Abstract
Purpose
Since differentiation between low-grade glioma (LGG) and high-grade glioma (HGG) remains challenging according to MRI criteria alone, we investigated the discriminative value of additional dynamic FET PET in patients with MRI-suspected LGG.
Methods
Included in this retrospective study were 127 patients with newly diagnosed MRI-suspected LGG and dynamic FET PET prior to histopathological assessment. FET PET lesions were visually classified as having reduced, normal, or increased tracer uptake. Maximal tumour uptake scaled to the mean background uptake (SUVmax/BG), mean tumour uptake (SUVmean/BG), biological tumour volume and kinetics were evaluated and correlated with individual histopathological findings.
Results
Histopathological analysis revealed 71 patients with LGG, 47 patients with HGG (including 5 glioblastoma multiforme), 2 patients with low-grade ganglioglioma and 7 patients with non-neoplastic lesions. Of the 127 patients, 97 had lesions with increased FET uptake, of which 93 were neoplastic. Increased uptake was found in 49/71 LGG (69 %) and 42/47 HGG (89 %). None of the conventional uptake parameters differed significantly between the HGG and LGG groups. Kinetic analysis reliably identified HGG (sensitivity 95 %, specificity 72 %, PPV 74 %, NPV 95 %). Normal tracer uptake was observed in 19 patients (15 with LGG, 1 with HGG and 3 with non-neoplastic lesions) and reduced uptake in 11 patients (7 with LGG and 4 with HGG).
Conclusion
Among the MRI-suspected LGG, kinetic but not conventional analysis of FET uptake enabled remarkably high sensitivity for detection of HGG. This held true even for lesions with low or diffuse tracer uptake. Lesions with reduced tracer uptake must be interpreted with caution, as they can also harbour HGG tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The optimal clinical management of a newly diagnosed glioma is highly dependent on accurate assessment of the tumour malignancy. Initial differentiation between low-grade glioma (LGG) and high-grade glioma (HGG) is usually based on MRI findings, especially on leakage of the blood–brain barrier (BBB) as defined by the presence of contrast enhancement. However, this diagnostic technique lacks accuracy in the non-invasive assessment of tumour grading as about 40–45 % of non-enhancing MR lesions are found subsequently to be malignant glioma [1–4].
In recent years, metabolic imaging by PET with the amino acid analogue [18F]fluoroethyltyrosine (FET) has gained increasing importance in the imaging of glioma. FET PET is of proven utility in treatment planning and therapy monitoring [5–8], and has furthermore been deemed to be useful for tumour grading [9–11]. The latter, however, is still a matter of controversy, and dynamic FET PET analysis has been suggested to be superior to conventional FET PET analysis. Although promising, the time-consuming dynamic method is not yet widely used, and the observations on tumour grading with dynamic FET PET were based on relatively small cohorts, which furthermore included patients with contrast-enhancing as well as non-contrast-enhancing lesions [9, 10].
In another study focusing on patients with non-contrast-enhancing brain lesions only, kinetic analysis of FET uptake was correlated with histopathological findings and proved to be particularly useful for the delineation of “hot spots”, representing the most malignant parts of heterogeneous gliomas [12], which are the optimal targets for stereotactic biopsy. However, a systematic evaluation of dynamic FET PET including lesions without or with only low FET uptake for non-invasive prediction of malignancy in patients with MRI-suspected LGG has not yet been performed.
The aim of this study was to determine the informative value and reliability of dynamic FET PET in a large cohort of patients presenting with non-contrast-enhancing MRI lesions suspicious for LGG. We also intended to identify key parameters for differentiation between LGG and HGG, and to obtain a more detailed quantitative assessment of the a priori reliability of dynamic FET PET for tumour detection and grading than has hitherto been available.
Patients and methods
Inclusion criteria
For this retrospectively designed single-centre study all patients who had received FET PET scans prior to neurosurgical intervention during the years 2005 to 2010 were identified. Inclusion criteria consisted of diagnosis of a brain lesion suspicious for LGG based on MRI findings (see section Magnetic resonance imaging). Those patients who had undergone previous interventions such as stereotactic biopsy or microsurgery, as well as chemo- and/or radiotherapy, were excluded from the study. MRI scans were performed at the Department of Neuroradiology, FET PET scans at the Department of Nuclear Medicine, neurosurgical interventions at the Department of Neurological Surgery and histopathological evaluation at the Institute of Neuropathology of Ludwig-Maximilians University of Munich.
Magnetic resonance imaging
MRI scans were performed with either a 1.5-T (Magnetom Symphony; Siemens, Erlangen, Germany) or a 3.0-T (Signa HDx, 3T; GE Healthcare, Milwaukee, WI) magnet. The MRI protocols for both scanners included acquisition of axial T2-weighted sequences with a slice thickness of 2 mm, and 3-D T1-weighted sequences with a slice thickness of 1 mm before and after intravenous administration of 0.1 mmol/kg gadobenate dimeglumine contrast agent (Gd-BOPTA, MultiHance; BraccoImaging, Milan, Italy). Images were interpreted by an experienced neuroradiologist using a PACS workstation (Sienet Magic View 1000; Siemens). The MRI-based criteria for World Health Organization (WHO) grade II gliomas are (1) no contrast enhancement, (2) hyperintense signal on T2-weighted sequences, (3) hypointense signal on T1-weighted sequences, and (4) no evidence of haemorrhage or necrosis. The neuroradiologist was blinded to the histopathological results in all cases.
FET PET image acquisition and assessment
In order to obtain standardized metabolic conditions, all patients fasted for a minimum of 6 h prior to PET scanning. PET data were acquired with a Siemens ECAT EXACT HR+ scanner. Following a 15-min transmission scan with a 68Ge rotating rod source, approximately 180 MBq [18F]FET was injected as a slow intravenous bolus. A 40-min dynamic emission recording in 3-D mode consisting of 16 frames was started on tracer injection. Images corrected for attenuation and scatter were reconstructed by filtered back-projection using a 5-mm Hann filter. For further evaluation, images were transferred to a Hermes workstation (Hermes Medical Solutions, Stockholm, Sweden).
For conventional semiquantitative evaluation, the maximal FET uptake (SUVmax) in the tumour was determined on a summation image (20–40 min after injection), and scaled to the mean background uptake (SUVmax/BG) in a large region of the healthy contralateral hemisphere. In a first visual analysis, tumours were categorized as (1) lesions with reduced uptake compared to that of healthy brain tissue, (2) lesions with uptake equal to that of healthy brain tissue (“normal” uptake), and (3) lesions with uptake exceeding the cortical background (“FET-positive”). For further characterization, the uptake pattern of FET-positive lesions was classified visually as diffuse, focal/compact or inhomogeneous (mixed form with diffuse as well as focal elements). The volume of increased FET uptake was determined by means of a semiautomatic threshold-based calculation (SUVmax/BG ≥1.8) in order to estimate the biological tumour volume (BTV). Finally, the mean FET uptake corrected for BG (SUVmean/BG) within the BTV was calculated.
Dynamic PET data were evaluated as described previously [2]. In brief, a 90 % isocontour threshold region of interest was automatically defined on each slice of the summation image (10–30 min after injection) with suspicious uptake. Each region of interest was applied to the dynamic PET data in order to extract the time–activity curves (TAC) for kinetic analysis.
Stereotactic biopsies, microsurgical resections and histological evaluation
Collection of serial stereotactic biopsies was indicated in patients with suspected de-novo glioma, a tumour located in an eloquent brain region and significant comorbidities. In brief, the biopsies were performed using a modified Riechert head ring (Freiburg, Germany). A workstation capable of multiplanar trajectory planning was employed to determine the best trajectory for biopsy, based on coregistration of CT (2-mm contrast-enhanced axial images), MRI (2-mm T1-weighted gadolinium-enhanced and 2-mm T2-weighted axial images) and FET PET data (i-plan stereotaxy; BrainLab, Heimstetten, Germany). Microsurgical resections were performed by experienced neurosurgeons using modern neurosurgical equipment including neuronavigation with MRI and PET image fusion (BrainLab) and intraoperative ultrasound if indicated. Histological classification and tumour grading were performed according to the WHO guidelines current at the respective date of histopathological assessment.
Statistical analysis
SPSS for Windows (version 17.0; SPSS, Chicago, IL) was used for statistical calculations. Individual results for the PET parameters described above were correlated with the histopathological results using the Mann-Whitney-U-test. The chi-squared test was used to evaluate the significance of differences in FET kinetics and uptake patterns. Continuous parameters are reported as means ± standard deviation and range. Statistical significance was defined as p-values ≤0.05. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for LGG and HGG.
Results
Study population
Included in this study were 127 patients (72 men) with MRI-suspected de novo intracranial LGG. The mean age at the time of PET scanning was 45.7 ± 14.2 years. FET PET scans and histopathological evaluation were successfully performed in all patients, stereotactic biopsy was performed in 111 patients (87.4 %), and 16 patients (12.6 %) were referred for surgical resection.
Histology and WHO grading
Histological analyses revealed 4 patients with a WHO grade I tumour (3.1 %; 3 pilocytic astrocytoma, 1 ganglioglioma), 69 with a grade II tumour (54.3 %; 1 ganglioglioma, 52 diffuse astrocytoma, 11 oligoastrocytoma, 5 oligodendroglioma), 42 with a grade III tumour (33.1 %; 35 anaplastic astrocytoma, 7 anaplastic oligoastrocytoma) and 5 with a grade IV tumour (3.9 %; 5 glioblastoma multiforme). Of the 7 remaining patients (5.5 %) in whom no tumour was detected, 5 received a histopathological diagnosis of reactive astrocytosis or gliosis, or cortical dysplasia (1 patient) and multiple sclerosis (1 patient). These findings are summarized in Table 1.
FET PET evaluation
Of the entire group of 127 patients, 97 (76.4 %) presented with FET uptake exceeding the cortical level (FET-positive lesion), 19 (14.9 %) had normal tracer uptake and 11 (8.7 %) showed reduced uptake (Table 2).
Lesions with normal FET uptake
Among the 19 patients with normal FET uptake, histopathological analysis showed 15 with LGG (14 diffuse astrocytoma, 1 oligoastrocytoma), 1 with HGG (anaplastic oligoastrocytoma) and 3 without proof of tumour tissue. Here, the histopathological diagnoses were cortical dysplasia (1 patient) and reactive changes of brain tissue without signs of neoplasia (2 patients). The NPV of normal FET PET in this setting was 15.8 % (Table 2).
Lesions with altered (reduced or increased) FET uptake
Among the 11 patients with reduced FET uptake, histopathological analysis revealed neoplastic lesions in all patients (7 diffuse astrocytoma, 4 anaplastic astrocytoma). Among the 97 patients with increased FET uptake, histopathological analysis revealed 51 with low-grade tumours, 42 with high-grade tumours and 4 with nontumoral lesions (3 reactive astrocytosis, 1 multiple sclerosis). Overall, in 104 of 108 patients with altered uptake, the uptake was associated with a neoplastic lesion. The PPV of pathological FET PET was 96.3 % (Table 2).
FET uptake in low-grade gliomas
FET PET was positive in 49 of 71 patients (69.0 %) with LGG. The proportion of FET-positive LGG was notably higher among patients with oligodendroglial tumours (15/16; 93.8 %) compared to that among patients with pure astrocytoma (34/55; 61.8 %). Of the 71 patients with LGG, 15 (21.1 %) showed normal uptake, and 7 (9.9 %) showed reduced uptake (Table 2). FET PET had a sensitivity of 78.9 % for the detection of LGG when considering the pathological FET findings together (increased and reduced uptake), as compared to 69.0 % for positive findings only.
FET uptake in high-grade gliomas
Among the patients with HGG, FET PET was positive in all 5 with glioblastoma and in 37 of 42 with anaplastic glioma (88.1 %) (Table 2). Among the remaining patients with anaplastic glioma, four showed reduced FET uptake, and a single patient showed normal FET uptake. FET PET had a sensitivity of 97.9 % for the detection of HGG when considering the pathological FET findings together (increased and reduced uptake), as compared to 89.4 % for positive findings only.
Comparison of FET-positive LGG and HGG
Detailed PET characteristics in the different WHO groups are shown in Table 2. Of the 97 FET-positive lesions, 27 showed diffuse uptake, 51 showed focal uptake, and 19 showed inhomogeneous uptake including diffuse and focal components. No significant differences in FET uptake pattern were found between LGG and HGG. Mean uptake parameters for HGG tended to exceed those for LGG, specifically 3.1 vs. 2.7 for SUVmax/BG, 12.1 ml vs. 7.2 ml for BTV, and 2.2 vs. 2.1 for SUVmean/BG, but these differences were not statistically significant. The interindividual variability in SUVmax/BG by WHO group was very high, ranging from 1.4 to 5.4 in LGG and from 1.6 to 7.0 in HGG (Fig. 1), such that no reliable cut-off value could be identified. Interestingly, upon excluding the oligodendroglial tumours, the difference between astrocytic HGG and LGG reached statistical significance (3.1 vs. 2.4, p < 0.05).
Kinetic analysis of FET-positive gliomas revealed decreasing TACs in 40 of 42 HGG (95.2 %; Figs. 2 and 3) and increasing TACs in 33 of 46 LGG (71.7 %). Among the 49 patients with FET-positive LGG, 13 (26.5 %) of the tumours (6 diffuse astrocytoma, 4 oligodendroglioma and 3 mixed oligoastrocytoma) exhibited decreasing TACs, despite their low-grade status. Thus, the occurrence of decreasing TACs had a PPV of only 74.1 % for detecting HGG, whereas the occurrence of increasing TACs had a NPV of 94.7 %. Among the 13 patients with false-positive FET PET findings, 9 showed a focal uptake pattern and two each showed a diffuse or inhomogeneous pattern. The two false-negative lesions exhibited focal uptake.
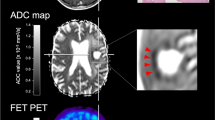
Representative a contrast-enhanced T1-weighted and b T2-weighted MR images suggest the presence of a LGG in the right temporal lobe. While the coregistered conventional PET image c shows diffuse FET uptake typical of LGG tissue, the kinetic analysis of FET-washout d indicates high-grade tumour tissue. Subsequent histopathological evaluation revealed a glioblastoma multiforme
Representative a contrast-enhanced T1-weighted and b T2-weighted MR images show the absence of contrast enhancement (a) and a hyperintense lesion (b) in the left frontal cortex. While the coregistered conventional PET image c shows high focal FET uptake, suggesting malignant glioma, increasing TAC in the kinetic analysis d indicates low-grade tumour tissue. Subsequent histopathological evaluation revealed a WHO grade II glioma
Discussion
In recent years, metabolic imaging with positron-emitting amino acid derivatives has emerged as a powerful complementary tool for the detection and treatment of gliomas [13–23]. Whereas the use of [methyl-11C]-l-methionine has a relatively long history [24], its widespread clinical use is hindered by the requirement for an on-site cyclotron, and it has consequently been largely supplanted by FET and other 18F-labelled amino acids [25–28]. Indeed, FET PET has been reported to be useful in diverse clinical oncology settings, notably for biopsy planning [12, 17, 29], treatment planning [8, 30–32], treatment monitoring, and for differentiation between benign therapy-induced changes and tumour recurrence [5–7, 33–35].
In addition, FET PET also provides clinically useful information for differential diagnosis of newly detected brain lesions, for prognostic evaluation, and for noninvasive tumour grading [10, 11, 36–39]. Whereas most studies have focused on the analysis of static FET uptake images, an additional analysis of FET kinetics has enabled more accurate tumour grading of newly diagnosed as well as recurrent gliomas in individual patients [9, 10]. This approach has been extended further in a study of FET hot spots which represent the most malignant regions of heterogeneous gliomas, and which are delineated with high accuracy through dynamic FET PET kinetic analysis [12]. These findings emphasize the value of dynamic FET PET for optimal target selection in the planning of stereotactic biopsy.
However, in this latter setting of MRI-suspected LGG, the value of dynamic FET PET might not be limited to planning of stereotactic biopsy, which is not performed at all neurosurgical centres. Especially in this setting, a reliable tool for noninvasive tumour grading would be of the utmost utility, since a notable percentage of brain lesions suspicious for LGG according to MRI criteria prove to be malignant upon histological examination [1–4], and early knowledge of malignancy is extremely helpful in achieving optimized patient management. However, previous studies showing superior predictive value of FET kinetics in tumour grading were conducted in relatively small groups of patients with both contrast-enhancing and non-enhancing lesions [9, 10], or focused on the relationship between FET kinetics and histological results in order to evaluate the distribution of kinetic and histopathological landscapes without further information on the FET uptake values or the uptake patterns [12].
Therefore, we elected to assess the informative value of dynamic FET PET and the reliability of kinetic analysis for tumour grading in a larger group of patients with MRI-suspected LGG, with the aim of estimating a priori probabilities for the presence of HGG. We also sought to determine the utility of FET kinetics in the interpretation of lesions with low or diffuse uptake, which can be formally ambiguous unless the full dynamics of tracer uptake and washout are known.
In the present study, more than one-third of our patients proved to have HGG, despite the lack of contrast enhancement on MRI, as also reported in several previous studies [1, 3]. More surprisingly, five of our patients were diagnosed with glioblastoma multiforme, thus stressing that evaluation of MRI alone is not sufficient for accurate tumour grading. We found that the neoplastic lesions in the great majority patients in this study showed positive FET uptake, although in 13 % of the patients the lesions showed normal uptake and in 9 % the lesions actually had reduced uptake. Interestingly, the lesions with reduced uptake were invariably associated with neoplastic histology, including both diffuse and anaplastic astrocytomas. However, the regions of reduced uptake were not associated with necrosis; any finding of necrosis on MRI had been an exclusion criterion, and there were in fact no histologically verified findings of necrotic tissue in the lesions with reduced FET uptake. Thus, the pathophysiological mechanisms leading to decreased FET uptake in certain gliomas remain to be explained, and further molecular and histological investigations would seem to be justified. For the present, we can conclude that not only increased but also reduced FET uptake, i.e. below background, must be considered a pathological finding, and indicates a considerable risk that high-grade tumour tissue is present.
In more than four-fifths of our patients (16/19) with a lesion showing normal FET uptake, the presence of neoplasia was proven through histology. Of all but 1 of these 16 patients, the lesion was graded as LGG. Our conventional PET analysis found increased FET uptake in the tumours of only 69 % of the entire group of 71 patients with LGG, a proportion nearly identical to that reported in an earlier FET PET study from another research group [39]. Notably, we found that almost two-thirds of pure astrocytic LGG showed FET uptake above background, whereas nearly every oligodendroglial LGG had increased uptake. Hence, this study revealed that the sensitivity of FET PET for detecting LGG, although considerably higher than that reported for FDG PET [40], still falls short of providing definitive exclusion of glioma tissue, especially in astrocytic LGG.
FET PET uptake was pathologically increased in 42/47 patients (89 %) with HGG. Although the values of SUVmax/BG, BTV and SUVmean/BG were all higher in HGG than in FET-positive LGG, there were no significant differences between the mean HGG and LGG values for the three parameters derived from static FET images. Upon excluding the oligodendroglial tumours, the differences between astrocytic LGG and HGG reached statistical significance. However, this latter finding does not enable noninvasive tumour grading in the individual patient without knowledge of the histopathological classification. Furthermore, tumour grading by conventional PET analysis was doubtlessly hampered by the occurrence of high interindividual variability in FET uptake and a large overlap between the appearance of FET PET images in LGG and HGG. This phenomenon has also been noted in previous FET PET studies of newly diagnosed gliomas with and without contrast enhancement [10, 11]. Surprisingly, we failed to find different incidences of diffuse, focal or heterogeneous uptake in the LGG and HGG, and therefore could not accurately predict tumour grading on the basis of visual assessment.
The most important factor for the differentiation of LGG and HGG proved to be the analysis of FET kinetics. We have previously described this approach in smaller patient cohorts with untreated or recurrent glioma [9, 10], which included patients with contrast enhancing lesions suspicious for HGG. In the earlier reports of FET kinetics we found increasing TACs in LGG patients, whereas HGG patients were characterized by decreasing TACs. The pathophysiological mechanisms resulting in different kinetic behaviours of LGG and HGG are still not fully clarified. Phenomena such as higher regional blood volume due to hypervascularization, increased angiogenesis and higher intratumoral microvessel density as well as the facilitated bidirectional amino acid transport have been suggested to influence the TACs as a result of high FET uptake in the early scan phase and rapid wash-out of intracellularly accumulated unmetabolized FET in HGG [9, 10]. In addition, disruption of the BBB has been considered a factor in the faster decrease in the TACs in HGG since it eases the passive back-diffusion of unbound FET [11]. Interestingly, in the present study with a focus on non-contrast-enhancing lesions, we found the same high sensitivity for detecting HGG as in the previous studies of cohorts with largely contrast-enhancing lesions. Since the BBB is disrupted only in patients with contrast-enhancing lesions, we can conclude that the slope of the FET TACs must be dependent on factors other than BBB integrity.
On the basis of decreasing TACs, our kinetic analysis identified HGG with a sensitivity of 95 %. It is a matter of particular interest that this very high sensitivity applied equally to the lesions with only low or diffuse uptake patterns in static images (Figs. 2 and 3). Consequently, we stress that FET kinetics can identify as HGG lesions that might otherwise not have been suspected of harbouring malignant tumour tissue. On the other hand, the presence of increasing TACs indicated LGG with high accuracy. Specifically, the NPV for identifying lesions with increasing TACs as HGG was 95 %. In contrast, the corresponding PPV for identifying lesions with a decreasing TACs as HGG was only 74 %. Here, it should be noted that half of the false-positive lesions histologically showed oligodendroglial components, in agreement with findings of another ongoing FET PET investigation, in which we observed a high false-positive rate in oligoastrocytomas and oligodendrogliomas. The rapid FET washout seen in many in oligodendrogliomas could be due to effects of blood flow, which tends to be higher in this tumour type than in astrocytomas [41, 42]. Considering the other false-positive lesions in our study, one patient progressed from diffuse astrocytoma to glioblastoma multiforme in only 10 months, and another patient showed remarkably high proliferative activity by Ki67 histology of 8 % despite a diagnosis of WHO grade II astrocytoma. Also, one of the patients with ganglioglioma (WHO grade I) and decreasing TACs progressed to gangliosarcoma (WHO grade IV) within 1.5 years. These findings imply that decreasing FET kinetics in low-grade tumours might represent an unfavourable prognostic factor, an intriguing notion which remains to be investigated in future prospective studies.
Notwithstanding the very high sensitivity of FET for detecting HGG, we do note that 4 of 97 FET-positive lesions were found to be non-neoplastic, indicating that increased FET uptake is not absolutely specific for neoplasia. Although FET generally exhibits only low uptake in most inflammatory cell types [43, 44], FET uptake may be enhanced in the presence of activated microglia and/or reactive astrocytes. This association is evident through the increased uptake seen in demyelinating lesions, brain abscesses, and ischaemic lesions [36, 39, 45, 46].
Conclusion
In the setting of newly diagnosed MRI-suspected LGG, positive FET PET provides an admirably high PPV (96 %) for the detection of neoplastic lesions. Whereas conventional FET PET analysis (uptake values and uptake pattern) failed to differentiate accurately between LGG and HGG, kinetic analysis showed that lesions with increasing TACs are most likely nonmalignant (NPV 95 %), and lesions with decreasing TACs are usually HGG (PPV 74 %). The sensitivity of kinetic analysis for identification of HGG (95 %) was equally high in lesions with only low or diffuse tracer uptake. Lesions with reduced tracer uptake must be interpreted with caution, as they can even be HGG, whereas a normal FET PET scan seems to bear the lowest risk for presence of HGG.
References
Ginsberg LE, Fuller GN, Hashmi M, Leeds NE, Schomer DF. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol. 1998;49:436–40.
Kondziolka D, Lunsford LD, Martinez AJ. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg. 1993;79:533–6.
Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology. 2002;59:947–9.
Barker 2nd FG, Chang SM, Huhn SL, Davis RL, Gutin PH, McDermott MW, et al. Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer. 1997;80:936–41.
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31:1464–70.
Rachinger W, Goetz C, Popperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505–11.
Popperl G, Goldbrunner R, Gildehaus FJ, Kreth FW, Tanner P, Holtmannspotter M, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2005;32:1018–25.
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, et al. FET-PET for malignant glioma treatment planning. Radiother Oncol. 2011;99:44–8.
Popperl G, Kreth FW, Herms J, Koch W, Mehrkens JH, Gildehaus FJ, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47:393–403.
Popperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34:1933–42.
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K, et al. O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging. 2005;32:422–9.
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13:307–16.
Coope DJ, Cizek J, Eggers C, Vollmar S, Heiss WD, Herholz K. Evaluation of primary brain tumors using 11C-methionine PET with reference to a normal methionine uptake map. J Nucl Med. 2007;48:1971–80.
Floeth FW, Pauleit D, Wittsack HJ, Langen KJ, Reifenberger G, Hamacher K, et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005;102:318–27.
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–22.
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10:7163–70.
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–87.
Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir (Wien). 2009;151:1377–83.
Thiele F, Ehmer J, Piroth MD, Eble MJ, Coenen HH, Kaiser HJ, et al. The quantification of dynamic FET PET imaging and correlation with the clinical outcome in patients with glioblastoma. Phys Med Biol. 2009;54:5525–39.
Ullrich RT, Kracht L, Brunn A, Herholz K, Frommolt P, Miletic H, et al. Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med. 2009;50:1962–8.
Vees H, Senthamizhchelvan S, Miralbell R, Weber DC, Ratib O, Zaidi H. Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging. 2009;36:182–93.
Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27:542–9.
la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13:806–19.
Langstrom B, Lundqvist H. The preparation of 11C-methyl iodide and its use in the synthesis of 11C-methyl-L-methionine. Int J Appl Radiat Isot. 1976;27:357–63.
Wienhard K, Herholz K, Coenen HH, Rudolf J, Kling P, Stocklin G, et al. Increased amino acid transport into brain tumors measured by PET of L-(2-18F)fluorotyrosine. J Nucl Med. 1991;32:1338–46.
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–12.
Ishiwata K, Kubota K, Murakami M, Kubota R, Sasaki T, Ishii S, et al. Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med. 1993;34:1936–43.
Langen KJ, Jarosch M, Muhlensiepen H, Hamacher K, Broer S, Jansen P, et al. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl Med Biol. 2003;30:501–8.
Plotkin M, Blechschmidt C, Auf G, Nyuyki F, Geworski L, Denecke T, et al. Comparison of F-18 FET-PET with F-18 FDG-PET for biopsy planning of non-contrast-enhancing gliomas. Eur Radiol. 2010;20:2496–502.
Plotkin M, Gneveckow U, Meier-Hauff K, Amthauer H, Feussner A, Denecke T, et al. 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperthermia. 2006;22:319–25.
Rickhey M, Koelbl O, Eilles C, Bogner L. A biologically adapted dose-escalation approach, demonstrated for 18F-FET-PET in brain tumors. Strahlenther Onkol. 2008;184:536–42.
Weber DC, Zilli T, Buchegger F, Casanova N, Haller G, Rouzaud M, et al. [(18)F]Fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol. 2008;3:44.
Popperl G, Gotz C, Rachinger W, Schnell O, Gildehaus FJ, Tonn JC, et al. Serial O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging. 2006;33:792–800.
Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6:648–57.
Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52:856–64.
Floeth FW, Pauleit D, Sabel M, Reifenberger G, Stoffels G, Stummer W, et al. 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med. 2006;47:776–82.
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ, et al. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48:519–27.
Floeth FW, Sabel M, Stoffels G, Pauleit D, Hamacher K, Steiger HJ, et al. Prognostic value of 18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific incidental brain lesions. J Nucl Med. 2008;49:730–7.
Pichler R, Dunzinger A, Wurm G, Pichler J, Weis S, Nussbaumer K, et al. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur J Nucl Med Mol Imaging. 2010;37:1521–8.
Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64:227–37.
Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging. 2003;30:868–73.
Nojiri T, Nariai T, Aoyagi M, Senda M, Ishii K, Ishiwata K, et al. Contributions of biological tumor parameters to the incorporation rate of L-[methyl-(11)C]methionine into astrocytomas and oligodendrogliomas. J Neurooncol. 2009;93:233–41.
Kaim AH, Weber B, Kurrer MO, Westera G, Schweitzer A, Gottschalk J, et al. (18)F-FDG and (18)F-FET uptake in experimental soft tissue infection. Eur J Nucl Med Mol Imaging. 2002;29:648–54.
Rau FC, Weber WA, Wester HJ, Herz M, Becker I, Kruger A, et al. O-(2-[(18)F]Fluoroethyl)-L-tyrosine (FET): a tracer for differentiation of tumour from inflammation in murine lymph nodes. Eur J Nucl Med Mol Imaging. 2002;29:1039–46.
Salber D, Stoffels G, Pauleit D, Reifenberger G, Sabel M, Shah NJ, et al. Differential uptake of [18F]FET and [3H]l-methionine in focal cortical ischemia. Nucl Med Biol. 2006;33:1029–35.
Salber D, Stoffels G, Pauleit D, Oros-Peusquens AM, Shah NJ, Klauth P, et al. Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med. 2007;48:2056–62.
Acknowledgments
The study was funded in part by the German Glioma Network, supported by German Cancer Aid (Deutsche Krebshilfe 70-3163-Wi 3).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jansen, N.L., Graute, V., Armbruster, L. et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET?. Eur J Nucl Med Mol Imaging 39, 1021–1029 (2012). https://doi.org/10.1007/s00259-012-2109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2109-9