Abstract
Meningiomas that progress after standard therapies are challenging with limited effective chemotherapy options. This phase II trial evaluated the efficacy of everolimus plus bevacizumab in patients with recurrent, progressive meningioma after treatment with surgical resection and local radiotherapy when appropriate. Patients with recurrent meningioma (WHO grade I, II, or III) following standard treatments with surgical resection and radiotherapy received bevacizumab (10 mg/kg IV days 1 and 15) and everolimus (10 mg PO daily) each 28 day cycle. Evaluation of response occurred every 2 cycles. The primary endpoint was progression-free survival (PFS). Secondary endpoints included response rate, overall survival and safety. Seventeen patients with a median age of 59 years (29–84) received study treatment. WHO grades at study entry included: I, 5 (29 %); II, 7 (41 %); III, 4 (24 %); unknown, 1 (6 %). Patients received a median of 8 cycles (1–37); all patients are off study treatment. A best response of SD was observed in 15 patients (88 %), and 6 patients had SD for >12 months. Overall median PFS was 22 months (95 % CI 4.5–26.8) and was greater for patients with WHO grade II and III compared to grade I tumors (22.0 months vs 17.5 months). Four patients discontinued treatment due to toxicity (proteinuria, 2; colitis, 1, thrombocytopenia, 1). However, other grade 3 toxicity was uncommon, and no patient had grade 4 toxicity. The combination of everolimus and bevacizumab was well-tolerated, and produced stable disease in 88 % of patients; the median duration of disease stabilization of 10 months (2–29). The median PFS from this prospective trial was similar to previous retrospective reports of bevacizumab in the treatment of recurrent meningioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are tumors that arise from the meninges and account for 13–26 % of central nervous system tumors [1]. The World Health Organization (WHO) criteria are used to classify these tumors by morphologic features into three categories: I—Benign; II—Atypical and III—Malignant [2]. Prognosis and treatment differ across categories. WHO grade I tumors are generally asymptomatic and slow growing, while WHO grade II and III tumors are more likely to be invasive, recur following initial treatment, and are associated with a shorter overall survival (OS) than WHO grade I tumors [3]. While the initial and subsequent treatment approaches for patients with meningioma includes surgical resection, if possible, and radiation, there are no defined standard treatments for patients whose disease recurs after these modalities fail.
Angiogenesis is vital to the growth of tumors and higher grade meningiomas are highly vascular tumors. These grade II and III tumors in particular are characterized by high microvascular density and display upregulation of hypoxia and angiogenesis-related factors [4, 5]. Reszec et al. reported that vascular endothelial growth factor (VEGF) expression (assessed by IHC) was increased in 29/43 high grade meningiomas (67.4 %) while only 29/93 low grade meningiomas (30.1 %) expressed VEGF. They noted that more intense VEGF expression was seen in recurrent and anaplastic meningiomas and also noted a statistically significant correlation between VEGF expression and tumor grading [6]. Other groups have correlated increased VEGF expression with increased peritumoral brain edema [7–9] in this patient population. Similarly, it is known that activation of mTORC1 occurs in high grade meningiomas, and a significant dose-dependent growth inhibition by temsirolimus and everolimus has been observed in all meningioma cell lines tested [10].
Clinical trials in patients with refractory meningioma have also suggested a possible role for angiogenesis inhibitors. Results from a phase two trial with sunitinib in grade II/III meningioma patients revealed a median progression-free survival (PFS)-6 of 36 % and a median PFS of 5.1 months [11]. Vatalanib, a tyrosine kinase inhibitor of the vascular endothelial growth factor receptor (VEGFR), showed modest activity in patients with refractory meningioma [12]. Two retrospective studies evaluating the role of bevazicumab have also been reported: the first one evaluated patients with atypical and anaplastic meningiomas treated with bevacizumab and showed a median PFS of 26 weeks, with a PFS-6 rate of 43.8 % [13]. In the second retrospective review, patients with recurrent meningioma who were treated with bevacizumab had an overall median PFS and a PFS-6 of 17.9 months and 85.7 %, respectively. Patients with grade II/III meningioma had a slightly longer median PFS (15.8 months) than grade I patients (12.2 months) [14].
Furtner et al. recently published a retrospective analysis of serial cranial MRI in patients with WHO II and III recurrent meningioma treated with systemic therapy [15]. They conducted a detailed analysis of the MRI images obtained before, during and after treatment and measured the tumor volume, maximum tumor diameter and volume of peritumoral edema. Using this information, they calculated growth rates of tumor diameter and tumor volume and observed that the most pronounced decrease in growth rates was seen in patients treated with bevacizumab. There was some drug induced tumor growth inhibition with other systemic therapies, albeit considerably smaller than seen with bevacizumab.
Inhibition of mTOR with everolimus and VEGF-A ligand with full dose bevacizumab has been a strategy that has shown some activity in kidney cancer and glioblastoma (GBM) patients. This combination was administered as maintenance therapy after concurrent radiotherapy, temozolomide and bevacizumab in the first-line setting [16]. Hence, this phase II trial was designed to evaluate the efficacy of this combination for patients with refractory meningioma, with PFS as the primary endpoint. Assessment of the safety of this regimen, which is well tolerated in other disease indications, was a secondary objective.
Patients and methods
This phase II multi-centered, open-label study (NCT00972335) was conducted in accordance with all applicable regulatory guidelines, and under the guidance of the Declaration of Helsinki [17]. The study was conducted at nine sites of the Sarah Cannon Oncology Research Consortium; institutional review boards at each site granted approvals prior to the consenting of patients. The primary and secondary endpoints were PFS and safety respectively.
Patient selection
Adult patients with symptomatic WHO grades I, II, or III progressive or refractory meningioma for which they had received up to one prior systemic regimen were enrolled. Patients must have undergone surgical resection, if possible, and definitive radiotherapy, when appropriate, for unresectable or recurrent disease. Resection and radiation must have been completed at least 4 and 2 weeks, respectively, prior to study treatment. Furthermore, patients were required to have measurable disease, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and adequate hematologic, renal, and hepatic function. The ability to swallow and retain whole pills was required, and prior treatment with non-approved or investigational agents must have been completed >4 weeks before beginning study treatment. Prior treatment with bevacizumab, other anti-angiogenesis agents, or mTOR inhibitors was not allowed. Women of child-bearing potential were required to have a negative serum pregnancy test.
Treatment and dose modification
Bevacizumab was provided by Genentech and was administered at 10 mg/kg by intravenous (IV) infusion on days 1 and 15 of each 28-day cycle. Patients were instructed to take everolimus 10 mg orally (PO), provided by Novartis, at the same time once daily, swallowed whole with a glass of water. Growth factors were not administered prophylactically; however, use of these agents to treat hematologic toxicity was at the discretion of the treating investigator.
Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3. No more than two dose reductions were allowed for the 10 mg daily dose of everolimus (first reduction, 5 mg daily; second reduction, 5 mg every other day). Patients who discontinued everolimus because of toxicity were allowed to continue treatment with bevacizumab as long as tumor progression was not evident and tumor-related symptoms were not present. With the exception of a > 10 % reduction in a patient’s baseline weight, there were no dose reductions allowed for bevacizumab. If bevacizumab was held because of toxicity, the dose remained the same if treatment resumed.
Patients were evaluated for response to treatment following the completion of 2 cycles (8 weeks) by magnetic resonance imaging (MRI) using the MacDonald criteria [18]. Treatment continued in the absence of disease progression or unacceptable toxicity, with MRI evaluations repeated every 2 cycles to assess response to treatment.
Assessments
Prior to the initiation of treatment, all patients underwent a complete medical history, physical examination, standard laboratory tests, assessment of vital signs and ECOG performance status. These assessments were repeated at the beginning of each 28-day treatment cycle and at the end of treatment. A single electrocardiogram was performed at screening and at the end of treatment. A computed tomography (CT) scan of the chest or chest radiograph was required every 2 cycles to monitor patients for pneumonitis.
There is no treatment for refractory meningioma that has been shown to be effective and the estimated PFS for these patients is 2 months. It was anticipated that the median PFS of patients in this study receiving the combination of bevacizumab and everolimus would be 4 months. To demonstrate this improvement in PFS at an alpha level of 0.05 and 85 % power, the treatment of 37 evaluable patients was required. In order to account for a 10 % rate of inevaluable patients, accrual of 41 patients was planned.
PFS was defined as the time from randomization until objective disease progression or death. Patients for whom no date of progression or death was captured were censored on the date of the last adequate disease assessment. Patients were also censored if subsequently treated following discontinuation from study. PFS was estimated using the method of Kaplan and Meier [19].
Tumor burden was assessed in selected patients by reviewing the MRI scans of the lesions. In eight patients, the radiologist selected the patients’ most prominent lesions (between one and seven lesions were selected per patient) and provided measurements in three dimensions for each lesion. For each lesion, an ellipsoid volume was calculated using the formula below and the overall tumor burden was determined by summing the volume of each lesion. Overall tumor burden was calculated for each scan assessed while the patient was on the study protocol as well as for scans preceding study treatment. The earliest overall tumor burdens were calculated 1188 ± 198 days prior to C1D1 of study treatment. Using a linear regression, the rate of increase or decrease of each patient’s overall tumor burden was calculated and found to be 0.0167 ± −0.0053 cm3/day prior to initiating treatment on the study protocol. However, overall tumor burden was found to decrease while on treatment at a rate of −0.0029 ± 0.261 cm3/day (p = 0.46 t test).
\(V=\frac{4}{3}\pi \frac{AP}{2}\frac{T}{2}\frac{CC}{2}\text{C}\)
Results
Patient characteristics and treatment received
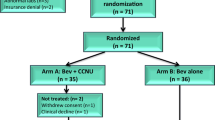
Between January 2010 and December 2011, 18 patients were enrolled in this study. Prior to treatment, 1 patient was found to be ineligible (no evidence of disease on baseline brain MRI) and is not included in the study analysis. The projected patient population of 41 was not reached as a result of the trial closing early due to slow accrual. Baseline characteristics of the 17 treated patients are summarized in Table 1. Twelve patients (70 %) had baseline ECOG performance status of zero or one and seven patients (41 %) had atypical (WHO grade II) tumors at study entry. Three patients (18 %) had undergone complete resection and subsequently relapsed prior to study entry; one patient (6 %) was unresectable due to tumor location. Thirteen patients (76 %) underwent partial surgical resection. Twelve patients (70 %) had received prior radiotherapy, ten patients (59 %) had received prior radiosurgery. Additional prior therapy details are included in Table 2.
Patients received a median of 8 cycles (range 1–37) of treatment with bevacizumab and everolimus. All patients are off treatment; reasons for treatment discontinuation include disease progression (six patients, 35 %), intercurrent illness (two patients, 12 %), patient request (two patients, 12 %), death due to disease, investigator discretion and patient withdrew consent (one patient, 6 % each). Four patients (22 %) discontinued treatment due to toxicity (proteinuria, 2; colitis, 1; thrombocytopenia, 1).
Efficacy
Sixteen patients (94 %) received at least 2 cycles and were evaluated for response to treatment (Table 3). One patient (6 %) expired due to disease after 1 cycle of treatment and was not evaluated. There were no objective responses to treatment. Fifteen patients (88 %) had stable disease with a median duration of 10 months (range 2–29 months). Figure 1 depicts the total disease burden relative to the tumor burden at baseline from the pre-treatment period through the duration on study treatment in eight patients. The data presented in the figure demonstrates tumor growth stabilization in some patients after receiving treatment. This is most clearly seen in patient number ten represented by the curve in red color in the figure.
Tumor growth stabilization during study treatment. The data presented in Fig. 1 shows that, in general, the tumor burden for the patients stabilized upon receiving study treatment (patient numbers in the graph correspond to the patient numbers noted in Table 2 in the manuscript). This is most clearly seen in patient number 2 represented by the curve in red color in the graph
The PFS is shown in Fig. 2. At a median follow up of 20 months (range 4–31), the median PFS was 22 months (95 % CI 4.5–26.8). The 6-, 12-, and 18-month PFS rates were 69, 57 and 57 %, respectively.
Patients with WHO grade II/III tumors appeared to have a longer median PFS than patients with grade I tumors (22 months vs 17.5 months). Figure 3 shows the OS. Median OS was 23.8 months (95 % CI 9.0–33.1). The OS rate at 18 months was 69 %.
Adverse events
Treatment-related toxicities are shown in Table 4. One patient experienced grade 3 thrombocytopenia. Grade 1/2 thrombocytopenia was the most common hematologic toxicity, observed in nine patients (53 %). Severe non-hematologic events were uncommon, with no grade 4 events, and proteinuria was the only adverse event observed in two or more patients (2, 12 %). Four patients discontinued treatment due to toxicity: Grade 3 colitis, Grade 3 chronic thrombotic microangiopathy/Grade 3 proteinuria, Grade 2 proteinuria/nephrotic syndrome, and Grade 3 thrombocytopenia (one patient each).
Discussion
There have been two retrospective studies published evaluating the role of bevacizumab in patients with recurrent meningioma [13, 14]. To our knowledge, this is the first prospective trial to evaluate a bevacizumab-based regimen in patients with recurrent meningioma, albeit with small patient numbers. The results from our prospective trial with the combination of bevacizumab and everolimus in refractory meningioma patients compare favorably to the two published retrospective trials, with a median PFS of 22 months (95 % CI 4.5–26.8) and a median OS of 23.8 months (95 % CI 9.0–33.1). Over two-thirds of the 17 patients treated with this combination had WHO grade II or III tumors at study entry. While there were no objective responses documented, 15 patients (88 %) had stable disease as the best response. In six of these 15 patients, the duration of stable disease was >12 months. The median PFS in patients with WHO grade II/III tumors was slightly higher than in patients with WHO grade I tumors (22 months vs 17.5 months).
We hypothesize that these higher grade tumors which are more vascular are also potentially more dependent upon downstream mTOR and AKT signaling may therefore also respond better to anti-angiogenic therapy and mTOR inhibition. Normally grade II and III meningiomas have a lower PFS than grade 1 tumors; but our study suggests that treatment of these higher grade meningiomas with the combination of bevacizumab and everolimus improves the median PFS similar to that of the low-grade meningiomas. However, the sample size is small and therefore conclusions ought to be drawn with caution. Larger trials in this population of patients would be needed to confirm this hypothesis.
Recurrent meningioma patents treated with bevacizumab and everolimus experienced tumor growth stabilization as seen from the data presented in Fig. 1. This is especially apparent in patient #2 who had a steady increase in tumor growth prior to study entry but this increase was stemmed upon receiving 2 cycles of study treatment; a similar but less dramatic change in the pace of tumor growth was observed in patient #14. Furtner’s retrospective analysis also performed volumetric analysis of tumor and edema during and after treatment and found the greatest tumor inhibition in patients receiving bevacizumab. These data corroborate with our observations of tumor growth stabilization in meningioma patients who received bevacizumab plus everolimus.
Adverse events did not significantly limit the ability for patients to receive the combination of bevacizumab and everolimus. Four patients (22 %) discontinued treatment due to toxicity, and there were no treatment-related deaths. The incidence and severity of reported events were consistent with the known toxicity profiles of bevacizumab and everolimus, both as single agents and when used in combination.
To summarize, the results from this prospective trial are consistent with the data from other small retrospective studies with bevacizumab in recurrent meningioma patients. Treatment with bevacizumab and everolimus did not produce any objective tumor responses, but resulted in prolonged disease stability by (>12 months) in six patients (35 %). The combination of the two targeted therapies was well tolerated in most patients; bevacizumab-related toxicities were limited to proteinuria and hypertension.
References
Whittle IR, Smith C, Navoo P, Collie D (2004) Meningiomas. Lancet 363:1535–1543
Perry A, Louis D, Scheithauer B et.al (2007) Meningiomas. In: Louis D, Ohgaki H, Wiestler O (eds) WHO classification of tumors of the central nervous system. IARC Press, Lyon, p 164
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99:307–314
Preusser M, Berghoff AS, Hottinger AF (2013) High-grade meningiomas: new avenues for drug treatment? Curr Opin Neurol 26:708–715. doi:10.1097/WCO.0000000000000035
Pistolesi S, Boldrini L, Gisfredi S et al (2004) Angiogenesis in intracranial meningiomas: immunohistochemical and molecular study. Neuropathol Appl Neurobiol 30:118–125
Reszec J, Hermanowicz A, Rutkowski R (2015) Expression of MMP-9 and VEGF in meningiomas and their correlation with peritumoral brain edema. Biomed Res Int 2015:646853
Otsuka S, Tamiya T, Ono Y et al (2004) The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol 70:349–357
Provias J, Claffey K, delAguila L et al (1997) Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery 40:1016–1026
Yoshioka H, Hama S, Taniguchi E et al (1999) Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer 85:936–944
Pachow D, Andrae N, Kliese N et al (2013) mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res 19:1180–1189
Kaley TJ, Wen P, Schiff D et al (2015) Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol 17:116–121
Raizer JJ, Grimm SA, Rademaker A et al (2014) A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol 117:93–101
Nayak L, Iwamoto F, Rudnick J et al (2012) Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol 109:187–193
Lou E, Sumrall A, Turner S et al (2012) Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol 109:63–70
Furtner J, Schopf V, Seystahl K et al (2016) Kinetics of tumor size and peritumoral edema before during and after systemic herapy in recurrent WHO grade II or grade III menigoma. Neuro Oncol 18:401–407
Hainsworth JD, Shih KC, Shepard GC et al (2012) Phase II study of concurrent radiation therapy, temozolomide and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin Adv Hematol Oncol 10:240–246
Declaration of Helsinki. In: www.wma.net/e/ethicsunit/helsinki.htm, World Medical Association, Ethics Unit 2007
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shih, K.C., Chowdhary, S., Rosenblatt, P. et al. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol 129, 281–288 (2016). https://doi.org/10.1007/s11060-016-2172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2172-3