (i.e. the standardized uptake value of the tumor divided by the mean background activity in the unaffected contralateral hemisphere) and the biological tumor volume (BTV) as calculated semiautomatically on a threshold basis of the volume of interest (TBRmax ≥1.8) as described previously [30]. Volumes of interest were classified as a single focus, if the threshold was exceeded at only one coherent area or as multifocal, if the threshold was exceeded at multiple disconnected areas. Additionally to the standard assessment of the summation images 20–40 min post-injection, the early maximal tumor-to-background ratio (early TBRmax) was investigated using summation images 5–15 min post-injection.
As also described previously [28], dynamic PET data was evaluated according to the standardized clinical procedures using the software PET Display Dynamic which is implemented in the Hermes workstation: early summation images (10–30 min post-injection, frames 13–15) were used to define a 90% isocontour threshold region of interest (ROI), which was semiautomatically generated for the area of suspicious 18F-FET uptake on each slice (thickness 3 mm) of the tumor. The individual TACs of each tumor slice within these ROIs were extracted and used for the kinetic analysis. The TAC patterns of each tumor were evaluated and classified according to the predominant kinetic structure into increasing vs. decreasing TACs analogous to our previous study [22]. Solely (G1) and predominantly (G2) increasing TACs were classified as increasing, whereas mixed pattern (G3) TACs as well as predominantly (G4) and solely (G5) decreasing TACs were classified as decreasing TACs.
Additionally, for each ROI and corresponding TAC the frame presenting the peak uptake was determined as previously published [23]: the starting time of the particular frame plus half the frame duration, corresponding to the respective peak value, was set as time to peak (TTP). Accordingly, TTP accounted for 4 min in frame 11, 7.5 min in frame 12, 12.5 min in frame 13, 17.5 min in frame 14, 25 min in frame 15, and 35 min in frame 16. The shortest TTP presenting in at least 2 consecutive slices was set as minimal TTP (TTPmin). In order to exclude noise artifacts at the beginning of the acquisition and due to low counting rates, only slices 11–16 (3–40 min post-injection) were analyzed in the dynamic evaluation.
Re-irradiation and concomitant use of bevacizumab
Re-RT was applied as described before [31] with a total dose of 36 Gy in conventional fractions of 2 Gy. 18F-FET PET images and an analysis of the tracer uptake kinetics were demonstrated by a nuclear medicine physician in the interdisciplinary neurooncologic tumor board, before the start of re-irradiation planning. 18F-FET PET (3 mm slice thickness) and gadolinium-enhanced MRI images (3 mm slice thickness) were fused with the planning computer tomography scan images (3 mm slice thickness) and dynamic 18F-FET PET information was utilized in the planning process. Generally, the gross tumor volume plus a 10 mm margin accounted for the planning target volume. In patients receiving concomitant bevacizumab therapy, 10 mg/kg body weight was applied at days 1 and 15 of Re-RT. Maintenance therapy with bevacizumab was initiated on a patient-specific basis by the interdisciplinary Neuro-Oncology Tumor Board of the University Hospital of Munich.
Statistical analysis
Statistical analysis was performed with IBM® SPSS® Statistics for Microsoft® Windows®, Version 23, Armonk, NY, USA. Descriptive statistics were used for patients’ characteristics and 18F-FET PET data including TACs, TTPmin, TBRmax, early TBRmax, BTV and 18F-FET PET positive foci. Post-recurrence survival (PRS) was defined as first day of Re-RT until death or until the last day of follow-up. Kaplan–Meier estimators and Log-rank tests were used for survival and univariate analysis. Continuous variables were evaluated by Cox regression analysis. A Mann–Whitney U test was used to assess association between the dynamic PET parameters TTPmin and TACs. Multivariate analysis was performed by using a backward stepwise exclusion model, based on the likelihood ratio test. Statistical significance was considered for two-tailed p-values below 0.05.
Consent
Written informed consent for scientific evaluation of 18F-FET PET and follow-up data was obtained for all patients before participating in the 18F-FET PET investigation.
Results
Patient characteristics
72 HGG patients who received Re-RT at recurrence with preceding dynamic 18F-FET PET examination at the University hospital of Munich were retrospectively analyzed (Table 1) of which 52 were previously examined in a former study with a reduced number of parameters [22]. Duration between 18F-FET PET and Re-RT was intended to be 3 weeks on average, therefore the mean time duration was 22 days ± standard deviation of 13 days. Exceptionally, three patients received 18F-FET PET shortly after beginning of Re-RT and one patient received 18F-FET PET two-and-a-half months before Re-RT. At the beginning of Re-RT median age of the patients was 49 years (range 18–73 years) and median Karnofsky performance score (KPS) was 80 (range 40–100). 57 patients (79.2%) suffered from WHO grade IV tumors at relapse. MGMT methylation status was available for 64 patients (88.9%) with methylated MGMT promoters in 37/64 (57.8%) patients and IDH1 mutational status was available for 42 patients (58.3%) with IDH1 mutation in 10/42 (23.8%) patients. Median prescribed radiation doses were 60 Gy at primary radiotherapy and 36 Gy during Re-RT, both with 2 Gy single fractions. Bevacizumab was applied concomitantly to Re-RT in 57 patients (79.2%). PRS was 8 months (95% CI 6.5–9.5). Applying the reverse Kaplan–Meier method the median follow-up was not reached, consequently mean follow-up was 30.5 months (95% CI 26.4–34.7).
18F-FET PET
18F-FET PET examination revealed a single focus in 59 patients (81.9 %) and multifocal tumor recurrence in the remaining 13 patients (range 2–4). Median TBRmax was 3.2 (range 1.6-7.0), median early TBRmax 3.7 (range 1.8–7.2) and median size of the BTV 11.1 cc (range 0.1–100.6). On kinetic analysis median TTPmin was 12.5 min with TTPmin below 12.5 min in 8 patients (11.1%), TTPmin in the range of 12.5 to 25 min in 53 patients (73.6%) and TTPmin above 25 min in 11 patients (15.3%). Tumoral TAC pattern was increasing in 13 (18.1%) patients, while decreasing TACs were detected in 59 (81.9%) patients. As expected by its definition increasing TACs were associated with longer median TTPmin values (35/12.5 min within the increasing/decreasing TAC groups, p < 0.001 on Mann–Whitney U test). Due to technical reasons early TBRmax could only be investigated in 71 of the 72 patients.
Univariate analysis including Log-rank test and Cox regression
Univariate analysis with Log-rank test and Cox regression was performed according to PRS including the factors age groups, gender, KPS, MGMT methylation status, IDH1 mutational status, WHO grade at relapse, concomitant bevacizumab therapy to Re-RT, number of foci, TBRmax, early TBRmax, BTV, TACs and TTPmin (Table 2).
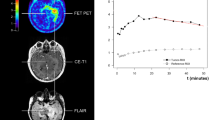
Factors with statistically significant influence on PRS were number of foci (p = 0.025), TTPmin (p = 0.027; continuous), TAC classifications (p = 0.019; increasing vs. decreasing), and gender (p = 0.028). In patients with one PET focus, PRS was 9 months (95% CI 6.8–11.2), whereas patients with two to four PET foci presented with a median PRS of only 6 months (95% CI 3.8–8.2, p = 0.028). In patients with TTPmin < 12.5 min median PRS was 6 months (95% CI 1.9–10.1), with TTPmin 12.5–25 min 7 months (95% CI 5.3–8.7) and for TTPmin ≥25 min 11 months (95% CI 6.7–15.3, p = 0.027, Fig. 1). Similarly in patients with increasing TACs median PRS was 11 months (95% CI 7.5–14.5) compared to patients with decreasing TACs with only 7 months of median PRS (95% CI 6.0–8.0, p = 0.019). Female patients presented with longer median PRS of 10 months (95% CI 7.4–12.6) than male patients with only 7 months (95% CI 4.6–9.4, p = 0.028). No significant influence on PRS could be shown for age categories of younger than 50 years or 50 years and older, KPS, WHO grade, bevacizumab therapy, BTV size, TBRmax. The early static parameter early TBRmax did not show significance in Log-Rank test (p = 0.997, Fig. 2b) or on Cox regression (p = 0.140). The time span between the 18F-FET PET and the beginning of the re-irradiation did not show significant correlation with TTPmin on Pearson’s correlation (p = 0.205).
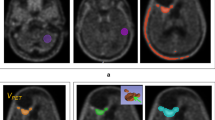
Patient examples a A male glioblastoma patient (WHO grade IV, 48 years) at Re-RT: static 18F-FET PET images show recurrent tumor tissue in the right temporal lobe with pathologic 18F-FET uptake, a TBRmax of 3.37 and an early TBRmax of 3.49, dynamic images reveal decreasing TACs with a TTPmin of 4 min. The patient had a PRS of 3 months. b A female glioblastoma patient (WHO IV, 36 years) at Re-RT: static 18F-FET PET images present temporally located tumor recurrence with intense tracer uptake, a TBRmax of 4.73 and an early TBRmax of 4.65, but with increasing TACs in the dynamic analysis. The TTPmin was accordingly set as 35 min. The patient had a PRS of 17 months
Multivariate analysis
Multifactorial analysis was performed for all factors with statistical significance or trend (p < 0.1) according to PRS on univariate analysis including TTPmin, TACs, number of foci, gender, KPS, BTV and WHO grade (see Table 3). Early TBRmax was not included into the multivariate analysis, since it did not show statistical significance or trend according to PRS.
Since TTPmin and kinetic categories showed significant intercorrelation on Mann–Whitney U test three multivariate models were calculated separately. For all variables without TACs TTPmin (p = 0.011; continuous) and KPS (p = 0.028; ≥70 vs.<70) were significant factors. For all variables without TTPmin, TACs (p = 0.008; increasing vs. decreasing) and KPS (p = 0.015; ≥70 vs. <70) were significant. In the model including both TACs and TTPmin with an interaction variable of TTPmin × TACs, merely one kinetic parameter (TACs p = 0.008) remained significant while the other was ruled out due to correlation effects.
Discussion
Molecular imaging with positron emission tomography (PET) visualizing metabolic pathways is increasingly used in the neurooncological treatment of patients suffering from both high and low grade glioma [32]. Particularly radiolabeled amino acids such as 18F-FET are of special interest in the clinical workup because of a high uptake in biologically active tumor tissue and low uptake in normal brain tissue [33] which enables the identification of vital tumor tissue even after preceding therapies [22]. 18F-FET PET implementation in the clinical routine helps to individualize and optimize the specific therapy because of its value in tumor grading, biopsy guidance and planning of surgery and radiotherapy [17–19, 29], therefore the Response Assessment in Neuro-Oncology working group and the European Association for Neuro-Oncology emphasize the clinical use of amino acid tracers such as 18F-FET PET [21].
Since conventional biomarkers such as MGMT promoter methylation status or IDH1 mutational status are lacking prognostic significance in the recurrence setting, there is an urgent need for novel imaging derived parameters with prognostic validity at this stage of the disease, especially as non-invasive methods have to be preferred in this treatment setting.
In this study, TTPmin in the pretherapeutic dynamic 18F-FET PET proved to be a strong prognostic factor in our cohort of patients with recurrent glioma treated with Re-RT. Patients with longer TTPmin value showed significantly longer PRS. Unlike the TACs, which only give qualitative information of the tracer uptake kinetics (increasing vs. decreasing) and usually refer to the entire tumor, TTPmin has the advantage of showing detailed information within the group of tumors with decreasing TACs by presenting the time point of curve decrease. This is particularly useful when high-grade tumors are evaluated, as most HGG are characterized by decreasing TACs, which points out the need for a more detailed risk stratification by dynamic parameters within these tumors with decreasing TACs. Furthermore, by evaluating the TTP layer by layer within the entire tumor, one may receive spatial information on tumor areas with a more aggressive biology, which would not be represented with the standard evaluation of increasing vs. decreasing TACs. This was particularly obvious in the observation, that there is a link between an early TTPmin and a short PRS and vice versa. Exact localization of the most aggressive parts within the tumor is another advantage of TTPmin over the TACs, which only describe the most prevalent kinetic type of the tumor in toto. As the study was conducted retrospectively, the timespan between the pre-therapeutic 18F-FET PET and the Re-RT varied with a median of 22 days with a standard deviation of 13 days. A longer timespan could have added a certain confounding to our results, since some tumors might have had a shorter TTPmin, if the PET had been conducted at a closer time to the radiation planning. Since some of the tumors could have been “understaged” in our retrospective analysis, we performed a Pearson’s correlation of the time span between the 18F-FET PET and the beginning of the re-irradiation, which was not statistically significant.
Conversely, none of the static 18F-FET PET parameters (i.e. TBRmax, BTV) but only the number of tumor foci showed significance on univariate analysis. A possible explanation for the insignificance of static parameters prior to Re-RT might be, that the summation of the images from minutes 20–40, which are commonly used for clinical reading and the standard calculation of TBRmax, were not able to represent tumors with a very aggressive tracer uptake accurately, because the peak uptake had already occurred at an earlier timepoint. Therefore, taking into account the high prognostic value of TTPmin, one might hypothesize that the assessment of TBRmax values in earlier time frames might be more promising for outcome prognostication than the standard evaluation in this recurrence scenario.
Nonetheless, the newly introduced static parameter early TBRmax, which is assessed by the summation of the early images from minutes 5–15 of the dynamic scans, did not reveal any association with the patients’ PRS in this setting prior to recurrent HGG Re-RT. These data indicate that the main application of this interesting new parameter might be the differentiation of LGG and HGG, e.g. at the initial diagnosis, as already demonstrated [21]. Whether relative changes of PET parameters derived from early summation images after Re-RT and during the maintenance therapy with bevacizumab have an additional predictive or prognostic value, as demonstrated in conventional static parameters during bevacizumab therapy [25, 26], has to be evaluated in further prospective settings. Unlike after primary surgery [34], static parameters derived from a single 18F-FET PET including early TBRmax, do not seem to have a prognostic value prior to Re-RT, as also described previously [22].
One might hypothesize that the tumoral uptake intensity does not have an impact on the patients’ PRS, since the mere intensity is most likely related to the amount and density of the tumor cells, whereas the dynamic parameter TTPmin enables to characterize the biological behavior in terms of tumoral aggressiveness, since patients with an early TTPmin in the dynamic evaluation had a significantly poorer outcome regarding the PRS. Further fields of application for early TBRmax have to be evaluated in future studies, e.g. the identification of malignant transformation in patients suffering from LGG. In the setting of HGG recurrence, this early static parameter does not seem to have a clinical impact so far.
On a future perspective, TTPmin has the potential to further personalize retreatment of HGG by adding individually tailored radiation boosts to tumor areas with aggressive kinetic uptake behavior, i.e. a short TTPmin. Additional voxelwise analysis of the tracer uptake and the quantitative TTPmin might enable even more elaborate radiation planning approaches such as dose-painting, where areas with a short TTPmin could be precisely treated with higher radiation doses, compared to areas with a late TTPmin.
Conclusion
The new dynamic parameter TTPmin has prognostic value prior to recurrent HGG Re-RT with concomitant bevacizumab; the earlier the TTPmin in the dynamic evaluation, the shorter the PRS after Re-RT and vice versa. In contrast, early TBRmax as derived from early summation images did not show prognostic value in this setting. Therefore, dynamic 18F-FET PET aims towards a future of personalized radiotherapy treatment planning in recurrent HGG with TTPmin as novel quantitative prognostic marker. Voxelwise TTPmin analysis with dose-escalation at aggressive tumor parts with short TTPmin as dose-painting could help to advance tumor control and survival in HGG in the future.
References
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/s1470-2045(09)70025-7
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB (2014) Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol 9: 130 doi:10.1186/1748-717x-9-130
Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth F-W, Tonn J-C, Belka C (2011) Therapeutic options for recurrent malignant glioma. Radiother Oncol 98:1–14
Flieger M, Ganswindt U, Schwarz SB, Kreth F-W, Tonn J-C, la Fougère C, Ertl L, Linn J, Herrlinger U, Belka C (2014) Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117:337–345
Hundsberger T, Brügge D, Putora PM, Weder P, Weber J, Plasswilm L (2013) Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol 112:133–139
Niyazi M, Jansen NL, Rottler M, Ganswindt U, Belka C (2014) Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat Oncol 9: 299 doi:10.1186/s13014-014-0299-y
Hartmann C, Hentschel B, Simon M, Westphal M, Schackert G, Tonn JC, Loeffler M, Reifenberger G, Pietsch T, von Deimling A (2013) Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res 19:5146–5157
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Ohgaki H, Kleihues P (2011) Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol 28:177–183
Harris RJ, Cloughesy TF, Pope WB, Nghiemphu PL, Lai A, Zaw T, Czernin J, Phelps ME, Chen W, Ellingson BM (2012) 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol 14:1079–1089. doi:10.1093/neuonc/nos141
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Geist C, Dahlbom M, Silverman DH, Satyamurthy N, Phelps ME, Chen W (2012) 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med 53:29–36. doi:10.2967/jnumed.111.092387
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, Elashoff D, Geist C, Silverman DH, Phelps ME, Chen W (2014) Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res 20:3550–3559. doi:10.1158/1078-0432.ccr-13-1440
Harris RJ, Cloughesy TF, Hardy AJ, Liau LM, Pope WB, Nghiemphu PL, Lai A, Ellingson BM (2015) MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bevacizumab. J Neurooncol 122:497–505. doi:10.1007/s11060-015-1755-8
Huang RY, Rahman R, Ballman KV, Felten SJ, Anderson SK, Ellingson BM, Nayak L, Lee EQ, Abrey LE, Galanis E, Reardon DA, Pope WB, Cloughesy TF, Wen PY (2016) The Impact of T2/FLAIR evaluation per RANO criteria on Response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res 22:575–581. doi:10.1158/1078-0432.ccr-14-3040
Leu K, Enzmann DR, Woodworth DC, Harris RJ, Tran AN, Lai A, Nghiemphu PL, Pope WB, Cloughesy TF, Ellingson BM (2014) Hypervascular tumor volume estimated by comparison to a large-scale cerebral blood volume radiographic atlas predicts survival in recurrent glioblastoma treated with bevacizumab. Cancer Imaging 14:31. doi:10.1186/s40644-014-0031-z
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, Schnell O, Suchorska B, Kreth F-W, Tonn J-C, Bartenstein P, Fougère Cl, Belka C (2011) FET–PET for malignant glioma treatment planning. Radiother Oncol 99:44–48. doi:10.1016/j.radonc.2011.03.001
Piroth M, Pinkawa M, Holy R, Stoffels G, Demirel C, Attieh C, Kaiser H, Langen K, Eble M (2009) Integrated-boost IMRT or 3-D-CRT using FET-PET based auto-contoured target volume delineation for glioblastoma multiforme: a dosimetric comparison. Radiat Oncol 4:57
Wyss M, Hofer S, Bruehlmeier M, Hefti M, Uhlmann C, Bärtschi E, Buettner U, Roelcke U (2009) Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol 95:87–93. doi:10.1007/s11060-009-9896-2
Miwa K, Matsuo M, Ogawa S, Shinoda J, Yokoyama K, Yamada J, Yano H, Iwama T (2014) Re-irradiation of recurrent glioblastoma multiforme using 11 C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 9: 181 doi:10.1186/1748-717x-9-181
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, Todica A, Brendel M, Tonn JC, Bartenstein P, la Fougere C (2016) Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur J Nucl Med Mol Imaging 43:1105–1114. doi:10.1007/s00259-015-3276-2
Niyazi M, Jansen N, Ganswindt U, Schwarz S, Geisler J, Schnell O, Büsing K, Eigenbrod S, la Fougère C, Belka C (2012) Re-irradiation in recurrent malignant glioma: prognostic value of [18F]FET–PET. J Neurooncol 110:389–395. doi:10.1007/s11060-012-0980-7
Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S, Niyazi M, Tonn J-C, Bartenstein P, Kreth F-W, la Fougère C (2015) Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med 56:9–15. doi:10.2967/jnumed.114.144675
Suchorska B, Tonn JC, Jansen NL (2014) PET imaging for brain tumor diagnostics. Curr Opin Neurol 27:683–688. doi:10.1097/wco.0000000000000143
Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, Muigg A, Virgolini IJ, Staffen W, Trinka E, Gotwald T, Jacobs AH, Stockhammer G (2011) O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med 52:856–864. doi:10.2967/jnumed.110.086645
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH, Sabel M, Langen KJ (2013) Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging 40:22–33. doi:10.1007/s00259-012-2251-4
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, DeGroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/jco.2009.26.3541
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S, Cumming P, Bartenstein P, Tonn J-C, Kreth FW (2012) MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging 39:1021–1029
Mehrkens J, Pöpperl G, Rachinger W, Herms J, Seelos K, Tatsch K, Tonn J, Kreth F (2008) The positive predictive value of O-(2-[18F] fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol 88:27–35
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, Simon M, Popperl G, Kreth FW, la Fougere C, Weller M, Tonn JC (2015) Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84:710–719. doi:10.1212/wnl.0000000000001262
Niyazi M, Ganswindt U, Schwarz SB, Kreth F-W, Tonn J-C, Geisler J, la Fougère C, Ertl L, Linn J, Siefert A (2012) Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 82:67–76
Jager PL, Vaalburg W, Pruim J, de Vries EGE, Langen K-J, Piers DA (2001) Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 42:432–445
Hutterer M, Nowosielski M, Putzer D, Jansen NL, Seiz M, Schocke M, McCoy M, Göbel G, la Fougère C, Virgolini IJ, Trinka E, Jacobs AH, Stockhammer G (2013) [18F]-fluoro-ethyl-l-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol 15:341–351. doi:10.1093/neuonc/nos300
Piroth MD, Holy R, Pinkawa M, Stoffels G, Kaiser HJ, Galldiks N, Herzog H, Coenen HH, Eble MJ, Langen KJ (2011) Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol 99:218–224. doi:10.1016/j.radonc.2011.03.006
Acknowledgements
We thank Alexander Nieto for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Daniel F. Fleischmann and Marcus Unterrainer have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fleischmann, D.F., Unterrainer, M., Bartenstein, P. et al. 18F-FET PET prior to recurrent high-grade glioma re-irradiation—additional prognostic value of dynamic time-to-peak analysis and early static summation images?. J Neurooncol 132, 277–286 (2017). https://doi.org/10.1007/s11060-016-2366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2366-8