Abstract
There is a lack of long-term information on the use of industry forest residues in Pinus taeda L plantations for purposes of enhancing sustainability. The study goal was to evaluate tree growth and nutrition, soil chemical properties, litter accumulation, and weed occurrence in a Pinus taeda L. system amended with alkaline residues from recycled paper. The residues were broadcasted at planting using different rates (0, 10, 20, 30, and 40 t ha−1) on a sandy soil with low fertility. Tree diameter and height along with soil chemical properties were monitored for 15 years. At 15 years samples of litter, weeds, and soil were collected for evaluation. Over 15 years of monitoring, no changes in tree growth or final yield were observed despite increased Ca and reduced Mn foliar tissue concentrations. Low Mg concentration in needles combined with symptoms resembling Mg deficiency and a high increase in Ca levels, suggest that this nutrient could be a limiting factor in tree response. At the same time, a reduction in weed mass with residue application was verified. Residue amendment increased soil Ca and attenuated acidity down to 60 cm after 15 years with the maximum effect on soil properties at ~ 13 years. Litter decreased from 36.2 (control) to 26.9 Mg ha−1 (highest rate) but increased Ca and diminished Al concentrations. The alkaline residue (rich in Ca) improved soil chemical properties at the surface and subsurface, decreased weed growth, increased Ca and reduced Mn in needles, but probably failed to increase tree yield due to an Mg deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World population growth and economic expansion can generate greater wood demand for diverse purposes (FAO 2020). In Brazil, 1.8 million hectares were destined for Pinus cultivation to produce pulp wood and saw timber (IBGE 2021). The majority of Brazilian Pinus taeda plantations were established on acidic soils with inherent natural low fertility without fertilizer and lime additions (Ferreira et al. 2001; Batista et al. 2015). In the US, the low adoption of fertilizer and lime use in Pinus taeda systems could be due to uncertainty of response and degree of tree growth, which varied widely with natural soil fertility and rate and time of application (Gregoire and Fisher 2004; Albaugh et al. 2012). Additionally, the use of fertilizers and soil acidity correctives in pine plantations was often unfeasible due to product costs (Vance 2000). In Brazil, Pinus growth enhancements have been observed (Moro et al. 2014; Consalter et al. 2021b) but the cost can be problematic since large portions of fertilizer are imported at a higher price (Simões et al. 2018).
An alternative to forest fertilization is the use of industrial residues since the forest industry generates large amounts of residue waste (Bellote et al. 1998). Furthermore, the disposal of waste residues in planted forests resolves legal, environmental, and financial issues by reducing or eliminating the need for the construction and maintenance of landfills (Maeda et al. 2011). Forest residue use has also been shown to improve soil fertility with or without tree yield enhancements (Paim 2007; Fonseca et al. 2012; Rodriguez et al. 2018; Pereira et al. 2021; Quadros et al. 2021). Recycled white paper scraps are a common residue near pulp and paper industries that has alkaline reactivity and high concentrations of calcium (Ca) (Balbinot Junior et al. 2006). This characteristic is very significant since Ca has been exported from forests in large amounts from previous harvests (Sixel et al. 2015) and Ca exhaustion can occur after a few harvest cycles (Gatiboni et al. 2020). Application of alkaline amendments in forest systems, with rates varying from 4 to 15 t ha−1, can have a long residual effect (up to 30 years) on soil and tree nutrition in terms of increasing foliar Ca and reducing toxic Al, Mn, and Fe levels (Borja and Nilsen 2009; Prietzel et al. 2008). The use of high rates of alkaline residues lacking a balanced composition of basic cations can result in nutritional imbalances (Sun and Payn 1999; Beets et al. 2004). Response to the use of amendments also depends on soil and climate characteristics. Application of wood ash to Swedish conifers on a mineral soil resulted in two distinct tree growth responses with increases on fertile sites and decreases on poor soil sites due to increased nitrogen (N) mineralization and immobilization, respectively (Jacobson 2003).
When acidity correctives and alkaline residues were surface applied to forest stands, the litter layer retained the majority of added bases and the alkalizing effect did not attain a full effect at the soil surface (Mizel et al. 2015; Rabel et al. 2021). On one hand, this retention can change litter decay and affect the organic horizon (Prietzel et al. 2008; Zucon et al. 2021), which can result in greater N and phosphorus (P) mineralization (Attiwill and Adams 1993). On the other hand, residue application can increase tree growth and litterfall amounts/maintenance on the forest floor (Wienand and Stock 1995). This change in litter and tree growth combined with shifts in soil fertility can impact weed occurrence. Vance (2000) found increases in weed occurrence when recycled paper waste was utilized. Busby et al. (2019) reported that the use of paper waste residues with low N levels favored the development of native species over invasive or unwanted weed species.
At the soil surface, acidity correctives and alkaline residues react and begin to affect lower soil layers, especially with sandy soils (Gargantini et al. 1982; Wang et al. 2016). This can be monitored by changes in soil pH, bases (Ca, Mg, and K), and exchangeable Al in lower soil layers (Vargas et al. 2019).
Few long-term studies have evaluated the effect of waste residue application on major components of forest systems under subtropical conditions. For this reason, our objective was to evaluate the long-term effects of applying alkaline paper waste on major components of a Pinus taeda forest system: soil, forest floor, herbaceous plants (weed growth and nutrition), and trees (growth and nutrition). Based on the above, we hypothesize that the whole system can be affected by alkaline residues due to improved soil chemical properties in sandy soils with low fertility. This improvement results in enhanced development and nutrition of Pinus taeda, weed suppression, and increased litter decay that diminishes organic forest floor accumulation.
Materials and methods
Study site
The study was conducted in a second rotation commercial area of Pinus taeda L. located in the municipality of Rio Negrinho, state of Santa Catarina, Brazil (26° 33′ 52′′ S and 49° 39′ 45′′ W). The site has an altitude of 1020 m, a Cfb climate (average temperature of 17.2 °C and 1760 mm of rainfall per year), and an undulating landscape relief with soil classified as a typical Humic Distryc Regosol with a sandy clay loam texture and a well-developed humic A horizon (> 50 cm depth) over a C horizon.
Experimental design
The experiment was initiated in 2006 and had randomized blocks (4 replications) with five treatments of increasing rates of alkaline residue waste from recycled paper (ARPR): T1 = 0 t ha−1, T2 = 10 t ha−1, T3 = 20 t ha−1, T4 = 30 t ha−1, and T5 = 40 t ha−1. Chemical characteristics of the material are shown in Table 1. Each experimental plot consisted of 25 trees (2.5 × 2.5 m spacing). For evaluations, the 9 central trees within each plot were utilized. Previous native vegetation in the study area was an Ombrophilous mixed subtropical Araucaria angustifolia (Bertol) forest. The native vegetation was cut and replaced by a secondary Mimosa scabrella Benth forest for several cycles. After mimosa harvest and wood extraction, Pinus elliottii seedlings were manually planted and harvested after 20 years. Following this, Pinus taeda seedlings were manually planted to establish the current study area. In addition to canopy shading, weeds were also controlled by two sequences of herbicide application followed by mowing during the initial two years. During this whole period, the experimental area never received fertilizer or lime.
Growth data
Biometric data (height and DBH-diameter at breast height) of the 9 central trees were collected annually until the year 2013, followed by measures in 2016, 2017, and 2020. Tree height measurements were initially conducted using an extension ruler. DBH was assessed with a tape measure at 1.30 m above the ground. Tree heights and volumes were estimated with adjusted equations used by the commercial company during forest inventories (Table 2). In April 2021, the dominant tree (greatest apparent height and DBH criterion) in each experimental plot was harvested to collect needles.
Soil
In April of 2021, samples for analyzing soil attributes were collected at 5 depths (0–5 cm; 5–10 cm; 10–20 cm; 20–40 cm; and 40–60 cm) and were composed of 4 subsamples collected from respective plots. Samples were oven dried at 65 °C for 48 h, crushed, and sieved (2 mm mesh). Based on the methodology described by Marques and Motta (2003), soil chemical analysis consisted of the following: pH in 0.01 M of CaCl2 (ratio 1:2.5), pH SMP, exchangeable aluminum (Al), calcium (Ca), magnesium (Mg), potassium (K), available phosphorus (P), manganese (Mn), copper (Cu), and zinc (Zn). Available P, K, and micronutrients were extracted by Mehlich I and determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, Varian, model 720-ES). K was determined by flame photometry (Digimed DM-62). Exchangeable Al was extracted by 1 M KCl and titrated with 0.2 M NaOH.
The fractions considered for Ca were acid soluble, exchangeable, residual, and soluble. The acid soluble fraction was composed of the exchangeable fraction plus what was soluble in a strong acid (residual); this fraction used a Mehlich I extraction and determinations were done by ICP-AES. The exchangeable fraction was extracted using 1 M KCl and determined by titration with 0.0125 M EDTA. The residual fraction was determined by the difference between the acid soluble fraction and the exchangeable fraction with KCl. The soluble fraction was extracted with water (1:5 ratio) and determined by ICP-AES (Ballard and Pritchett 1975). Soil texture analysis was performed using the densimeter method (Gee and Bauder 1986).
Due to the large residue influence on Mn concentration in foliar tissue, soil Mn fractionation was done by sequential extractions described by Sims (1986), which consisted of five extraction forms: exchangeable (1 M Mg(NO3)2), organic (5.3% NaOCl, pH 8.5), manganese oxide (0.1 M NH2OH · HCl, pH 2.0), amorphous oxides (0.25 M NH2OH. HCl + 0.25 M HCl), and crystalline oxides fractions (0.2 M (NH4)C2O4 + 0.2 M H2C2O4, pH 3.0). This sequential extraction was done for the 0–5 cm soil layer since this depth was the most affected by residue amendment. All extracts were determined by atomic absorption spectrophotometry (Varian AA 240/280) using standards in the same matrix of each sample.
Over the course of the experiment, soil samples were collected from each plot in 2007, 2008, 2012, and 2016. Samples were collected at the 0–20 cm depth and analyzed for pH in 0.01 M of CaCl2 (ratio 1:2.5), pH SMP, exchangeable aluminum (Al), calcium (Ca), magnesium (Mg), potassium (K), and available phosphorus (P). The aluminum saturation or percentage of effective CEC load (effective CEC = Ca + Mg + K + Al) occupied by Al was calculated from the equation: m% = Al/effective CEC*100. The base saturation (V%) or percentage of base in CEC pH 7.0 (pH 7.0 CEC = Ca + Mg + K + H + Al) was established by the following equation: V% = Ca + Mg + K/CEC pH 7.0 *100.
Needles
To determine the concentration of nutrients in needles, 6 branches of the upper third crown were collected from each cardinal point within the canopy of felled trees. Needles were separated in first and second needle flushes. Samples were washed with deionized water and dried in a forced ventilation oven (65 °C) until they reached constant weight. One hundred needles were separated and weighed to determine dry mass using a precision scale. After drying, the material was ground in an electric grinder and analyzed (Martins and Reissman 2007). For obtained extracts, levels of Ca, Mg, P, K, Fe, Cu, Mn, Zn, Ni, and Al were determined by ICP-AES (Varian, model 720-ES). Needle pH was also determined by placing crushed material in water (1:10 ratio) and leaving samples in equilibrium for 30 min after shaking prior to pH determinations (Melvin et al. 2013).
Litter
Litter samples were randomly collected from 4 points in each experimental plot using a 20 × 20 cm template and a saw knife for residue cutting. In the laboratory, material fractions were separated and characterized as fresh litter (newly deposited material, without signs of decomposition corresponding to the Oi horizon) and decomposed litter (corresponding to the Oe horizon). O horizons were dried in an oven with forced air ventilation (65 °C) until constant weight prior to dry mass determinations. After drying, samples were ground in an electric grinder. For obtained extracts, levels of Ca, Mg, P, K, Fe, Cu, Mn, Zn, Ni, and Al were determined by ICP-AES (Varian, model 720-ES). Litter pH was also determined using the same methodology described above (Melvin et al. 2013).
Weed cover
Within each plot, herbaceous plants were randomly collected from a 1 m2 area by cutting plants at ground level. Samples were dried in an oven with forced air circulation (65 °C) until attaining a constant weight. After drying, samples were ground in an electric grinder. For obtained extracts, levels of Ca, Mg, P, K, Fe, Cu, Mn, Zn, Ni, and Al were determined by ICP-AES (Varian, model 720-ES) (Martins and Reismann 2007).
Statistical analysis
Data were submitted to a residual normality test (Shapiro–Wilk) and homogeneity of variance (Bartlett) test. ANOVA was performed and data were analyzed by regression and Pearson correlation. Soil collection depths were analyzed as subplots. For soil attributes and biometric measurements over the years, data were analyzed by a maximum likelihood linear mixed model approach, with plot as a random effect and a first order-auto regressive covariance structure. Statistical analyses were performed using R software (version 4.0.0.).
Results
Growth data
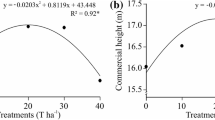
There was no effect of ARPR on tree growth (CH and DBH) parameters during the 15 yr monitoring period (Fig. 1). In addition, there was no change in tree growth at the final evaluation (15th yr); overall mean values were 15.36 m, 21.58 cm2, and 0.30 m3 for the commercial height, DBH, and volume per tree, respectively (Table 3).
Soil
Soil showed a high degree of acidity with a pH lower than 4.0 in the control (Table 4). Residue use increased pH to a 20 cm depth suggesting a diminishing effect as a function of depth (Table 4). For the highest ARPR rate, the greatest increases were in the 0–5 cm soil layer with an increase of approximately 3 pH units followed by decreases of 1.27, 0.30, and 0.16 units for the 5–10, 10–20, and 20–40 cm soil layers, respectively.
The low soil pH resulted in very high Al values in the control, indicating high buffering power down to the 40–60 cm soil layer. Furthermore, the soil showed a high percentage of exchange points occupied by Al with a saturation above 90% along the profile (Table 4). In contrast, very low values of base saturation (close to 1%) indicated an almost absolute predominance of H and Al at exchange points. The use of residue resulted in the complete neutralization of Al in the 0–5 cm soil layer and decreases in the other soil layers; the same occurred for Al saturation given the decrease in Al combined with an increase in Ca. This indicated a decrease in Al, even for the 40–60 cm soil layer (Table 4), while base saturation was affected down to a depth of 20 cm.
Available Mg was very low at the soil surface and decreased even more with depth in the control (Table 4). Residue amendment enhanced exchangeable Mg only within the 0–5 cm soil layer. At the highest ARPR rate, the Mg concentration reached the highest level in the upper soil layer. Levels of K also showed no differences, but concentrations showed a decreasing trend with depth; levels were considered medium in the first two soil layers and decreasing values were noted in the lower soil layers (Table 4). For P, there was a significant difference at 5–10 cm where the control had a higher value than the treatment receiving the highest residue rate. High values of organic C were observed down to the 40–60 cm depth layer, which suggested a well-developed A horizon. There was no significant difference at any depth for variable organic carbon (C-org). Exchangeable Ca values in the control had very low records (< 0.5 cmolc dm−3) along the soil profile, whereas plots receiving residues displayed increases since Ca was the most abundant element in the applied residue. Residue use led to increases in Ca values from very low to high and medium levels within the 0–5 and 5–10 cm soil layers, respectively.
Regarding Ca forms in soil, significant differences in exchangeable (to 20 cm depth), soluble, and acid-soluble fractions (to 10 cm depth) were verified (Table 5). The majority of Ca was in the exchangeable fraction, with the residual fraction concentrating the highest values primarily near the surface. The linear regression fit for the surficial soil layers in relation to soluble Ca (Table 5), also suggested a residual effect of ARPR material. When using only water as an extractor, it was still possible to notice differences between treatments.
For micronutrients (Table 6), differences in Fe levels were noted and ARPR use resulted in decreased Fe levels to a depth of 20 cm. For Mn, ARPR use promoted an increase only in the first 5 cm of soil, however, changes in Mn forms were noted (Fig. 2, supplementary Table 1). The use of ARPR diminished exchangeable Mn forms, especially at the 30 and 40 t ha−1 rates, which correlated with pH increases. There was an increase in Mn oxide and amorphous oxide forms, but this effect was not significant (Supplementary Material Table 1). Increased pH was accompanied by a percentage decline in Mn exchangeable forms, which led to an increase in the amorphous oxides fraction (Fig. 2). There were no significant correlations between Mn forms in soil and levels found in plants. However, a correlation between soil pH and plant Mn was noted (i.e., r = − 0.50 and − 0.62 for needles at the first and second flush, Supplementary Fig. 3).
Soil sampling timing effect
There was a direct relationship between soil pH and exchangeable Ca based on the amount of residue applied (r = 0.99 Fig.7, Table 7). Soil pH and exchangeable Ca showed a smooth reduction with time (Table 8). The highest ARPR rate continued to promote an increase in pH (even 15 years after application), indicating that the residue reaction was a slow process (Table 9). Furthermore, the time needed to reach maximum pH and Ca values were directly proportional to applied ARPR rates; these values were 6.4, 7.1, and, 8.4 years for pH and 7.7, 10.1, and 9.6 years for exchangeable Ca at rates of 10, 20, and 30 t ha−1, respectively (Fig. 3). Base saturation data indicated intermediate times to reach a maximum value; i.e., 7.4, 8.5, 9.4, and 12.8 years for ARPR rates of 10, 20, 30, and 40 t ha−1, respectively (Fig. 3, Table 9).
Fitted (line) versus observed values (box-plot) in soil pH, Ca content, and base saturation the during 15 years after the application of increasing rates of alkaline residue from recycled paper (ARPR) at a depth of 0–20 cm in the Pinus taeda study in Rio Negrinho, Santa Catarina state, Brazil (n = 4)
Needles
As seen with soil, there was a pronounced effect of ARPR on Ca levels in the first needle flush (Table 10). The opposite was observed for Mn where a decrease in concentration was accompanied by a decrease in soil acidity and exchangeable Ca. Thus, an antagonistic interaction possibly exists between Ca and Mn. However, the same was not true for Al, which was not impacted by residue application despite the decrease in soil acidity and exchangeable Al. Observed concentrations were above or close to the critical levels for Pinus, except for Mg which was below 0.8 g kg−1 (Albaugh et al. 2010); this occurred in both the control and trees receiving ARPR (Table 10). Needle symptoms observed in the field support the possibility of a Mg deficiency (Fig. 4). Other nutrients did not show any response to treatment with ARPR.
Litter
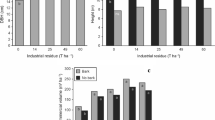
The amount of total litter (Oi + Oe) varied from 25 and 35 t ha−1 and there was a litter decrease of 0.21 t ha−1 for each ton of ARPR applied after 15 years (Fig. 5). The Oe was the most abundant (more than 90% of total litter mass) and displayed a linear decrease close to 0.20 t ha−1 per ton of applied residue. However, the Oi was not influenced by the ARPR application (Fig. 5).
Following results found for soil, there was a large increase in Ca concentration in the Oi (more than two-fold) and Oe (more than three-fold), suggesting that increases in plant uptake influence litter maintenance and fragmentation dynamics (Table 11).
Both litter fractions showed high acidity (pH close to 4) and an acidity decrease associated with ARPR use (Fig. 5). It is important to consider that ARPR was applied at planting, and the litter collected for evaluation was deposited after this application (at 15 year-old). Decreases in acidity occurred both with the Oe directly contacted by ARPR during application and Oi not directly contacted by the application. However, increases in observed pH were less than 0.5 pH units, even with the use of 40 t ha−1. That is, increases in litter pH were much smaller in relation to those observed in soil (Fig. 5).
Weed cover
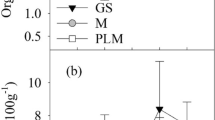
The use of ARPR resulted in a change in weed occurrence in the study area. There was a rapid decrease in weed incidence at the first-rate, followed by an increase in weed occurrence at the highest rate (Fig. 6). Differences between the control and ARPR use (Fig. 6, Table 12) suggest that the nutritional composition of weeds may have been influenced by ARPR use. There was more than a twofold increase in Ca concentration, some increase in Al concentration, a small increase in Cu, and a reduction in Ni.
Correlations
The Ca in the soil that was most correlated with levels in the first needle flush was exchangeable Ca in the 0–20 soil layer (r = 0.64; Supplementary Fig. 4). Needle and weeds Ca level were strongly correlated with Ca in litter (r = 0.79 Oi; r = 0.85 Oe, respectively). Litter pH was correlated with litter Ca (r = 0.88 for Oi; r = 0.93 for Oe) (Fig. 7).
Correlations between Ca levels in soil (exchangeable), needles, litter, weed cover and pH 15 years after application of increasing rate of alkaline residue from recycled paper (ARPR) in the Pinus taeda study located Rio Negrinho, Santa Catarina state, Brazil (n = 20). I = 20 cm soil depth. *** p value < 0.001; ** p value < 0.01; * p value < 0.05; nsnot significant
Discussion
The main effects of ARPR on soil parameters were pH, Ca concentration, base saturation, and Al saturation. Somewhat similar effects have been reported in several studies evaluating the use of waste from pulp and recycled paper industries, and use of these waste stream materials which share corrective characteristics similar to actions displayed by lime applications (Pértile et al. 2012, 2017; Maeda et al. 2011; Rabel et al. 2021).
When soil acidity correctives are broadcasted on the surface, the pH rise occurs gradually within the soil profile. Initially, the surface layer will display a rapid pH increase that is later transmitted to lower depths from the formation and movement of an alkalization front (i.e., due to OH− and HCO3− anion formations). When this zone is corrected (especially at a pH above 5.0), large portions of Al will be hydrolyzed (Caires et al. 2005). Therefore, the use of higher rates of pH correctives has a greater impact on depth due to the rapid formation of an alkalization front on the surface (Hansen et al. 2017). Collectively, these findings corroborate the results obtained in our study.
After 14 years, the effects on exchangeable soil acidity (Al) were observed down to the 60 cm soil layer. Al saturation (m), the major parameter for evaluating Al toxicity, also diminished due to reduction of Al as a result of Al3+ hydrolysis and base elevation (Al saturation considers the bases). With the use of the same waste residue in another Pinus taeda system, Rabel et al. (2021) found a corrective effect only down to the 10 cm soil layer after 10 years on clayey soil. This may be related to the fact that residue application occurred in the third year after planting, whereupon there was already an accumulation of litter from deposition of needles and other plant materials. In the current study, waste residue (ARPR) was used at study initiation thereby providing greater contact with the soil surface, which may have increased waste reactivity and subsequent corrections to soil acidity. Other factors that may have been influential were the sandy soil texture and the continuing reactivity of the waste residue over time (Wang et al. 2016; Vargas et al. 2019).
Another factor that could affect the slow and continuing reactivity of waste residue, was the pH in the zone where the corrective was deposited. Magdoff and Barlett (1985) observed a pH buffering zone around 7.0 (H2O) when corrected with the lime application (carbonate predominating over bicarbonate and hydroxyl groups). In addition, coarse fractions of corrective material can remain intact without reacting with soil (Miller 2015). Residue structure is also important since large residue clumps of white paper residue scraps (a mixture of organic and mineral components) can hinder reactivity with soil (Supplementary Fig. 1). Although this structure was broken down over 15 years, sizable remnants could still be seen during sampling and handling (Supplementary Fig. 2). These factors combined with results on soil pH and Ca content over time indicate that after 15 years, some residue material remains for reacting with soil in terms of alkalization. Borja and Nilsen (2009) related effects of liming even after 35 years, with Ca increasing in needles, and Al, Mn, and Fe decreasing as a function of the pH amendment. The effect over time was dependent on the rate used since only the higher rate showed a linear trend over time, which was similar to ash or lime studies that showed a sustained effect over time at higher application rates (Gascho and Parker 2001; Hansen et al. 2017). The acidic extraction of Mehlich I was capable of evaluating the amount of residual from the waste residue. The alkalization front will continue to gradually and slowly correct deeper soil layers as a result of hydroxyl and bicarbonate leaching.
In this study, no differences were found ion Pinus taeda growth in relation to ARPR use on a low-fertility soil. This occurred despite improvements in soil chemical properties and foliar composition. Responses of Pinus taeda to residue application in Brazil have been variable, with absences of response observed with the use of vegetable ash and green liquor dregs residue from Kraft pulp mills (DREGS) (Pértile et al. 2012; Quadros et al. 2021). On the other hand, increases in Pinus taeda log volume up to 16% have been reported with the same residue waste used in our study (Rabel et al. 2021), while increases up to 127% in log volume have been found with the use of composted cellulosic waste (Rodriguez et al. 2018). It is important to highlight that the two previous studies did not use fertilizer and liming.
Such variability in response may be related to residue (quality and rate), soil, and plant factors. The quality of the residue in terms of supplying nutrients and reducing toxic elements must be considered. Cellulosic residues, especially when composted (e.g., Rodriguez et al. 2018), have high levels of N and P when compared to residues of ash, DREGS, and GRITS (residues from the quicklime slaking process) and the white paper scrap residues used in our study. Furthermore, the response can vary according to the natural fertility of the soil, which is influenced by weathering of primary parent minerals that release non-exchangeable forms of bases in soil solution that contribute to the nutrition of long-cycle crops (Melo 1995). Residues can also be used as a carrier for soluble fertilizer resulting in a slow release of nutrients in the cycle of cultivated forests (Khan et al. 2008). Lack of response may be due to existing limiting factors such as high acidity or low levels or excesses of one or more nutrients. In relation to soil acidity, the enhancement of soil pH could be a major factor in increasing plant growth and yield in many crops (Li et al. 2019b). Although Pinus spp. have a high tolerance to soil acidity, the absence of a supply of exchangeable bases such as Ca and Mg in highly weathered soils can be limiting when there are inherently low levels of these nutrients (Rocha et al. 2019). The lack of an Al concentration change in needles seems to indicate a low influence of this factor. This fact was not expected since the application of alkaline residue (Rabel et al. 2021; Pereira et al. 2021) and limestone (Borja and Nilsen 2009) resulted in a decrease in Al in needles.
The low level of Ca and Mg exchangeable forms suggests a possible deficiency. Analysis of needle tissue suggests this possibility since Ca levels were below a critical level of 1.5 g kg−1 in the control (Albaugh et al. 2010) and waste residue use improved Ca levels in the first flush by reaching the critical level at the 30 t ha−1 rate. However, since there was no improvement in growth and yield, something other than Ca could have been a limiting factor.
Although Mn is not usually considered a toxic element under high soil acidity, Mn can reach high availability levels that could be toxic or lead to imbalances with other nutrients (Millaleo et al. 2010; Li et al. 2019a). The increase in soil pH from alkaline residue use can reduce the absorption of Mn by plants as shown in several studies (Sass et al. 2020; Consalter et al. 2021a; Pereira et al. 2021; Quadros et al. 2021; Rabel et al. 2021). The use of ARPR converges more available forms of exchangeable Mn to lower available amorphous fractions. This was expected due to increased soil pH, which changes exchangeable Mn forms to organic and oxides fractions (Sims 1986; Alvarez et al. 2006; Walna et al. 2010); these reactions are more influenced by microorganisms than abiotic factors (Alejandro et al. 2020). However, the absence of a correlation between Mn levels in soil and needles was probably due to the shallow depth of our soil analysis since Pinus taeda roots can reach deeper soil layers (Albaugh et al. 2006) that showed lower pH levels. Another factor that could have impacted results is the natural variability of nutrition among trees in a forest system (Reid et al. 2004; Bussotti and Pollastrini 2015). The extraction with Mehlich-1 (acid extractor) presented a contradictory result due to the solubilization of ARPR (which has low Mn levels) and an increase in Mn at the 0–5 cm soil depth.
The reduction in Mn at both needle flushes shows a high Mn sensitivity to soil acidity, thereby suggesting that Mn levels in foliar tissue could be used as an indicator for the soil acidity amendment since Mn is more readily transported to shoots compared to Al (Marschner 2011). Despite the large reduction in needle Mn concentrations, the level reached at the highest rate was far superior to the critical level of 20 mg kg−1 (Albaugh et al. 2010) and could not be related to a lack of response. However, benefits of reduced Mn could not be confirmed since there was no yield enhancement in our study.
Concentrations of Mg were well below the critical level (0.8 g kg−1; Albaugh et al. 2010) in all treatments, which was expected due to low levels in soil (0.1 cmolc dm−3 in the 0–20 cm layer). Thus, the increase in exchangeable Mg within the 0–5 cm soil depth was not sufficient since observed levels continued to be low. Additionally, there is a well-known strong interaction between Ca versus Mg and K during plant absorption (Marschner 2011). This is especially important when levels of these elements are very low or low (Quaggio 2000) as was observed in our study. Soil Ca reached high levels and enhanced concentrations in needle tissue compared to small increases in Mg levels, which continue to be low in both the soil and plant tissue. Occurrence of needle chlorosis symptoms resembled Mg deficiency in all treatments; i.e., yellowish chloroses of needle tips especially in the first flush and on lower third branches (Fig. 4; Beets and Jokela 1994; Chaves and Corrêa 2005). There was a clear indication that Mg could be a limiting factor associated with the waste residue response. The deficiency of Mg has been recognized as a widespread issue in conifers worldwide (Hüttl and Schaaf 2012) and has also been found in Brazil (Rocha et al. 2019). Rabel et al. (2021) indicated that the use of waste residues in a 3 year Pinus taeda cultivation led to increased productivity with no symptoms of Mg deficiency, but needle Mg concentrations were slightly higher than the observed in our study. Additions of Mg sources in the ARPR could attenuate this deficiency since residues tend to have toxic elements or an excess of an element in regard to others; therefore, chemical improvement management could be used (Gomes et al. 2016).
Another limitation of our study area was low soil P, which resulted in suboptimal foliar concentrations (1.2 g kg−1; Albaugh et al. 2010). Amendments resulting in increased soil pH usually lead to improved P availability (Penn and Camberato 2019); however, this was not the case in our study. In contrast, K concentration displayed values above the critical level (4 mg kg−1) despite low soil levels possibly related to extraction from low release structures as proposed by Alves et al. (2013). Shadowing other soil and tree nutrition observations, our study results suggested that Mg and P could be limiting tree growth.
Similar to needle tissue, the Oi and Oe displayed increases in Ca concentration corroborating findings of Rabel et al. (2021). The increase in pH of Oi could be related to increases in bases in organic tissue. Noble et al. (1996) noted low amounts of base, excess cations, and ash alkalinity for Pinus compared to other plants, but observed a positive correlation between Ca in litter and ash alkalinity for different tree species. This could suggest that the pH (below to 4.0) observed in needles and the pH rise in litter fractions was due to the accumulation of Ca and the low level of retranslocation (Albaugh et al. 2008).
The increase in Ca concentration in litter fractions, compared to needles from the first and second flush, was likely due to mass loss from litter degradation and the consequent release of more soluble compounds (Berg et al. 2017). Although some soluble fractions are leached without prior decomposition, release of Ca largely depends on decomposition of litter since Ca is strongly bound as calcium pectates (Staaf 1980). For micronutrients, Mn showed a greater decrease (than Zn and Cu) in relation to Oi and Oe horizons; this possibly could be related to the sorption and maintenance of these metals in organic matter components (e.g., humic acid). Copper exhibits higher sorption in humic acid compared to Zn, with Mn being the metal with lower sorption (Kerndorff and Schinitzer 1980). These facts can explain why Cu exhibits a lower decay or even an immobilization in litter (Bockheim et al. 1991; Gurlevik et al. 2003; Bueis et al. 2018).
Levels of P and K in the litter were not affected and were lower than those found in needles, which could be due to their greater mobility within plants and their rapid release during the degradation of plant tissues (Albaugh et al. 2008). Contents of Fe and Al were much higher (especially in the Oe horizon) suggesting contamination via soil (Rodrigues et al. 2019; Rabel et al. 2021).
The poverty of Ca and Mg in Oi of our study can be highlighted when compared to values obtained by Viera and Schumacher (2010). These authors reported respective concentrations of 7.88 and 1.20 g kg−1 for Ca and Mg in Oi from Pinus taeda grown under soils formed from basalt. Baietto et al. (2021) reported concentrations of 3.83 and 0.87 g kg−1 for Ca and Mg in Pinus taeda on a silty soil in Uruguay. While Ca levels were similar to results found in our study (in any ARPR treatment), their Mg levels were much higher.
Also, Rabel et al. (2021) using the same waste residue as in our study, reported a value of 0.87 g kg−1 for Mg in litter on a clayey soil and this level corresponded to more than twice the value found in our study on a sandy soil. This confirmed the importance of natural soil fertility and its relationship with the chemical composition of needles that can be later manifested in the litter layer.
The reduction of total litter amount from ARPR application corroborates the findings of others (Marschner and Wilczynski 1991; Jandl et al. 2003; Huber et al. 2006). However, the 24% reduction (or 0.57 t ha−1 year−1) in forest floor mass observed in our study (15 years) was smaller than reported by Jandl et al. (2003) for a year 20 period (69% or 2.6 t ha−1 year−1) who used lime and fertilizer applications. For the same time period, Huber et al. (2006) found 48% or 0.92 t ha−1 year−1 of C from the forest floor receiving 4 t ha−1 of lime. Similarly, Marschner and Wilczynski (1991) found a reduction of 24% (2.3 t ha−1 year−1) in forest floor mass by using 6 t ha−1 of lime in three years. It is important to highlight that litter dynamics are highly variable among climatic conditions and species (Prescott et al. 2000). The lack of increased growth observed in our study could be a major cause for the reduction in the total amount of litter. Greater vegetative development of forests resulting from fertilization (Wienand and Stock 1995; Consalter et al. 2021b) or residue application (Rabel et al. 2021; Pereira et al. 2021) could be a cause for increasing amounts of litter. However, there is difficultly in establishing a causative effect due to the large number of parameters involved as illustrated by Rizvi et al. (2012) who reported a decrease in total litter on a clayey soil and an increase on a sandy soil when using 2.5 t ha−1 of lime.
The increase in litter pH provides greater solubility of organic carbon compounds, thus facilitating the process of microbial decomposition (Kalbitz et al. 2000; Melvin et al. 2013). The greater litter decomposition was possibly due to the increased pH of the material that facilitated decomposition. In relation to substrates poor in Ca, higher levels of Ca in organic material may favor decomposition due to greater ligninolytic fungi activity (Berg 2000).
There were noticeable differences in weed cover between treatments, indicating a response to waste utilization. The sampling approach was limited to a single point within the plot, chosen as the most representative area; however, this may not have captured the entire spatial variability, highlighting the need for further evaluation in future studies. Areas receiving rates of 10 t ha−1 and 20 t ha−1 had lower plant biomass values. Greater volumes of litter on soil for these treatments likely inhibited weed establishment and development. Another factor limiting vegetation cover intensely could have been related to reduced luminosity or light penetration through the tree canopy. However, some works have shown that residue use can cause soil chemical improvements that promote greater weed development and increased “weed competition” in early years (Vance 2000). This could help explain the quadratic response observed in our study.
Ca nutrition in Pinus taeda showed better correlations with exchangeable Ca in the surficial soil layer, since residual Ca was likely not available to alter plant nutrition. Ca in litter was strongly correlated with Ca levels in needles, which was expected, due to the high abundance of roots on forest floors with poor soils (Consalter et al. 2021b; Rabel et al. 2021). Also, litter pH was correlated with Ca in the litter, indicating that an increase in base amounts could explain the pH rise in these tissues. We also noted a strong correlation between Ca levels in weed-cover plants and fractionated litter, indicating that this was a possible source of Ca for these plants.
Conclusion
Despite great improvements in soil chemical properties (especially Ca and acidity), the application of ARPR alone may not result in yield enhancements for soils lacking in other nutrients such as Mg and P. Thus, ARPR combined with products that supply limiting elements (i.e., dolomitic lime for Mg and natural reactive phosphate for P) is recommended. ARPR rates under 40 t ha−1 rate showed slow reactions with incomplete solubility after 15 years. Calcium evaluations by acid extraction and monitoring of soil pH and Ca over 15 years demonstrated a long residual effect of ARPR. Thus, the 40 t ha−1 application rate would not be required for the second Pinus cycle planting. Since the 10 t ha−1 rate had the highest Ca and pH values at ~ 7 years and reached the same initial level at 15 years, use of this rate could require an ARPR reapplication after 15 years. The lack of a tree yield response prevents a precise recommendation rate. The high rate of 40 t ha−1 also demonstrated that this could increase Ca levels and attenuate acidity down to the 60 cm soil layer. Increases in soil pH and Ca promoted an elevation of Ca in needles and a reduction of Mn, confirming the high sensitivity of these nutrients to acidity. ARPR caused a 25% reduction in litter accumulation in this forest system that did not compromise soil protection. ARPR use influenced weed incidence, with reductions occurring at rates of 10 and 20 t ha−1. Calcium in the exchangeable form at a depth of 0–20 cm was more correlated with Ca in needles of Pinus taeda. Strong correlations were also found between Ca level in needles and Ca in litter and between litter pH and Ca in the litter. These findings confirm that the application of ARPR was useful to attenuate soil acidity and presence of Al while improving long-term Ca availability. However, since Ca is a major component of ARPR, application of this residue should be managed with other fertilization practices to avoid nutritional imbalances due to excess Ca (especially at high rates). The absence of fertilization in this study likely limited the scope of our results.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
References
Albaugh TJ, Allen HL, Kress LW (2006) Root and stem partitioning of Pinus taeda. Trees 20(2):176–185. https://doi.org/10.1007/s00468-005-0024-4
Albaugh TJ, Allen HL, Fox TR (2008) Nutrient use and uptake in Pinus taeda. Tree Physiol 28:1083–1098. https://doi.org/10.1093/treephys/28.7.1083
Albaugh JM, Blevins L, Allen HL, Albaugh TJ, Fox TR, Stape JL, Rubilar RA (2010) Characterization of foliar macro-and micronutrient concentrations and ratios in loblolly pine plantations in the southeastern United States. South J Appl for 34:53–64. https://doi.org/10.1093/sjaf/34.2.53
Albaugh TJ, Stape JL, Fox TR, Rubilar RA, Allen HL (2012) Midrotation vegetation control and fertilization response in Pinus taeda and Pinus elliottii across the southeastern United States. South J Appl for 36:44–53. https://doi.org/10.5849/sjaf.10-042
Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from acquisition to subcellular allocation. Front Plant Sci 11:300. https://doi.org/10.3389/fpls.2020.00300
Alvarez JM, Lopez-Valdivia LM, Novillo J, Obrador A, Rico MI (2006) Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 132(3–4):450–463. https://doi.org/10.1016/j.geoderma.2005.06.009
Alves MJF, Melo VDF, Reissmann CB, Kaseker JF (2013) Reserva mineral de potássio em Latossolo cultivado com Pinus taeda L. Rev Bras Ciênc Solo 37(6):1599–1610. https://doi.org/10.1590/S0100-06832013000600016
Attiwill PM, Adams MA (1993) Nutrient cycling in forests. New Phytol 124:561–582. https://doi.org/10.1111/j.1469-8137.1993.tb03847.x
Baietto A, Hirigoyen A, Hernández J, del Pino A (2021) Comparative dynamics of nutrient release through litter decomposition in Eucalyptus grandis Hill ex Maiden and Pinus taeda L. stands. Forests 12(9):1227. https://doi.org/10.3390/f12091227
Balbinot Junior AAB, Tôrres ANL, Fonseca JÁ, Teixeira JR, Nesi CN (2006) Alteração em características químicas de um solo ácido pela aplicação de calcário e resíduos de reciclagem de papel. Revista De Ciências Agroveterinárias 5:16–25
Ballard R, Pritchett WL (1975) Soil testing as a guide to phosphorus fertilization of young pine plantations in the Coastal Plain. Agricultural Experiment Stations, Institute of Food and Agricultural Sciences, Gainesville, FL.
Batista AH, Motta ACV, Reissmann CB, Schneider T, Martins IL, Hashimoto M (2015) Calagem e adubação em plantios de Pinus taeda com severa deficiência nutricional em solos de cerrado. Acta Scientiarum-Agronomy 37:117–125. https://doi.org/10.4025/actasciagron.v37i1.18061
Beets PN, Jokela EJ (1994) Upper mid-crown yellowing in Pinus radiata: some genetic and nutritional aspects associated with its occurrence. New Zealand J for Sci 24(1):35–50
Beets PN, Oliver GR, Kimberley MO, Pearce SH, Rodgers B (2004) Genetic and soil factors associated with variation in visual magnesium deficiency symptoms in Pinus radiata. For Ecol Manag 189(1–3):263–279. https://doi.org/10.1016/j.foreco.2003.08.013
Bellote AFJ, Silva HD, Ferreira CA, Andrade GC (1998) Resíduos da indústria de celulose em plantios florestais. Boletim De Pesquisa Florestal 37:99–106
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22. https://doi.org/10.1016/S0378-1127(99)00294-7
Berg B, Johansson M, Liu C, Faituri M, Sanborn P, Vesterdal L, Ni X, Hansen K, Ukonmaanaho L (2017) Calcium in decomposing foliar litter—a synthesis for boreal and temperate coniferous forests. For Ecol Manag 403:137–144. https://doi.org/10.1016/j.foreco.2017.08.022
Bockheim JG, Jepsen EA, Heisey DM (1991) Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can J for Res 21(6):803–812. https://doi.org/10.1139/X91-113
Børja I, Nilsen P (2009) Long term effect of liming and fertilization on ectomycorrhizal colonization and tree growth in old Scots pine (Pinus sylvestris L.) stands. Plant Soil 314:109–119. https://doi.org/10.1007/s11104-008-9710-5
Bueis T, Bravo F, Pando V, Turrión MB (2018) Local basal area affects needle litterfall, nutrient concentration, and nutrient release during decomposition in Pinus halepensis Mill. plantations in Spain. Ann for Sci 75(1):1–12. https://doi.org/10.1007/s13595-018-0699-5
Busby RR, Torbert HA, Prior SA (2019) Soil and vegetation responses to amendment with pulverized classified paper waste. Soil Tillage Res 194:104328. https://doi.org/10.1016/j.still.2019.104326
Bussotti F, Pollastrini M (2015) Evaluation of leaf features in forest trees: methods, techniques, obtainable information and limits. Ecol Ind 52:219–230. https://doi.org/10.1016/j.ecolind.2014.12.010
Caires CE, Alleoni L, Cambri MA, Barth G (2005) Surface application of lime for crop grain production under a no-till system. Agron J 97:791–798. https://doi.org/10.2134/agronj2004.0207
Chaves RDQ, Corrêa GF (2005) Macronutrients in the soil-Pinus caribaea Morelet system with yellowing of the needles followed by senescence and death. Revista Árvore 29:691–700. https://doi.org/10.1590/S0100-67622005000500004
Consalter R, Barbosa JZ, Prior AS, Vezzani FM, Bassaco MVM, Pedreira GQ, Motta ACV (2021a) Mid-rotation fertilization and liming effects on nutrient dynamics of Pinus taeda L. in subtropical Brazil. Eur J Forest Res 140:19–35. https://doi.org/10.1007/s10342-020-01305-4
Consalter R, Motta ACV, Barbosa JZ, Vezzani FM, Rubilar RA, Prior SA, Nisgoski S, Bassaco MVM (2021b) Fertilization of Pinus taeda L. on an acidic oxisol in southern Brazil: growth, litter accumulation, and root exploration. Eur J for Res 140(5):1095–1112. https://doi.org/10.1007/s10342-021-01390-z
FAO-Food And Agriculture Organization of the United Nations (2020) Global forest resources assessment 2020: main report. FAO, Roma. https://doi.org/10.4060/ca9825en
Ferreira CA, Silva HD, Reissmann CB, Bellote AFJ, Marques R (2001) Nutrição de Pinus no sul do Brasil: diagnóstico e prioridades de pesquisa. Embrapa Forestry, Colombo. Available online at https://ainfo.cnptia.embrapa.br/digital/bitstream/item/17070/1/doc60.pdf
Fonseca JA, Hanisch AL, Backes RL, Gislon I (2012) Evolução de características químicas de um Latossolo Vermelho Distrófico típico até o quinto ano após aplicação de resíduos da indústria de celulose. Revista Agropecuária Catarinense 25:73–79
Gargantini H, Mello FAF, Arzolla S (1982) Efeitos da calagem sobre os teores de cálcio mais magnésio de perfis de solos de cerrado. Anais Da Escola Superior De Agricultura Luiz De Queiroz 39:1115–1136. https://doi.org/10.1590/S0071-12761982000200026
Gascho GJ, Parker MB (2001) Long-term liming effects on Coastal Plain soils and crops. Agron J 93(6):1305–1315. https://doi.org/10.2134/agronj2001.1305
Gatiboni LC, Silva WC, Mumbach GL, Schmitt DE, Iochims DA, Stahl J, Vargas CO (2020) Use of exchangeable and nonexchangeable forms of calcium, magnesium, and potassium in soils without fertilization after successive cultivations with Pinus taeda in southern Brazil. J Soils Sediments 20:665–674. https://doi.org/10.1007/s11368-019-02460-x
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods. Soil Science Society of America, American Society of Agronomy, Madison, WI, USA, pp 383–411. https://doi.org/10.2136/sssabookser5.1.2ed.c15
Gomes HI, Mayes WM, Rogerson M, Stewart DI, Burke IT (2016) Alkaline residues and the environment: a review of impacts, management practices and opportunities. J Clean Prod 112:3571–3582. https://doi.org/10.1016/j.jclepro.2015.09.111
Gregoire N, Fisher RF (2004) Nutritional diagnoses in loblolly pine (Pinus taeda L.) established stands using three different approaches. For Ecol Manag 203:195–208. https://doi.org/10.1016/j.foreco.2004.07.049
Gurlevik N, Kelting DL, Allen HL (2003) The effects of vegetation control and fertilization on net nutrient release from decomposing loblolly pine needles. Can J for Res 33(12):2491–2502. https://doi.org/10.1139/X03-182
Hansen M, Bang-Andreasen T, Sorensen H, Ingerslev M (2017) Micro vertical changes in soil pH and base cations over time after application of wood ash on forest soil. For Ecol Manage 406:274–280. https://doi.org/10.1016/j.foreco.2017.09.069
Huber C, Baier R, Göttlein A, Weis W (2006) Changes in soil, seepage water and needle chemistry between 1984 and 2004 after liming an N-saturated Norway spruce stand at the Höglwald, Germany. For Ecol Manag 233(1):11–20. https://doi.org/10.1016/j.foreco.2006.05.058
Hüttl RF, Schaaf WW (2012) Magnesium deficiency in forest ecosystems. Springer Science & Business Media, Cham
IBGE-Instituto Brasileiro de Geografia e Estatística (2021) Produção da Extração Vegetal e da Silvicultura 2020. Available online at https://www.aen.pr.gov.br/sites/default/arquivos_restritos/files/migrados/0610pevs_2020_v35_informativo.pdf
Jacobson S (2003) Addition of stabilized wood ashes to Swedish coniferous stands on mineral soils-effects on stem growth and needle nutrient concentrations. Silva Fenn 37(4):437–450. https://doi.org/10.14214/sf.483
Jandl R, Kopeszki H, Bruckner A, Hager H (2003) Forest soil chemistry and mesofauna 20 years after an amelioration fertilization. Restor Ecol 11(2):239–246. https://doi.org/10.1046/j.1526-100X.2003.00179.x
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304. https://doi.org/10.1097/00010694-200004000-00001
Kerndorff H, Schnitzer M (1980) Sorption of metals on humic acid. Geochim Cosmochim Acta 44:1701–1708. https://doi.org/10.1016/0016-7037(80)90221-5
Khan MA, Mingzhi W, Lim BK, Lee JY (2008) Utilization of waste paper for an environmentally friendly slow-release fertilizer. J Wood Sci 54:158–161. https://doi.org/10.1007/s10086-007-0924-6
Li J, Jia Y, Dong R, Huang R, Liu P, Li X, Wang Z, Liu G, Chen Z (2019a) Advances in the mechanisms of plant tolerance to manganese toxicity. Int J Mol Sci 20(20):5096. https://doi.org/10.3390/ijms20205096
Li Y, Cui S, Chang SX, Zhang Q (2019b) Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: a global meta-analysis. J Soils Sediments 19(3):1393–1406. https://doi.org/10.1007/s11368-018-2120-2
Maeda S, Silva HD, Costa ERO, Bognola IA (2011) Aplicação de lodo celulósico em plantios de Pinus. Embrapa Forestry, Colombo. Available online at https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/898070/1/CT283.pdf
Magdoff FR, Bartlett RJ (1985) Soil pH buffering revisited. Soil Sci Soc Am J 49:145–148. https://doi.org/10.2136/sssaj1985.03615995004900010029x
Marques R, Motta ACV (2003) Análise química do solo para fins de fertilidade. In: Lima MR, Sirtoli AE, Serrat BM, Wisniewski C, Almeida L, Machado MAM, Marques R, Motta ACV, Krieger KI, Oliveira AC, Ferreira FV (eds) Manual de diagnóstico da fertilidade e manejo dos solos agrícolas, 2nd edn. Universidade Federal do Paraná, Curitiba, pp 81–102
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic Press, Cambridge
Marschner B, Wilczynski AW (1991) The effect of liming on quantity and chemical composition of soil organic matter in a pine forest in Berlin, Germany. Plant Soil 137:229–236. https://doi.org/10.1007/BF00011201
Martins APL, Reissmann CB (2007) Material vegetal e as rotinas laboratoriais nos procedimentos químico-analíticos. Scientia Agraria 8:1–17. https://doi.org/10.5380/rsa.v8i1.8336
Melo VF (1995) Formas de potássio e de magnésio em solos do Rio Grande do Sul, e sua relação com o conteúdo na planta e com a produção em plantios de eucalipto. Rev Bras Ciênc Solo 19:165–171
Melvin AM, Lichstein JW, Goodale CL (2013) Forest liming increases forest floor carbon and nitrogen stocks in a mixed hardwood forest. Ecol Appl 23:1962–1975. https://doi.org/10.1890/13-0274.1
Millaleo R, Reyes-Díaz M, Ivanov AG, Mora ML, Alberdi M (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10(4):470–481. https://doi.org/10.4067/S0718-95162010000200008
Miller L (2015) How fast is lime moving and is it treating acidity at depth? Southern Farm Syst 8:133–135
Mizel NL, Sharpe WE, Swistock BR (2015) Efficacy of pelletized lime versus limestone sand for forest regeneration enhancement in Pennsylvania, USA. Open J for 5:221. https://doi.org/10.4236/ojf.2015.52020
Moro L, Gatiboni LC, Simonete MA, Cassol PC, Chaves DM (2014) Resposta de Pinus taeda com diferentes idades à adubação NPK no Planalto Sul Catarinense. Rev Bras Ciênc Solo 38:1181–1189. https://doi.org/10.1590/S0100-06832014000400014
Noble AD, Zenneck I, Randall PJ (1996) Leaf litter ash alkalinity and neutralisation of soil acidity. Plant Soil 179(2):293–302. https://doi.org/10.1007/BF00009340
Paim RM (2007) Efeito do uso de lama de cal e cloreto de potássio no solo, estado nutricional e crescimento do Pinus taeda L., sobre Latossolo. Dissertation, Universidade Federal do Paraná. Available online at https://acervodigital.ufpr.br/bitstream/handle/1884/24090/RICARDO%20MAYVORME%20PAIM.pdf?sequence=1&isAllowed=y
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9(6):120. https://doi.org/10.3390/agriculture9060120
Pereira M, Bassaco M, Motta ACV, Maeda S (2021) Influence of industrial forest residue applications on Pinus taeda: soil, litter, growth, nutrition, and wood quality characteristics. New for 1:1–24. https://doi.org/10.1007/s11056-021-09902-w
Pértile P, Albuquerque JA, Gatiboni LC, Costa A, Warmling MI (2012) Application of alkaline waste from pulp industry to acid soil with pine. Rev Bras Ciênc Solo 36:939–950. https://doi.org/10.1590/S0100-06832012000300024
Pértile P, Albuquerque JA, Gatiboni LC, Costa A, Luciano RV (2017) Corrective potential of alkaline residue (dregs) from cellulose industry in an acid soil cultivated under no-tillage. Commun Soil Sci Plant Anal 48:1868–1880. https://doi.org/10.1080/00103624.2017.1407427
Prescott CE, Zabek LM, Staley CL, Kabzems R (2000) Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Can J for Res 30(11):1742–1750. https://doi.org/10.1139/x00-097
Prietzel J, Rehfuess KE, Stetter U, Pretzsch H (2008) Changes of soil chemistry, stand nutrition, and stand growth at two Scots pine (Pinus sylvestris L.) sites in Central Europe during 40 years after fertilization, liming, and lupine introduction. Eur J Forest Res 127:43–61. https://doi.org/10.1007/s10342-007-0181-7
Quadros LP, Ducheiko HAS, Maeda S, Prior SA, Araújo EM, Gomes JBV, Bognola IA, Soares MTS, Magri E, Frigo C, Kawasaki A, Motta ACV (2021) Effects of wood ash application on tree nutrition and soil dynamics in a Pinus taeda system. Forest Science 67:618–628. https://doi.org/10.1093/forsci/fxab030
Quaggio JA (2000) Acidez e calagem em solos tropicais. Instituto Agronômico, Campinas
Rabel DO, Maeda S, Araújo EM, Gomes JBV, Bognola IA, Prior SA, Magri E, Frigo C, Brasileiro BP, Santos MC, Pedreira GQ, Motta ACV (2021) Recycled alkaline paper waste influenced growth and structure of Pinus taeda L. forest. New for 52:249–270. https://doi.org/10.1007/s11056-020-09791-5
Reid DE, Lieffers VJ, Silins U (2004) Growth and crown efficiency of height repressed lodgepole pine; are suppressed trees more efficient? Trees 18(4):390–398. https://doi.org/10.1007/s00468-003-0317-4
Rizvi SH, Gauquelin T, Gers C, Guérold F, Pagnout C, Baldy V (2012) Calcium–magnesium liming of acidified forested catchments: Effects on humus morphology and functioning. Appl Soil Ecol 62:81–87. https://doi.org/10.1016/j.apsoil.2012.07.014
Rocha JHT, Toit B, Gonçalves JLM (2019) Ca and Mg nutrition and its application in Eucalyptus and Pinus plantations. For Ecol Manag 442:63–78. https://doi.org/10.1016/j.foreco.2019.03.062
Rodrigues ANA, Motta ACV, Melo VF, Goularte GD, Prior SA (2019) Forms and buffering potential of aluminum in tropical and subtropical acid soils cultivated with Pinus taeda L. J Soils Sediments 19(3):1355–1366. https://doi.org/10.1007/s11368-018-2144-7
Rodriguez DRO, Andrade C, Bellote A, Tomazello Filho M (2018) Effect of pulp and paper mill sludge on the development of 17-year-old loblolly pine (Pinus taeda L.) trees in Southern Brazil. For Ecol Manag 422:179–189. https://doi.org/10.1016/j.foreco.2018.04.016
Sass AL, Bassaco MVM, Motta ACV, Maeda S, Barbosa JZ, Bognola IA, Gomes JBV, Goularte GD, Prior SA (2020) Cellulosic industrial waste to enhance Pinus taeda nutrition and growth: a study in subtropical Brazil. Sci for 48:1–16. https://doi.org/10.18671/scifor.v48n126.13
Simões DC, Caixeta-Filho JV, Palekar US (2018) Fertilizer distribution flows and logistic costs in Brazil: changes and benefits arising from investments in port terminals. Int Food Agribus ManagRev 21:407–422. https://doi.org/10.22434/IFAMR2017.0037
Sims JT (1986) Soil pH effects on the distribution and plant availability of manganese, copper, and zinc. Soil Sci Soc Am J 50(2):367–373. https://doi.org/10.2136/sssaj1986.03615995005000020023x
Sixel RMM, Arthur JC, Gonçalves JLM, Alvares CA, Andrade GRP, Azevedo AC, Stahl J, Moreira AM (2015) Sustentabilidade da produtividade de madeira de Pinus taeda com base na exportação e no estoque de nutrientes na biomassa e no solo. Rev Bras Ciênc Solo 39:1416–1427. https://doi.org/10.1590/01000683rbcs20140297
Staaf H (1980) Release of plant nutrients from decomposing leaf litter in a South Swedish beech forest. Ecography 3(2):129–136. https://doi.org/10.1111/j.1600-0587.1980.tb00719.x
Sun OJ, Payn TW (1999) Magnesium nutrition and photosynthesis in Pinus radiata: clonal variation and influence of potassium. Tree Physiol 19(8):535–540. https://doi.org/10.1093/treephys/19.8.535
Vance ED (2000) Recycling paper mill by-products on forest lands: By-product composition, potential applications, and industry case studies. The Forest Alternative: Principles and Practice of Residuals Use. College of Forest Resources, University of Washington, Seattle, p 193–207
Vargas JPR, Santos DR, Bastos MC, Schaefer G, Parisi PB (2019) Application forms and types of soil acidity corrective: changes in depth chemical attributes in long term period experiment. Soil Tillage Res 185:47–60. https://doi.org/10.1016/j.still.2018.08.014
Viera M, Schumacher MV (2010) Teores e aporte de nutrientes na serapilheira de Pinus taeda L., e sua relação com a temperatura do ar e pluviosidade. Revista Árvore 34:85–94. https://doi.org/10.1590/S0100-67622010000100010
Walna B, Spychalski W, Ibragimow A (2010) Fractionation of iron and manganese in the horizons of a nutrient-poor forest soil profile using the sequential extraction method. Pol J Environ Stud 19(5):1029–1037
Wang X, Tang C, Baldock BC (2016) Long-term effect of lime application on the chemical composition of soil organic carbon in acid soils varying in texture and liming history. Biol Fertil Soils 52:295–306. https://doi.org/10.1007/s00374-015-1076-2
Wienand KT, Stock WD (1995) Long-term phosphorus fertilization effects on the litter dynamics of an age sequence of Pinus elliottii plantations in the southern Cape of South Africa. For Ecol Manag 75:135–146. https://doi.org/10.1016/0378-1127(95)03528-I
Zucon A, Dominschek R, Motta ACV (2021) Can fertilization and liming affect the amount of litter and roots on Pinus taeda forest floor? Sci For/For Sci 48:1–12. https://doi.org/10.18671/SCIFOR.V48N128.21
Acknowledgements
The authors thank the Cahdan Volta Grande Brazilian paper companies and staff (Forest Eng. Daniel Maros), Embrapa Floresta for field work support and the laboratory technicians, Fabiana Gavelaki and Maria Aparecida de Carvalho Santos, who helped in the analysis of materials. Antônio Carlos Vargas Motta is grateful to the National Council for Scientific and Technological Development (CNPq) for financial support (project n° 311304/2019-2) and to the Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarship support.
Funding
National Council for Scientific and Technological Development (CNPq) for financial support (project n° 311304/2019-2) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarship support (process n° 88887.604660/2021-00).
Author information
Authors and Affiliations
Contributions
Antonio Carlos Vargas Motta, Shizuo Maeda, João Bosco Vasconcellos Gomes and Itamar Antonio Bognola contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nicolas dos Santos Trentin, Antonio Carlos Vargas Motta, Shizuo Maeda and Carla Gomes de Albuquerque. The first draft of the manuscript was written by Nicolas dos Santos Trentin and Stephen Arthur Prior, Antonio Carlos Vargas Motta and Tamires Maiara Ercole commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trentin, N.d.S., Motta, A.C.V., Maeda, S. et al. Long-term effects of recycled paper alkaline waste use on soil, litter, weeds, and development and nutrition of Pinus taeda L.. New Forests (2024). https://doi.org/10.1007/s11056-024-10045-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11056-024-10045-x