Abstract
Purpose
The aim of this meta-analysis was to investigate the interactive effects of environmental and managerial factors on soil pH and crop yield related to liming across different cropping systems on a global scale.
Materials and methods
This study examined the effects of liming rate, lime application method, and liming material type on various soil chemical properties and crop yield based on data collected from 175 published studies worldwide since 1980.
Results and discussion
The most important variables that drive changes in soil pH and crop yield were liming rate and crop species, respectively. Soil conditions, such as initial soil organic matter and soil pH, were more important for increasing soil pH in field-based experiments, while lime material type and application method were more important for improving crop yield. To effectively neutralize soil acidity, the optimum liming duration, rate, and material type were < 3 years, 3–6 Mg ha−1, and Ca (OH)2, respectively. Averaged across different crop species, the application of CaO, CaCO3, Ca (OH)2, and CaMg (CO3)2 increased yield by 13.2, 34.3, 29.2, and 66.5%, respectively.
Conclusions
This meta-analysis will help design liming management strategies to ameliorate soil acidity and thus improve crop yield in agroecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global production and use of synthetic nitrogenous fertilizers had increased considerably since 1960, which resulted in a significant increase in crop production and severe negative environmental and agronomic consequences for soil health, e.g., nitrate-N leaching and soil acidification (Smil 2002). Acid soils are considered soils with a pH < 5.5 in their surface horizons (0–20 cm). About 3950 million hectares of land area has been estimated to be affected by acidity, occupying nearly 30% of the global land surface (Sumner and Noble 2003) and accounting for approximately 50% of the global arable land area (Dai et al. 2017). Soil acidity is one of the most yield-limiting factors that affect crop productivity (McLaren and Cameron 1996; Sumner and Noble 2003; Fageria and Nascente 2014). This is mainly because acidified soil reduces the availability of several vital nutrient elements (e.g., phosphorous, P), while exacerbating the toxicity level of others (e.g., aluminum, Al) by altering numerous chemical and biological reactions in the soil (Sumner and Noble 2003). Various factors could contribute to soil acidification, such as natural processes, industrial pollution, and agricultural production (Fageria and Nascente 2014; Holland et al. 2018). Particularly, more than 50% of the world’s cropland has been acidified by agricultural intensification alone, mainly via monoculture farming and excessive use of synthetic fertilizers (Von Uexküll and Mutert 1995). In response of the continually increasing population and increasing energy costs, the development of alternative management practices that could effectively alleviate soil acidification and maintain crop productivity in the long term is imperative for achieving agricultural sustainability.
Liming is the most effective managerial practice for reducing soil acidity and consequentially improves crop production (Fageria and Baligar 2008; Kunhikrishnan et al. 2016). Adding various types of liming materials could neutralize excessive hydrogen ions in soil solution (Bolan et al. 2003), reduce the availability of mineral elements that are less soluble at higher pH values (e.g., aluminum ions, Al3+, and ferric iron, Fe2+; Fageria and Baligar 2008), and increase the availability of essential elements (e.g., P, K, and S) that are more available at higher pH values (Thomas and Hargrove 1984). Furthermore, liming can directly supply many cations that are important for crop production as part of the constituents included in liming materials (e.g., calcium ion, Ca2+, and magnesium iron, Mg2+; Fageria and Nascente 2014). Liming can also influence both the transformation and uptake of nutrients by plants through its indirect impact on the soil microbial community/activity (Cheng et al. 2013; Fageria 2002). Additionally, previous reviews have extensively reviewed how liming could affect heavy metal concentration (Bolan et al. 2003), nutrient use efficiency (Fageria and Baligar 2008; Fageria and Nascente 2014), and greenhouse gas emissions from acidic soils (Kunhikrishnan et al. 2016).
A broad array of mechanisms has been described with which plant-soil-microbe interactions tremendously impact on nutrient availability, acquisition, and crop productivity (Haynes 1984; Fageria and Baligar 2008). However, unraveling such complex mechanisms can be difficult. Clear evidence has been reported for how liming could be effectively used to enhance agronomic productivity, but the detailed information on the quantitative relationship between the liming management regime (i.e., both liming rate and material) and crop yield remains limited. Additionally, very little research has investigated how changes driven by liming management affect soil pH and the nutrient status (as well as potential feedbacks effects) on the yield of various crop species. This information gap impedes the efficient amelioration of soil acidity within different cropping systems. Therefore, a global-scale meta-analysis is needed to investigate/compare different crop and soil responses to liming and eventually help develop/generalize optimal management practices in an integrated manner. In this study, we adopted a meta-analysis approach based on published data and quantified the effects of liming on (1) the yield of various row crop and forage species, (2) soil chemical properties (pH and cation exchange capacity (CEC)) and nutrient status, as well as (3) the effect of different liming rates, methods, and liming materials on soil pH. We acknowledge that there exist complex plant-soil-microbe interactions/mechanisms that are important drivers for crop productivity and soil health; however, for the purpose of this study, we concentrated on identifying key factors that benefit soil pH and crop yield increase rather than postulating underlying mechanisms.
2 Materials and methods
2.1 Data collection
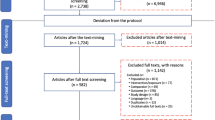
Published journal articles from 1980 to 2017 were searched using the ISI Web of Science (http://apps.webofknowledge.com/) and China Knowledge Resource Integrated Database (http://www.cnki.net/) using the topic “soil amendment” or lime or amelioration and topic “acid soil” or “soil acidification.” The crop yield, soil pH, and soil nutrients in the analysis were obtained from published tables and texts of all selected research articles, and a subset of data were extracted from published figures using Get-Data Graph Digitizer software (ver. 2.24, Russian Federation). To minimize bias, the following criteria were used:
-
(1)
Paired observations between a no-liming control and a liming treatment under identical experimental conditions were included. If an individual study used more than one level or material type of lime application at the same site, measurements of different application rates or liming materials were separately paired with no-liming control.
-
(2)
The mean and standard deviation (or standard error) of the achieved yield (either grain yield or biomass), soil pH value, and at least two replications were provided either as part of the experimental design or in figure captions.
-
(3)
Liming management practices were included as treatments, while other essential agronomic practices such as cropping intensity, fertilizer management, and irrigation were similar.
Pot-based studies (laboratory incubation or greenhouse pot studies) were also included to illustrate the integrative effect of liming on crop yield and soil chemical characteristics. In total, 175 peer-reviewed manuscripts were included in this meta-analysis, including 403 data points for yield, 643 data points for soil pH, and 291 data points for soil nutrient concentration under different liming practices. Details of the selected studies and references are presented in the supplementary material.
For each site, we extracted information about soil properties (e.g., texture and initial pH) and the duration of lime application. The yield data sets were organized based on crop species and type of liming materials [including dolomite (CaMg (CO3)2), calcium hydroxide (Ca (OH)2), calcium carbonate (CaCO3), and calcium oxide (CaO)]. The soil pH data set was organized based on the experimental conditions (pot-based or field-level research), the duration of the lime application (< 3, 3–6, 6–10, > 10 years), the type of liming materials, the initial soil pH, the initial soil organic matter (SOM), the soil texture (i.e., sandy (sand, loamy sand, and sandy loam), silty (silty loam and silt), loamy (sandy clay loam, medium loam, clay loam, and silty clay loam), or clayey (clay, sandy clay, and silty clay)) (Chen et al. 2018), the lime application method (subsoiling, plow, and surface), and the land use type (farmland (referenced as arable land too), grassland, and forest).
2.2 Data analysis
Based on the international ISO standard and modern soil classifications, we used the following equations to convert soil pH 1:2.5 KCl, 1:2.5 H2O, or 1:5 CaCl2 to 1:5 H2O (Kabala et al. 2016; Minasny et al. 2011) prior to performing the meta-analysis.
The response ratio (RR) of liming on yield and soil pH between treatment and control was analyzed according to Eq. (4) (Osenberg et al. 1999).
where RR was calculated as the natural log of the ratio between mean of lime amendment (Xt) and control (Xc) groups.
The variance (v) of the RR is calculated with Eq. (5):
where St and Sc represent the standard deviations of treatment and control groups, respectively, and nt and nc represent the number of replicates of treatment and control groups, respectively.
To derive the overall response effect of the treatment group relative to the control group, the weighted response ratio (RR++) between treatment and control was calculated using Eq. (6) (Hedges et al. 1999; Luo et al. 2006):
where RR++ represents the weighted response ratio, m represents the number of groups compared, k represents the number of comparisons in the corresponding group, and wi represents the weighting factor. The higher the weight, the more important the factor is for the comprehensive evaluation process. The standard error of RR++ was calculated via Eq. (7):
The 95% confidence interval (95% CI) was calculated via Eq. (8):
When the 95% CI value of the response variable did not overlap with 0, the liming effect on the variable was considered to be significantly different between control and treatment groups (Gurevitch and Hedges 2001). Additionally, the percentage change could be easily derived from RR++ using Eq. (9):
The meta-analysis was performed using the using the restricted maximum likelihood estimator (RMLE) estimation in the rma.unl model for “metafor” package of the R statistical software (version 3.4.2) (Viechtbauer 2010). For each group (crop species, liming material, experimental condition, liming duration, soil texture, liming practice, and land use type), the mean effect size (RR) and its 95% confidence interval (CI) were calculated with bias-correction generated via bootstrapping. A chi-square test was used to determine whether the heterogeneity among the RR of changes to yield and soil pH under liming treatments (Qtotal) significantly exceeded the expected sampling error. A randomization test was used to determine the significance of the between-group heterogeneity (Qb) (Adams et al. 1997). Chi-square tests were also used to determine whether the remaining within-group heterogeneity (Qw) was significant (see Tables S1–S3, Electronic Supplementary Material).
The “randomForest” package (Liaw and Wiener 2002) was used to determine the importance of variables on the response ratio of soil pH and crop yield to liming. This nonparametric method allowed to consider all observations for assessing the relationship of predictors to the change in response to the ratio of soil pH and crop yield and various environmental and experimental factors. Basically, this method randomly assigns observations at nodes where splits are based on the variable of interest; then, the mean squared error of the resulting trees is compared to that of the original trees with the observations correctly assigned to each split. The identified variables were used for classification and regression trees by the “partykit” package (Therneau et al. 2015) to account for differences of the response of liming within various environmental and experimental conditions. The method of classification and regression trees is a powerful yet simple technique for modeling a response that depends on several variables that interact in a hierarchical manner. Briefly, classification and regression trees conduct binary splits of the data according to explanatory variables; thus, the values of the dependent variable (y) that fall into the resulting node are modeled by the mean value of the node. Furthermore, a meta-regression analysis was performed using the “metafor” package of the R statistical software (Viechtbauer 2010) to relate the response ratio of yield or soil pH to the liming rate. A meta-regression was conducted with the RMLE using the Knapp and Hartung (KH) adjustment, and model comparisons were based on Akaike’s Information Criterion (AIC). The lowest AIC indicated the best-fitting model (Anderson and Burnham 2002). We also plotted frequency distributions of RR and fitted these with a Gaussian function (Luo et al. 2006).
3 Results
3.1 Liming effects on soil pH under different environmental and experimental conditions
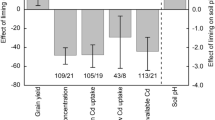
The differences in the effects of liming on soil pH under different scenarios are presented in Fig. 1. Regardless of environmental and experimental conditions, application of lime always significantly increased soil pH. The effect of liming on soil pH was more pronounced in pot conditions than in field conditions by 36% (Fig. 1 (a)). In field conditions, Ca (OH)2 indicated the best effect for improving soil pH with ∆pH = 0.6 (Fig. 1 (b)), while only using CaO achieved a significantly greater effect than CaMg (CO3)2 in pot trials (Fig. 1 (c)). The effect size of liming on soil pH decreased from < 3- to 3–6-year studies (Fig. 1 (d)). No significant difference was found when liming duration exceeded 3 years as well as if it exceeded 6 years or was shorter than 3 years. No significant differences in effect size were detected among various initial soil pH conditions, while lower initial pH values resulted in increased variances (indicated by the large CI; Fig. 1 (e)). Only SOM < 10 g kg−1 had a significantly higher effect than SOM > 30 g kg−1 (Fig. 1 (f)); otherwise, there were no statistical differences among various SOM contents. For soil texture, the liming effect size in sandy soil was 32% higher than in clayey soils (Fig. 1 (g)). Application methods showed no statistical differences regarding the effect size of liming (Fig. 1 (h)). There were no differences in how different land use types affected the effect of liming on soil pH (Fig. 1 (i)); however, a large variance was observed in forest agroecosystems.
Soil pH response to liming compared with no-liming treatments in different categories. Categories are (a) method, (b) liming material based on field trial, (c) liming material based on pot trial, (d) liming duration, (e) initial soil pH, (f) initial soil organic matter content, (g) soil texture, (h) application method, and (i) land use types. Effect size stands for the weighted response ratio between treatment and control. Bars represent the 95% confidence intervals. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals do not overlap 0 means P < 0.05
Figure 2 summarizes the effect of liming materials and rate on soil pH. Regardless of liming materials, the increasing application rate of lime improved soil pH. Additionally, the effect of application rate of > 6 Mg ha−1 significantly exceeded that of < 3 Mg ha−1. The effect of application rate of 3–6 Mg ha−1 significantly exceeded that of 1–3 Mg ha−1 with the exception of CaO and significantly exceeded that of ≤ 1 Mg ha−1 with the exception of CaMg (CO3)2. However, no differences were found when application rates exceeded 3 Mg ha−1 with the exception of CaMg (CO3)2 (Fig. 2 (d)).
Soil pH response to application rate of liming compared with no-liming treatments in different categories. Categories are (a) calcium oxide (CaO), (b) calcium hydroxide (Ca (OH)2), (c) calcium carbonate (CaCO3), and (d) dolomite (CaMg (CO3)2). Effect size stands for the weighted response ratio between treatment and control. Bars represent the 95% confidence intervals. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals do not overlap 0 means P < 0.05
3.2 Liming effects on soil nutrient status
The effects of liming on soil exchangeable AI, Ca, and Mg are indicated in Fig. 3. Both in field and pot conditions, lime application significantly increased soil exchangeable Ca and Mg concentrations (exchangeable elements are often adsorbed to the soil surfaces, which can be readily exchanged with other cations in the soil solution; McLaren and Cameron 1996), thus decreasing soil exchangeable AI (Fig. 3). In field conditions, liming significantly decreased exchangeable AI, while increasing exchangeable Ca and Mg by 75.2% (95% CI − 1.74 to − 1.05), 120% (95% CI 0.69 to 0.89), and 98% (95% CI 0.51 to 0.86), respectively (Fig. 3 (a–c)). In pot conditions, the percentage change was 80.9% (95% CI − 1.91 to − 1.41), 372.9% (95% CI 1.36 to 1.74), and 90.6% (95% CI 0.41 to 0.88), respectively (Fig. 3 (d–f)). For available elements (elements that are available for uptake by biota; McLaren and Cameron 1996), liming significantly decreased the soil available AI concentration in both field and pot conditions by 75.3% (95% CI − 1.86 to − 0.93) and 88.5% (95% CI − 3.07 to − 2.25), respectively (Fig. 4 (a, d)). Liming significantly increased the soil available Ca concentration in both field and pot conditions by 74.2% (95% CI 0.27 to 0.84) and 263.2% (95% CI 0.85 to 1.73), respectively (Fig. 4 (b, e)) as well as Mg concentration in pot condition by 73.9% (95% CI 0.23 to 0.87; Fig. 4 (c)). These effects were much higher in pot conditions compared to field conditions.
Frequency distributions of response ratios for soil elements responding to liming compared with no-liming treatments. Soil elements are (a) exchangeable aluminum (Al) with field trial, (b) exchangeable calcium (Ca) with field trial, and (c) exchangeable magnesium (Mg) with field trial and (d) exchangeable AI with pot trial, (e) exchangeable Ca with pot trial, and (f) exchangeable Mg with pot trial. The fitted curve is an estimated Gaussian distribution in frequency; dashed line is at RR = 0. Bars represent the 95% confidence intervals of response ratio. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals do not overlap 0 means P < 0.05
Frequency distributions of response ratios for soil elements responding to liming compared with no-liming treatments. Soil elements are (a) available aluminum (Al) with field trial, (b) available calcium (Ca) with field trial, and (c) available magnesium (Mg) with field trial and (d) available AI with pot trial, (e) available Ca with pot trial, and (f) available Mg with pot trial. The fitted curve is an estimated Gaussian distribution in frequency, dashed line is at RR = 0. Bars represent the 95% confidence intervals of response ratio. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals does not overlap 0 means P < 0.05
With regard to the total elemental concentration, liming only significantly improved the soil TK concentration in field conditions by 2.6% (95% CI 0.002 to 0.05) (Fig. S1a). However, lime application significantly increased soil available nitrogen (AN) and available phosphorus (AP) in field conditions by 7% (95% CI 0.01 to 0.13) and 9.3% (95% CI 0.04 to 0.14), respectively (Fig. 5 (b, c)). In pot conditions, the effect sizes of soil available potassium (AK), AN, and AP were 0.09 (95% CI − 0.03 to 0.21), 0.07 (95% CI − 0.04 to 0.19), and 0.09 (95% CI − 0.04 to 0.23), respectively (Fig. 5 (d–f)).
Frequency distributions of response ratios for soil elements responding to liming compared with no-liming treatments. Soil elements are (a) available potassium (K) with field trial, (b) available nitrogen (N) with field trial, and (c) available phosphorus (P) with field trial and (d) available K with pot trial, (e) available N with pot trial, and (f) available P with pot trial. The fitted curve is an estimated Gaussian distribution in frequency, dashed line is at RR = 0. Bars represent the 95% confidence intervals of response ratio. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals do not overlap 0 means P < 0.05
Liming significantly increased soil CEC in field conditions by 14.4% (95% CI 0.05 to 0.22) (Fig. S2a, b, Electronic Supplementary Material). The effect size of SOC was 0.02 (95% CI − 0.002 to 0.05; Fig. S2c) in field conditions and − 0.01 (95% CI − 0.13 to 0.12; Fig. S2d) in pot conditions.
3.3 Yield response to liming
Figure 6 demonstrates the effects of liming on crop yield. Liming significantly increased the yield of all tested species except for sorghum (Sorghum bicolor), tuber crops, and tobacco (Nicotiana tabacum) (Fig. 6 (a)). The largest percentage change of yield occurred for legume forages (Fabaceae spp.), while the smallest was reported for tobacco with percentage changes of 410.4% and 4.7%, respectively (calculated using Eq. (9); Fig. 6 (a)). For soil texture, the liming effect size on yield in silty soils was significantly higher than in clayey, loamy, and sandy soils by 306%, 295%, and 186%, respectively (Fig. 6 (b)). Liming significantly increased crop yield regardless of liming materials (Fig. 6 (c)) and the largest effect size occurred under CaMg (CO3)2, while the smallest effect size occurred under CaO and the percentage changes were 66.5% and 13.2%, respectively (Fig. 6 (c)). No significant differences in effect size were detected among various liming application methods, while plow increased both effect size and variances (indicated by the large CI; Fig. 6 (d)).
Yield response to liming compared without amelioration treatment in different categories. Categories are (a) crop species, (b) soil texture, (c) lime material and (b) application method. Effect size stands for the weighted response ratio between treatment and control. Bars represent the 95% confidence intervals. The number of observations of each variable is noted beside the bar. RR ± 95% confidence intervals do not overlap 0 means P < 0.05. Lime materials include calcium oxide (CaO), calcium hydroxide (Ca (OH)2), calcium carbonate (CaCO3), and dolomite (CaMg (CO3)2)
3.4 Comparing effects of liming on both yield and soil pH
Figure S3 (Electronic Supplementary Material) shows the importance of variables in changing in response ratio of soil pH and crop yield. Soil pH and yield resulted in different rankings. Liming rate was the most important for changes in the response ratio of soil pH in field-based experiments, followed by initial SOM, initial soil pH, and liming methods (Fig. S3a, Electronic Supplementary Material). This was consistent with the outputs of regression trees, indicating the initial split node as the liming rate (Fig. 7). Crop species was the most important for changes of the response ratio of crop yield, followed by soil texture, lime material, and liming method (Fig. S3b, Electronic Supplementary Material). This also agreed with outputs of regression trees, which indicated crop species as the initial split node (Fig. 8).
Classification and regression trees illustrated the influence of environmental and experimental variables (liming rate, initial soil organic matter (SOM) content, initial soil pH, liming method, crop species, soil texture, and liming material) on the dependent variables of soil pH. The numbers and the shading in the boxes represent the mean value at each decision point; the percentages indicate the percentage of all values considered at that decision point
Classification and regression trees illustrated the influence of environmental and experimental variables (liming rate, initial soil organic matter (SOM) content, initial soil pH, liming method, crop species, soil texture, and liming material) on the dependent variables of crop yield. The numbers and the shading in the boxes represent the mean value at each decision point; the percentages indicate the percentage of all values considered at that decision point
Meta-regression results indicated that the response ratio of soil pH significantly increased with increased liming (p = 0.001; Fig. S4a, Electronic Supplementary Material), and the quadratic equation explained a large amount of variation of the regressed data (R2 = 0.26). The response ratio of yield also indicated a significantly positive linear relationship with increased liming rate (p < 0.001; Fig. S3a, Electronic Supplementary Material). However, the linear model indicated poor fitting to the entire data (R2 = 0.02).
4 Discussion
4.1 Liming-induced changes in soil pH vary with environmental and experimental conditions
As the most important and effective managerial practices for the reduction of soil acidity (Pagani and Mallarino 2012b), the primary purpose of liming is to neutralize excessive hydrogen ions from the soil solution (Bolan et al. 2003). As indicated in this study, liming rate was the most important factor for changes in the response ratio of soil pH to liming (Fig. 7). A significant quadratic relationship also exists between the response ratio of soil pH and the liming rate, and the model explains more than 26% of the variation alone (Fig. S4a, Electronic Supplementary Material). The R2 value was remarkable considering the complexity of various factors that might affect soil pH. Interestingly, 3–6 Mg ha−1 was the effectiveness threshold of liming rate, regardless of environmental and experimental conditions (Figs. 2 and 7). The effect of liming on soil pH greatly depends on the property of liming materials (e.g., particle size and material types; Álvarez et al. 2009) as well as the initial chemical/physical properties of soil (e.g., pH buffering capacity and soil organic matter; Bolan et al. 2003) as indicated in our findings. This is primarily caused by the complex soil-plant-environmental interactions, as well as the heterogeneity of soil physical and chemical properties (Pagani and Mallarino 2015). The subgroup of meta-analysis (Fig. 1 (e–g)) and regression tree analysis (Fig. 7) highlights the impacts of initial soil pH and SOM. The effect of liming tended to be greater when the initial soil pH was lower (Fig. 1 (e)). Furthermore, high SOM often leads to high soil CEC and pH buffer capacity (Briedis et al. 2012); therefore, the effects of lime tended to be greater when the initial soil SOM was lower as indicated by our study (Fig. 1 (f)). Soil texture determines the buffering capacity of soil. Fine-textured soils typically have a superior buffering capacity. Hence, changes in chemical properties caused by liming are not as pronounced as for coarse-textured soil (Fageria and Baligar 2008) (see Fig. 1 (g)). Thus, liming has to consider other factors in addition to the liming rate, especially when the initial soil pH ranges from 4.5 to 5, or the initial soil SOM is approximately 26 g kg−1 or greater.
We found that the optimal liming duration should be approximately 3 years (Fig. 1 (d)), because the maximum pH effect was typically found within the initial 3 years after liming, which is similar to results obtained across many studies with a variety of managerial and environmental conditions (Rippy et al. 2007; Kassel 2009; Woodard and Bly 2010). However, to our knowledge, this is the first study to identify this 3-year critical time window for liming based on a meta-analysis synthesis. These results will be helpful for producers to reduce the costs of liming on a global scale. Additionally, short-term (shorter than 1 year) rapid increases of soil pH were also reported (Pagani and Mallarino 2012a). The overall dependence of the liming effect on the liming duration involves considerable inconsistency between different environmental/ecological conditions over time.
No differences were detected among various application methods on soil pH or crop yield (Figs. 1 (h) and 6 (d)), indicating that producers have the liberty to adopt any method that would incur less costs and operational difficulties. However, after taking account for variability in environmental and experimental factors such as site, lime material, and crop differences, the results differed with regression tree, which showed application method was a split node (Figs. 7 and 8). A possible explanation of the lack of differences is because our meta-analysis included pot-based studies in changing soil pH, which are generally conducted with more responsive species. Thus, the results obtained in pot conditions represent the ideal effects of liming. Similar responses were found for other soil nutrient indices (Figs. 3, 4, and 5). The response differences between field-based vs. pot-based studies have been well documented in various crop and soil studies (Liu et al. 2016). However, a systematic comparison on the magnitude of differences remains lacking. The results of this study showed that liming has a greater effect for improving the soil pH in pot conditions than in field conditions (Fig. 1 (a)), indicating the uncertain magnitude or implication of pot experiments and emphasizing the necessity of field-scale studies in the future. This finding could be attributed to highly complex environmental conditions that both soil and plants underwent in the less controlled environment of field conditions (Liu et al. 2016).
Interestingly, Ca (OH)2 appeared to be more effective for neutralizing acidity in field soils than in potting soils and CaO seemed to be a superior liming material for increasing the pH in potting soils (Fig. 1 (b, c)). These findings could be caused by the fact that Ca (OH)2 has stronger alkalinity even in complicated field conditions and CaO has indicated higher neutralizing value (Thomas and Hargrove 1984), particularly under controlled environment. Similar to our findings, Conyers et al. (1996) found that CaCO3 performed better than CaMg (CO3)2 in increasing soil pH, which was primarily ascribed to its greater solubility.
The differences in response of land use types to liming were insignificant in our study (Fig. 1 (h)), which could be the result of the heterogeneity in responses to liming under different agroecosystems. For instance, farmlands dominated by row-crop systems have a simple plant community structure but high land use intensity. Grasslands featuring high species diversity and complex animal-plant-microbe interactions often feature a wide array of responses to liming. Particularly, forest ecosystems (e.g., silvopasture operations with diverse production outputs, increased soil protection, and abundance of organic matter return) have a strong buffering capacity that leads to delayed or reduced liming effect on soil pH (Jiang et al. 2016).
4.2 Effects of liming on soil nutrient status
It is widely accepted that liming can neutralize excessive acidic ions in the soil including proton ions and other acidic mineral cations (e.g., Al3+), while simultaneously supplying basic cations to the root zones (e.g., Ca2+ and Mg2+; Fageria and Baligar 2008). In this meta-analysis, liming was found to decrease both exchangeable and available AI (Figs. 3 (a, d) and 4 (a, d)), while increasing both exchangeable and available Ca and Mg (Figs. 3 (b–f) and 4 (b, e, f)), with the exception of available Mg in field conditions. The response of increased Ca availability was anticipated primarily due to a reduction in contrasting actions of Al-Ca once exchangeable or soluble Al3+ is decreased by liming. Similarly, Mg is a poor competitor with Al and Ca as the weak affinity coefficient of absorbent surfaces and is furthermore prone to leaching and accumulation in the solution phase (Myers et al. 1988). Therefore, liming could also increase Mg availability in the soil solution. As indicated in this study, the effect of liming on K availability lacks consistency and has been debated over the years. The current consensus is that liming could reduce K leaching at low soil pH conditions, while it can also cause increased plant uptake of K (Bekker et al. 1994), leading to a small effect on the soil solution. Likewise, the availability and quantity of different forms of N in soils are governed by the complex microbe-driven N cycle, which could be influenced by many external factors. This partly explains why limited soil N responses were observed in this study. Furthermore, liming indicated little effect on soil available P (Fig. 5 (f)). Fageria and Baligar (2008) found that liming of Oxisols greatly improved P uptake and utilization efficiency of crops, thus, offsetting the effect of increased release and leaching reduction when soil pH increases. Additionally, we found that liming significantly increased soil CEC in field conditions by 14.4% (Fig. S2a, Electronic Supplementary Material). This is because liming improves the soil surface charge, which is part of the soil CEC pool and critically important for soil nutrient availability and mobility as well as N-cycle dynamics (Haynes and Naidu 1998). Additionally, both the availability and effectiveness of pH-dependent CEC could be greatly reduced when levels of iron and aluminum hydrous oxides are high. Thus, when soils are limed, total CEC increases.
4.3 Liming improves crop yield
Liming increases crop production primarily through direct effects on improving soil physical, chemical, and biological characteristics, which lead to increased availability and mobility of many plant essential nutrients (Chan and Heenan 1998; Bolan et al. 2003; Jaskulska et al. 2014). The results of this study verified that liming increased yield regardless of environmental and experimental conditions (Fig. 6) and the enhanced crop yield caused by liming depends greatly on crop species, liming material, liming application method, and soil texture (Fig. 8; Fig. S3, Electronic Supplementary Material). Crop species differ significantly in relation to their tolerance to soil acidity and their sensitivity to soil pH (Holland et al. 2018). Therefore, lime requirements also vary greatly among species and even among different cultivars (Fageria and Nascente 2014). Our results showed that the yield of sorghum, tuber crops, and tobacco was less affected by liming (Fig. 6 (a)). Tobacco root growth is typically directly affected by soil physical/chemical conditions, and the ideal soil pH is slightly acidic (Abruña-Rodríguez et al. 1970). Thus, as long as the soil pH is above the level where excessive acidity could delay the absorption of K, Ca, P, and Mg, moderate tobacco yield could always be achieved (Fageria and Nascente 2014). Similarly, tuber crops such as potato generally achieve the optimal yield at a lower soil pH compared to other common crops (Holland et al. 2018). Therefore, the great acid tolerance of tuber crops decreased the effect of liming on yield. Given appropriate symbiosis with N2-fixing bacteria, soybean can grow on soils that are exceedingly acidic for many other legume species. Furthermore, P and K are two key elements that are required for successful pod-filling processes (da Costa and Crusciol 2016); thus, liming alone can increase the grain yield through increasing soil P and K availability after an increase of pH values. Therefore, a small but significant liming effect was detected for soybean production. Additionally, our results indicated that maize is more responsive to lime treatment than soybean, which matches the report by Pagani and Mallarino (2015), but contrasts with the general belief that lime treatment should exert a greater impact on soybean yield than on corn yield, particularly in semiarid environments (Pierce and Warncke 2000; Kassel 2009). Interestingly, pasture forages (grasses and legumes) indicated great variability in response to liming. This is primarily caused by the wide variety of species under each forage genus group, the complexity of nutrient cycling processes involved in animal-incorporated forage systems, and the high species richness and resilience in multicultural forage production (Hooper et al. 2005). Forage legumes also showed much greater effect size than grasses because the microbial activity of rhizobia in nodules is extremely sensitive to soil pH, nutrient availability, and mineral toxicity (e.g., Al and Mn; MacAdam and Nelson 2017). In particular, soil molybdenum (Mo) availability could be greatly increased when soil pH was increased and Mo plays an essential role in the symbiotic-N2 fixation of legumes (Sumner and Noble 2003; Jean et al. 2013).
Interestingly, CaMg (CO3)2 treatment had greater effects on improving crop yield compared to both CaCO3 and CaO. This response is largely due to the Mg application contained in CaMg (CO3)2 (Fageria and Baligar 2008). Furthermore, this also indicated that the majority of the research fields are low in Mg, thus leading to a superior crop response to Mg input. The efficiency and effectiveness of liming are largely determined by the quantity and quality of the liming materials. In general, it is widely accepted that finer liming materials increase soil pH more rapidly than coarser materials (Thomas and Hargrove 1984; Pagani and Mallarino 2012b). However, we suggest that the residual effect of coarse/slow-dissolving liming materials on yield might exceed that of finer liming materials because these lime particles react slowly in neutralizing soil acidity and tend to remain in the soil for longer. For example, within a given fineness level, both CaO and Ca (OH)2 would dissolve more rapidly than other liming materials (Fageria and Baligar 2008), thus having similar or even smaller effect sizes for improving yield as found in our results (Fig. 6 (c)). Additionally, the CaO reaction is an exothermic reaction (Smith et al. 1998), which could result in heating, consequentially impacting soil microbial community, root metabolic activity, and other soil chemical processes.
For soil texture, the liming effect size on yield in silty soils significantly exceeded that of other textures (Fig. 6 (b)); this is different from the liming effect size on soil pH (Fig. 1 (g)). This could be partly caused by higher initial soil pH values found in studies conducted in silty-soil fields. Furthermore, silty-type soil typically has well-balanced soil physical traits (e.g., sufficient plant available water and porosity), making it well suited for the production of the majority of crop species globally.
In addition to soil chemical property, liming influences the soil physical property such as soil texture/structure, nutrient provision, and others that could affect the crop yields (Fageria and Baligar 2008), especially when application rate and duration of liming are high. For instance, Ca in liming materials helps in the formation of soil aggregates, hence improving soil structure (Chan and Heenan 1998) and thus dispersion and flocculation of soil particles (Bolan et al. 2003). Liming can also influence both transformation and uptake of nutrients by plants (Fageria 2002; Cheng et al. 2013) and, additionally, nutrient use efficiency (Fageria and Nascente 2014).
Although CaMg (CO3)2 has a higher neutralizing capacity compared to CaCO3, it is typically less effective for neutralizing soil acidity as indicated by our results (Fig. 1 (c)). This is particularly obvious for particle sizes at low fineness levels (Pagani and Mallarino 2012b; Thomas and Hargrove 1984). However, this study showed that these soil pH responses could not be directly translated into similar crop yield responses (Fig. S4, Electronic Supplementary Material). Regressing the response ratio of yield and liming rate yielded a significant linear relationship but with a very small R2 value of 0.02 (Fig. S4b, Electronic Supplementary Material). This indicates that liming indeed increases yield across all crop species; however, the fundamental mechanisms for controlling crop yield integrate far more environmental and managerial factors than just liming alone (Fig. 8). The result differences observed between meta-analysis and regression tree approaches, specifically the liming application method, greatly supported this.
5 Conclusions
An extensive amount of research has been conducted for evaluating the effects of liming on crop production. However, synthesized analysis on the effectiveness and efficacy of liming under different environmental and experimental conditions is rarely reported. Taken altogether, our study demonstrated that the most important drivers for changing soil pH and improving crop yield generally were liming rate and crop species. Particularly, soil conditions such as initial SOM and soil pH were more important for increasing soil pH in field-based experiments, while liming practices, such as lime material and method, were more important for improving crop yield. Most importantly, the presented meta-analysis offers the opportunity to draw quantitative-synthetic conclusions on a global scale that would not be possible based on an individual study alone. In particular, the effects for improving soil pH were significantly greater in sandy soils. No differences were detected among various initial values of soil pH, soil textures, liming methods, and land use types. This indicates that producers could adopt any method that would incur less costs and operational difficulties, and relevant future studies could concentrate more on other aspects of liming. To effectively neutralize soil pH, the optimum liming duration, rate, and material were found to be <3 years, 3–6 Mg ha−1, and Ca (OH)2, respectively. Additionally, liming significantly ameliorated soil Al3+, increased basic cations (Ca2+ and Mg2+), neutralized soil pH across different cropping systems, and improved the soil nutrient status (AN, AK, and AP). Liming also significantly increased crop yield, particularly when CaMg (CO3)2 was used across various studies, highlighting the necessity of future studies to separate the effect of Mg supplementation and liming on crop yield. Finally, the complexity of responses identified in this study emphasizes the need for adopting more advanced data-science algorithms coupled with high-dimensional data sets in the future that could enhance our understanding of the underlying mechanisms of liming on an agroecosystem basis.
Abbreviations
- Al:
-
Aluminum
- AN:
-
Available nitrogen
- AP:
-
Available phosphorus
- AK:
-
Available potassium
- Ca:
-
Calcium
- CEC:
-
Cation exchange capacity
- Mg:
-
Magnesium
- N:
-
Nitrogen
- P:
-
Phosphorus
- K:
-
Potassium
- SOC:
-
Soil organic carbon
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- TK:
-
Total potassium
References
Abruña-Rodríguez F, Vicente-Chandler J, Pearson RW, Silva S (1970) Crop response to soil acidity factors in Ultisols and Oxisols: I. Tobacco. Soil Sci Soc Am J 34(4):629–635
Adams DC, Gurevitch J, Rosenberg MS (1997) Resampling tests for meta-analysis of ecological data. Ecology 78(4):1277–1283
Álvarez E, Viadé A, Fernández-Marcos ML (2009) Effect of liming with different sized limestone on the forms of aluminium in a Galician soil (NW Spain). Geoderma 152:1):1–1):8
Anderson DR, Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. J Wildlife Manage 66(3):912–918
Bekker AW, Hue NV, Chase RG (1994) Effects of liming, K fertilization and leaching on K retention, nutrient uptake and dry matter production of maize grown on a Samoan Oxic Inceptisol. Fertil Res 38(2):123–130
Bolan NS, Adriano DC, Curtin D (2003) Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Advances in agronomy. Academic Press, pp 215–272
Briedis C, de Moraes Sá JC, Caires EF, de Fátima Navarro J, Inagaki TM, Boer A, de Oliveira Ferreira A, Neto CQ, Canalli LB, Bürkner dos Santos J (2012) Changes in organic matter pools and increases in carbon sequestration in response to surface liming in an Oxisol under long-term no-till. Soil Sci Soc Am J 76(1):151–160
Chan KY, Heenan DP (1998) Effect of lime (CaCO3) application on soil structural stability of a red earth. Soil Res 36(1):73–86
Chen Y, Camps-Arbestain M, Shen Q, Singh B, Cayuela ML (2018) The long-term role of organic amendments in building soil nutrient fertility: a meta-analysis and review. Nutr Cycl Agroecosyst 111(2):103–125
Cheng Y, Wang J, Mary B, Zhang JB, Cai ZC, Chang SX (2013) Soil pH has contrasting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biol Biochem 57(Supplement C):848–857
Conyers MK, Heenan DP, Poile GJ, Cullis BR, Helyar KR (1996) Influence of dryland agricultural management practices on the acidification of a soil profile. Soil Till Res 37(2):127–141
da Costa CHM, Crusciol CAC (2016) Long-term effects of lime and phosphogypsum application on tropical no-till soybean–oat–sorghum rotation and soil chemical properties. Eur J Agron 74:119–132
Dai Z, Zhang X, Tang C, Muhammad N, Wu J, Brookes PC, Xu J (2017) Potential role of biochars in decreasing soil acidification-a critical review. Sci Total Environ 581–582:601–611.
Fageria NK (2002) Soil quality vs. environmentally-based agricultural management practices. Commun Soil Sci Plant Anal 33(13–14):2301–2329
Fageria NK, Baligar VC (2008) Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv Agron 99:345–399
Fageria NK, Nascente AS (2014) Management of soil acidity of South American soils for sustainable crop production. In: Sparks DL (ed) Adv Agron 128:221–275
Gurevitch J, Hedges L (2001) Meta-analysis: combining the results of independent experiments. In: Scheiner J (ed) Design and analysis of ecological experiments. Oxford University Press, pp 347–369
Haynes RJ (1984) Lime and phosphate in the soil-plant system. Adv Agron 37:249–315
Haynes RJ, Naidu R (1998) Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review. Nutr Cycl Agroecosyst 51(2):123–137
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80(4):1150–1156
Holland JE, Bennett AE, Newton AC, White PJ, McKenzie BM, George TS, Pakeman RJ, Bailey JS, Fornara DA, Hayes RC (2018) Liming impacts on soils, crops and biodiversity in the UK: a review. Sci Total Environ 610-611:316–332
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75(1):3–35
Jaskulska I, Jaskulski D, Kobierski M (2014) Effect of liming on the change of some agrochemical soil properties in a long-term fertilization experiment. Plant Soil Environ 60(4):146–150
Jean M-E, Phalyvong K, Forest-Drolet J, Bellenger J-P (2013) Molybdenum and phosphorus limitation of asymbiotic nitrogen fixation in forests of Eastern Canada: influence of vegetative cover and seasonal variability. Soil Biol Biochem 67(Supplement C):140–146
Jiang J, Wang YP, Yu M, Li K, Shao Y, Yan J (2016) Responses of soil buffering capacity to acid treatment in three typical subtropical forests. Sci Total Environ 563-564:1068–1077
Kabala C, Musztyfaga E, Galka B, Labunska D, Manczynska P (2016) Conversion of soil pH 1:2.5 KCl and 1:2.5 H2O to 1:5 H2O: conclusions for soil management, environmental monitoring, and international soil databases. Pol J Environ Stud 25(2):647–653
Kassel PC (2009) Report on the effects of eggshells and aglime on soil pH and crop yields, Iowa State University, Iowa State Research Farm Progress Reports
Kunhikrishnan A et al (2016) Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv Agron 139:1–71
Liaw A, Wiener M (2002) Classification and regression by randomForest. Forest 2(3):18–22
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J (2016) Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy 8(2):392–406
Luo Y, Hui D, Zhang D (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87(1):53–63
MacAdam JW, Nelson CJ (2017) An introduction to grassland agriculture. In: Moore KJ (ed) Physiology of forage plants Blackwell Publishing, Ames, IA, pp 73–98
McLaren RG, Cameron KC (1996) Soil science sustainable production and environmental protection. Oxford University Press
Minasny B, McBratney AB, Brough DM, Jacquier D (2011) Models relating soil pH measurements in water and calcium chloride that incorporate electrolyte concentration. Eur J Soil Sci 62(5):728–732
Myers JA, McLean EO, Bigham JM (1988) Reductions in exchangeable magnesium with liming of acid Ohio soils. Soil Sci Soc Am J 52(1):131–136
Osenberg CW, Sarnelle O, Cooper SD, Holt RD (1999) Resolving ecological questions through meta-analysis: goals, metrics, and models. Ecology 80(4):1105–1117
Pagani A, Mallarino AP (2012a) Comparison of methods to determine crop lime requirement under field conditions. Soil Sci Soc Am J 76(5):1855–1866
Pagani A, Mallarino AP (2012b) Soil pH and crop grain yield as affected by the source and rate of lime. Soil Sci Soc Am J 76(5):1877–1886
Pagani A, Mallarino AP (2015) On-farm evaluation of corn and soybean grain yield and soil pH responses to liming. Agron J 107(1):71–82
Pierce FJ, Warncke DD (2000) Soil and crop response to variable-rate liming for two Michigan fields. Soil Sci Soc Am J 64(2):774–780
Rippy JFM, Nelson PV, Hesterberg DL, Kamprath EJ (2007) Reaction times of twenty limestones. Commun Soil Sci Plant Anal 38(13–14):1775–1783
Smil V (2002) Nitrogen and food production: proteins for human diets. Ambio 31(2):126–131
Smith KA, Goins LE, Logan TJ (1998) Effect of calcium oxide dose on thermal reactions, lime speciation, and physical properties of alkaline stabilized biosolids. Water Environ Res 70(2):224–230
Sumner M, Noble A (2003) Handbook of soil acidity. In: Rengel Z (ed) Marcel Dekker, New York, pp 1–28
Therneau T, Atkinson B, Ripley B (2015) rpart: recursive partitioning and regression rrees. R package version 4:1–10
Thomas GW, Hargrove WL (1984) The chemistry of soil acidity. In: Adams F (ed) Soil acidity and liming. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI, pp 3–56
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Statistical Softw 36(3):1–48
Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In: Date RA, Grundon NJ, Rayment GE, Probert ME (eds) Plant-soil interactions at low pH: principles and management: Proceedings of the Third International Symposium on Plant-Soil Interactions at Low pH, Brisbane, Queensland, Australia, 12–16 September 1993. Springer Netherlands, Dordrecht, pp 5–19
Woodard H, Bly A (2010) Soil pH change and crop responses with long-term liming applications in tilled and untilled systems. Commun Soil Sci Plant Anal 41(14):1723–1739
Acknowledgments
We thank all the researchers whose data were used in this meta-analysis.
Funding
This research was supported by grants from the China Agriculture Research System (No. CARS-35-32), the Shandong Agricultural Science and Technology Innovation Project (CXGC2016B03), the Forage Industrial Innovation Team, the Shandong Modern Agricultural Industrial and Technical System (SDAIT-23-02), and the China Scholarship Council (File no. 201606180104).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Electronic supplementary material
ESM 1
(DOCX 810 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Cui, S., Chang, S.X. et al. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: a global meta-analysis. J Soils Sediments 19, 1393–1406 (2019). https://doi.org/10.1007/s11368-018-2120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2120-2