Abstract
The low availability of phosphorus (P) in tropical soils may hinder the initial establishment of forest species planted in disturbed areas. However, the effects of P fertilization as a strategy to improve the survival, growth and photosynthesis-related traits of Amazonian tree species in P-limited soils remain unclear. Here, we investigate how contrasting levels of P fertilization affect the establishment of native Amazonian tree species with different successional statuses. We measured the survival, growth (height and root collar diameter) and leaf traits (e.g., chlorophyll a fluorescence parameters, leaf nutrients and gas exchange) of ten species (five pioneers and five nonpioneers) subjected to contrasting P supplies (−P = 0 kg P2O5 ha−1 and +P = 160 kg P2O5 ha−1) in the field over 24 months. Phosphorus fertilization improved the availability of P in the soil. Pioneers species were more responsive to fertilization. Erythrina fusca showed high mortality under −P, while Cedrela fissilis, Cordia alliodora and Guazuma ulmifolia (pioneers) exhibited the highest increases in growth rates after fertilization. Fertilization improved the P content in the leaves in the noonpioneers group, while both ecological groups had the highest K content in the leaves under no P fertilization. Carbon capture (stomatal conductance) increased only in pioneers species. Conversely, positive effects on carbon assimilation at the leaf level were not observed. P fertilization reduced the P use efficiency of nonpioneers species. Fertilization is an important management practice to relieve the effects of stress induced by soil phosphorus starvation on the establishment of Amazonian trees and is modulated by successional status and species identity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a widely limiting nutrient of aboveground plant production in terrestrial ecosystems (Hou et al. 2020). Most Amazonian soils, for example, have low fertility and are characterized mainly by high acidity, low exchange capacity and low P availability (Walker and Syers 1976; Moreira and Fageria 2009; Quesada et al. 2012). The soils of Amazonas state (Brazil) can exhibit values < 5.4 mg P kg−1 (Moreira and Fageria 2009). In addition to natural low fertility, deforestation can exacerbate P limitation by restricting mobilization and increasing fixation in soils in disturbed areas (McGrath et al. 2001; Ketterings et al. 2002).
The input of P by fertilization has positively affected the growth and survival of tree species (Resende et al. 1999; Uddin et al. 2009; Tng et al. 2014). However, there are cases in which even after an increase in available P in the soil through the addition of chemical fertilizer, the species did not exhibit a growth response (Driessche 1980). Likewise, different responses between species submitted to fertilization have been observed (Barrow 1977). These differences may be associated with successional status (Resende et al. 1999) and may vary between species and their characteristics (Groves and Heraitis 1976; Barrow 1977; Resende et al. 2005).
Pioneers species have higher growth rates than nonpioneers species and can show greater nutrient use efficiency, maintaining their growth even in soils limited by P (Hidaka and Kitayama 2013; Zhang et al. 2018). However, when submitted to fertilization treatments, pioneers species tend to show positive responses to the input of P (Resende et al. 1999; Fonseca e Cruz et al. 2011). Conversely, nonpioneers species have slow growth and may show low nutrient use efficiency (Zhang et al. 2018). The effects of P fertilization are normally less significant for the growth of nonpioneers species due to the nutrient reserves of seeds and conservative life-history strategies (Huante et al. 1995; Milberg and Lamont 1997; Resende et al. 1999). The conservative strategies of nonpioneers species result in low growth rates with high survival during their initial establishment (Poorter et al. 2008; Chave et al. 2009; Wright et al. 2010). These responses are related to resource utilization or the leaf economic spectrum, which consists of the leaf strategies of plants and how they change their chemical, structural, and/or physiological properties to maintain their growth or survival (Wright et al. 2004; Hidaka and Kitayama 2013; Zhang et al. 2018).
Leaf traits representative of the leaf economic spectrum can reflect the trade-off between growth and survival (Sterck et al. 2006; Martínez-Garza et al. 2013; Luo et al. 2016; Guimarães et al. 2018). Plants growing in sites limited by P may have reduced photosynthetic capacity (Wright et al. 2004; Warren et al. 2011), low leaf nutrient content (Wright et al. 2004; Hidaka and Kitayama 2009), and consequently, slow growth rates (Warren et al. 2011; Zavisic and Polle 2018). However, tropical tree species adapted to infertile soils can maintain their photosynthetic capacity by increasing their P allocation to metabolic foliar P fractions (Mo et al. 2019). The relevance of using nutrient use efficiency as a measured trait to determine the interspecific growth differences during the initial establishment of plants on infertile soils has been highlighted for plantations of Amazonian trees (Santos Jr et al. 2006; Guimarães et al. 2018). However, how the controlled supply of P by fertilization drives the growth and photosynthetic mechanisms of native species in the field is still unknown.
In this study we tested the hypothesis that the effects of P fertilization in a mixed plantation of Amazonian trees are modulated by successional status and species identity. Specifically, we aim to answer the following questions: how variable are the establishment responses of Amazonian trees under contrasting P conditions in the soil? Are these responses related to successional status and species identity? We used a two-year-old mixed plantation of ten Amazonian tree species growing in the field under contrasting levels of P supply (0 and 160 kg P ha−1). In additional to soil analyses, we measured demographic rates and photosynthesis-related traits.
Materials and methods
Study site
The experiment was conducted at the Experimental Farm of the Federal University of Amazonas (02° 38′ S and 60° 03′ W), Manaus, Amazonas, Brazil. The Central Amazon region is characterized by an annual precipitation of 2350 mm, monthly air temperature ranging from 26.4 to 28.5 °C and air humidity reaching an average value of 75% in the dry season and 85 % in the wet season (data from 1988 to 2018; INMET 2019). The precipitation seasonality is generally moderate, with a dry season from August to September (Sombroek 2001). The soil is clayey yellow Latosol (Chauvel 1982). The surrounding area is a rainforest with an enclosed canopy (Guillaumet and Kahn 1982). The site is a disturbed area where the mature forest was removed in 1985; the site experienced several changes in soil use (mainly agricultural activities) until it was completed abandoned. The site was dominated by weeds (Brachiaria spp.) before planting, and these weeds acted as a strong barrier to the establishment of natural regeneration.
Experimental installation: species selection and soil preparation
Ten native forest species were selected based on their socioeconomic and ecological importance in the region and on the availability of seeds in the planting year. The species were divided into two successional statuses, with five pioneers species and five nonpioneers species (Table 1).
Before the experimental installation, the soil had a mean clay content of 75%, pHH2O = 4.4 and available phosphorus (P) = 3.12 mg kg−1 (Luvison, 2018). For soil acid correction, 108.8 g of dolomitic limestone (PRNT 92%; Ca 32%; Mg 15%) was applied 60 days before planting in each pit. Fertilizers were applied to all plants during planting. We used 10 g of FTEBR12 (Mo 0.001%, B 1,8%, Cu 0.8%, Mn 2%, and Zn 0.7%), 16 g of urea (45% N) and 11.5 g of potassium chloride (58% K20). The same amounts of urea and potassium chloride were applied thirty, sixty and ninety days after planting. The dosages and methods that guaranteed the minimum nutrient requirements of native tropical forest species were obtained from a literature review (Furtini Neto et al. 2000; Resende et al. 2005; Campoe 2014; Alvarado 2015).
Experimental design and phosphorus fertilization treatments
The experiment consisted of a factorial design in randomized blocks with three replications. The factors were constituted by five pioneers species and five nonpioneers forest species at two levels of P fertilization. The contrasting levels of P supply (0 and 160 kg P ha−1, named −P and +P, respectively) were applied using a triple superphosphate fertilizer (46% P2O5) to each sapling in alternated lines of pioneers and nonpioneers species (Nave and Rodrigues 2007). The planting lines (pioneers or nonpioneers) were planted with a total of 15 saplings, with three pseudoreplicates of each species. The planting spacing was 3 × 2 m. The planting was carried out in 40 × 50 cm pits prepared with a soil drill attached to a tractor. The control of ants was performed before and after planting through the application of granulated formicide. The control of weed competition was performed by manually crowning semi-mechanized mowing between the lines every three months.
Soil analyses
Two years after planting, soil samples were collected from the pits of both treatments at a depth of 0–20 cm. Three simple samples were collected per planting line to comprise one homogeneous sample.
The soil samples were analyzed for pH (H2O and KCl), potential acidity (H+Al), soil organic matter (SOM) and concentrations of carbon (C), macronutrients such as phosphorus (P), potassium (K), magnesium (Mg), calcium (Ca), and micronutrients such as iron (Fe), zinc (Zn) and manganese (Mn). Subsequently, base saturation and the sum of bases and (V%, SB) were calculated. The pH was determined using an electrode (Thomas 1996). The extraction of potential acidity (H + Al) from the soil was carried out with a solution of calcium acetate and alkalimetric titration of the extract (Mclean 1965). The levels of SOM and C were determined by the volumetric method using potassium dichromate (Walkley and Black 1934). The concentrations of Ca and Mg were obtained by atomic absorption spectrophotometry (Mclean 1965) after extraction in 1 M KCl (Miyazawa et al. 1999). The macronutrients (P and K) and micronutrients (Fe, Zn, and Mn) were extracted with the Mehlich solution (0.05 M HCl, 0.0125 M H2SO4). The available P was determined by visible spectrophotometry (660 nm), while the K and micronutrients were quantified by atomic absorption spectrophotometry (Thomas 1996).
Demographic rate measurements
The temporal variation in survival and growth was monitored quarterly for 24 months from April 2017 to April 2019. In each monitoring campaign, we recorded the number of live saplings, and the root collar diameter (D) (5 cm above the ground) and total height (H) of each sapling were measured.
We calculated the annual survival rate (SR) according to Poorter and Bongers (2006). The daily survival rate (DSR) of the seedlings for each species consisted of the slope of the log10 regression (percentage of surviving seedlings) against time (in days). The annual survival rate was calculated as 10365DSR.
The relative growth rates were calculated as follows: RGRheight = (lnH2 − lnH1)/(t2 − t1) and RGRdiameter = (lnD2 − lnD1)/(t2 − t1) according to Hunt (1990) and Bugbee (1996), where H1 = height (cm) at the time of planting; H2 = height after two years; D1 = diameter (mm) at the time of planting; D2 = diameter after two years; and t2 and t1 = interval between two years.
Leaf nutrient contents
The nutrient analyses were performed on three to six leaves per plant. All leaves of each plant were ground to form a homogeneous composite sample. The leaf nitrogen content was determined by the Kjeldahl method with digestion, distillation and titration (Bremner 1996). Leaf P was determined by spectrophotometry (λ = 750 nm) after the molybdate method (Murphy and Riley 1962). The leaf content of potassium was determined by atomic absorption spectrometry (1100B, PerkinElmer, Ueberlingen, Germany).
Fluorescence parameters
Chlorophyll a fluorescence analyses were performed with a portable fluorimeter (PEA, MK2-9600, Hansatech, Norfolk, UK) adjusted to emit a saturating light pulse of 3000 µmol m−2 s −1 at a wavelength of 650 nm for 1 s. Measurements were made on three leaves per plant at 4:30 am (predawn). The fluorescence data from typical chlorophyll a polyphasic fluorescence transient rise O–J–I–P used by the JIP-test were the minimal fluorescence intensity (F0 = 50 µs); the maximal fluorescence intensity (Fm); the variable fluorescence (Fv=Fm − F0) and the maximum quantum yield of PSII (Fv/Fm) (Strasser et al. 1995, 1999, 2010; Tsimilli-Michael and Strasser 2008).
Photosynthesis and photosynthetic nutrient use efficiency
The net photosynthesis per unit leaf area (An), stomatal conductance (gs) and transpiration rate (E) were measured by using an infrared gas analyzer (LI-6400XT, LI-COR, USA). The measurements were made on one leaf per plant. Before measurements, we performed experimental tests on three leaves per plant to ensure the selection of leaves with maximum values of photosynthesis at light saturation (Amax). The measurements were made between 08:00 am and noon. The chamber was adjusted to a flow of 400 µmol s−1; CO2 concentration of 400 µmol mol−1; 21 mmol mol−1 H2O vapor concentration; leaf temperature of 31 °C; and photosynthetic photon flux density (PPFD) of 2000 µmol m−2 s−1 (except for Rd when PPFD was 0 µmol m−2 s−1). The maximum photosynthesis per unit mass (Amass) was calculated by multiplying the specific leaf area (SLA) by Amax and by 0.1 depending on the differences between the units of each variable (µmol m−2 s−1 × cm2 g−1). The SLA was calculated from the ratio of the fresh leaf area to the dry leaf mass at 65 °C until obtaining a constant leaf mass using leaf discs of known area (0.283 cm2) (Evans and Poorter 2001). The photosynthetic nutrient use efficiency (PnutrientUE)—for macronutrients (P; N; K)—was calculated by the ratio between the rates of photosynthesis per unit mass (Amax * SLA * 0.1) and the respective leaf nutrient content of each plant (Field and Mooney 1986; Ferreira et al. 2015).
Statistical analysis
The data were previously submitted to the assumptions of normality and homoscedasticity by Shapiro-Wilk and Levene tests, respectively. Nonnormality and heterogeneity were corrected by log transformation.
Demographic rates and photosynthetic characteristics were analyzed by two-way ANOVA to test the effects of fertilization treatments, ecological group and their interaction (2 × 2), as well as fertilization treatments, species and their interactions, separately for each ecological group (5 × 2). In the case of significance (P ≤ 0.05), Duncan’s post hoc test was performed. All analyses were performed using Statistica 9.0 software.
Results
Effects of fertilization on the chemical properties of the soil
Two years after fertilization, the pH values increased in both treatments. In addition, in the fertilized treatments, there was a sevenfold increase in available P and an increase of 27% in the Fe concentrations (Table 2).
Effects of P fertilization on survival and growth
Survival had the effect of all factors (T = P ≤ 0.001; S = P ≤ 0.001; T×S = P ≤ 0.001), due to the high mortality of the species E. fusca, which showed a survival of 2% when not fertilized. This caused a significant difference in group-level survival for all factors (T= P ≤ 0.001; S = P ≤ 0.001; T×S = P ≤ 0.001). Despite the high mortality of E. fusca, the other pioneer species showed high survival in both treatments, ranging between 87 and 100%, and 77 and 100% for nonpioneers, with no significant effect of fertilization at the species and group level. The dynamics of survival behavior over the months, we can observe that, E. fusca (pioneer species) under −P had significant reductions in its survival values after 10 months, reaching 0% at 24 months (Fig. 1a).
A. leiocarpa exhibited the highest height values in both −P and +P at 15 months after planting. C. alliodora and E. fusca increased their height under +P over time (Fig. 1c, d). The interspecific differences in absolute values in diameter over time were more evident under +P. C. alliodora, E. fusca and C. fissilis showed the largest diameters, while (A) leiocarpa and S. macrophylla exhibited intermediate diameters, and H. courbaril, D. odorata, C. guianensis and (B) excelsa had the smallest diameters (Fig. 1e, f).
Survival (pioneers: a, nonpioneers: b), height (pioneers: c, nonpioneers: d) and diameter (pioneers: e, nonpioneers: f) (mean ± SD) of plants subjected to fertilization treatments with contrasting levels of P (filled geometric figures = −P; unfilled geometric figures = + P) over 24 months. Pioneers species: Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; Ef = Erythrina fusca; and Gu = Guazuma ulmifolia; Nonpioneers species: Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla
The growth rates for height and diameter were affected in different ways by fertilization and species according to successional status (Figs. 2 and 3). For nonpioneers species (NP), the relative growth rate (RGR) for height was not affected by fertilization, while there were differences among species. The RGR height values for D. odorota were approximately two times higher than those for C. guianensis. For the RGR of diameter, NP exhibited no significant effect on any of the treatments. Conversely, the pioneers group showed increasing on average values of RGRs in height and diameter of 1.4 and 1.3-fold, respectively, as affected by fertilization. C. alliodora and C. fissilis had height and diameter values that were 44.5% and 36% higher, respectively, in the +P treatment than in the −P treatment. A. leiocarpa showed average height values that were 27% and 46% higher than those of G. ulmifolia in the +P and −P treatments, respectively.
Relative growth rates related to height (mean ± SD) of nine Amazon tree species, four pioneers species (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia; Erythrina fusca was excluded due to high mortality) and five nonpioneers species (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (−P and +P) (n = 3), where T = treatment; S = species; and T × S = the interaction between treatment and species. Relative growth rates related to height (mean ± SD) of ecological groups, pioneers and nonpioneers, where T =treatment; EG= ecological group; and T × EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05 ; P < 0.01**; and P < 0.001***). The mean values of treatments for each species followed by asterisks are different (P < 0.05*; P < 0.01**; and P < 0.001***) according to Duncan’s test
Relative growth rates related to diameter (mean ± SD) of nine Amazon tree species, pioneers (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia) and nonpioneers (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (−P and +P) (n = 3), where T = treatment; S = species; and T × S = the interaction between treatment and species. Relative growth rate in related to diameter (mean ± SD) of ecological groups, pioneers and nonpioneers, where T = treatment; EG= ecological group; and T × EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05*; P < 0.01**; and P < 0.001***). The mean values of treatments for each species followed by asterisks are different (P < 0.05*; P < 0.01**; and P < 0.001***) according to Duncan’s test
Effects of phosphorus fertilization on photosynthesis-related traits
Fluorescence and gas exchange
Fertilization did not significantly affect the maximum quantum yield of photosystem II (Fv/Fm) of species during the predawn measurements (Fig. 4a). The values of maximum photosynthesis per unit mass (Amass) were different only for nonpioneers species and were not significantly affected by fertilization (Fig. 4b). H. courbaril had values 43% and 38% higher than those of C. guianensis for −P and +P, respectively. Fertilization affected the stomatal conductance (gs) (Fig. 4c) and transpiration (E) of pioneers species (Fig. 4d). Pioneers group exhibited increasing of 1.3-fold on gs after fertilization. The species most positively affected by fertilization was C. alliodora, with values that were 37% (gs) and 31% (E) higher when fertilized. A. leiocarpa showed the highest values of gs and E, which were between 70 and 50% (−P and +P) higher than those for C. fissilis for gs and between 42 and 28% (−P and +P) for E compared to the same species.
Maximum quantum yield of photosystem II (Fv/Fm; a), Maximum photosynthesis per unit mass (Amass; b) and stomatal conductance (gs; c), transpiration (E; d), (mean ± SD) of nine Amazon tree species, pioneers (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia) and nonpioneers (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (-P and +P) (n = 3), where T = treatment; S = species; and T × S = the interaction between treatment and species. Maximum photosynthesis per unit mass (Amass; a) and stomatal conductance (gs; b) of ecological groups, pioneers and nonpioneers, where T = treatment; EG= ecological group; and T × EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05*; P < 0.01**; and P < 0.001***). The mean values of treatments for each species followed by asterisks are different (P < 0.05*; P < 0.01**; and P < 0.001***) according to Duncan’s test
Leaf nutrient content
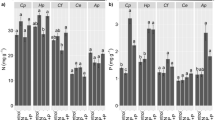
The leaf P content was significantly affected by fertilization treatment in nonpioneers group (Fig. 5a), with average values 1.6-fold higher in +P than −P. C. guianensis, H. courbaril and S. macrophylla exhibited values approximately 93%, 61% and 73% higher when submitted to P fertilization. H. courbaril had values 21 and 37% higher than those of D. odorata in −P and +P, respectively. The leaf nitrogen content was significantly affected by the species in both the pioneers and the nonpioneers groups. B. excelsa, D. odorata and H. courbaril had the highest values of nitrogen leaf content among nonpioneers species, while C. fissilis, A. leiocarpa and C. alliodora had the highest values among pioneers species (Fig. 5b). The N/P ratio decreased significantly across species as affected by P fertilization (Fig. 6). The highest reductions were observed in C. alliodora and D. odorata, which also exhibited the highest values of this parameter under −P.
Leaf K concentration had an effect of the treatment and species for the pioneers (T = P ≤ 0.001; S = P ≤ 0.05). For nonpioneers there was an effect of treatment (T = P ≤0.001). At group level, both groups had treatment effect (T = P ≤0.001). Pioneers and nonpioneers groups had K leaf content 1.5-fold higher under −P than + P. C. alliodora (under −P) exhibited the highest values among pioneers species.
Macronutrient (P; a, N; b) contents in the leaves, (mean ± SD) of nine Amazon tree species, pioneers (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia) and nonpioneers (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (-P and +P) (n = 3), where T = treatment; S = species; and T × S = the interaction between treatment and species. Macronutrient contents in the leaves (mean ± SD) of ecological groups, pioneers and nonpioneers, where T = treatment; EG= ecological group; and T × EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05*; P < 0.01**; and P < 0.001***). The mean values of treatments for each species followed by asterisks are different (P < 0.05*; P < 0.01**; and P < 0.001***) according to Duncan’s test
The N/P ratio, (mean ± SD) of nine Amazon tree species, pioneers (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia) and nonpioneers (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (-P and +P) (n = 3), where T = treatment; S = species; and T × S = the interaction between treatment and species. The N/P ratio (mean ± SD) of ecological groups, pioneers and nonpioneers, where T = treatment; EG= ecological group; and T × EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05*; P < 0.01**; and P < 0.001***). The mean values of treatments for each species followed by asterisks are different (P < 0.05*; P < 0.01**; and P < 0.001***) according to Duncan’s test
Photosynthetic nutrient use efficiency
P fertilization decreased the photosynthetic P use efficiency of nonpioneers species, with the exception of B. excelsa and D. odorata, which also had the highest values for this parameter among nonpioneers species (Fig. 7a). B. excelsa was the only species that increased its values of nitrogen use efficiency after P fertilization (Fig. 7a). Fertilization with P increased the potassium use efficiency in all species, pioneers (T = P ≤ 0.01) and nonpioneers (T = P ≤ 0.001), but the increase was more accentuated for the nonpioneers group.
Phosphorus (PPUE; a) and nitrogen (PNUE; b) use efficiency (mean ± SD) of nine Amazon tree species, pioneers (Cf = Cedrela fissilis; Ca = Cordia alliodora; Al = Apuleia leiocarpa; and Gu = Guazuma ulmifolia) and nonpioneers (Ca = Carapa guianensis; Be = Bertholletia excelsa; Do = Dipteryx odorata; Hc = Hymenaea courbaril; and Sm = Swietenia macrophylla), subjected to fertilization with contrasting levels of P (−P and +P) (n = 3), where T = treatment; S = species; and T x S = the interaction between treatment and species. Nutrient use efficiency (mean ± SD) of ecological groups, pioneers and nonpioneers, where T = treatment; EG= ecological group; and T x EG = the interaction between treatment and ecological group according to two-way ANOVA (P > 0.05 ns; P < 0.05 *; P < 0.01 **; and P < 0.001 ***). The mean values of treatments for each species followed by asterisks are different (P < 0.05 *; P < 0.01 **; and P < 0.001 ***) according to Duncan’s test
Discussion
Phosphate fertilization improved the availability of P in the soil. The highest P concentrations (seven-fold higher than the control) were provided after the correction of acidity and by the efficiency of the applied mineral (Sanchez and Uehara 1980; Haynes 1982). The increase in P availability significantly improved the survival and growth in diameter of pioneers species (Fig. 1a; Table 2). Specifically, Erythrina fusca showed greater sensitivity to fertilization, with high mortality when not fertilized (Fig. 1a). The sensitivity of this genus to low P availability was also observed by Radomski and Oliveira (2018) in a restoration project of pasture areas. In accordance with our results for pioneers species, seedlings of native Amazon forest species were also positively affected by P supply under greenhouse conditions (Resende et al. 1999; Santos et al. 2006; Fontes et al. 2013). These results reinforce the importance of correction and fertilization practices to improve the chemical quality of Amazonian soils, since the soils in this region generally have high acidity and a low natural availability of P, which can compromise the productivity of forests (Falcão and Silva 2004; Quesada et al. 2012). In a general way, corroborating Resende et al. (1999), pioneers species have shown more growth plasticity to P supply than nonpioneers species. Differences in responses between groups may be associated with their characteristics. Pioneers species have small seeds and a high growth rate, thus they can respond quickly to fertilization; however, nonpioneers species adopt conservative strategies for maintaining their survival under low P availability. Thus, fertilization treatments are important for relieving the effects of stress induced by soil P starvation on the establishment of Amazonian trees, especially for pioneers species.
Nonpioneers species showed significant accumulation of leaf P under fertilization treatment (Fig. 6b), while both successional groups exhibited the highest values of K in the leaves under low P availability (Fig. 6c). Despite the changes in the nutritional status of the plants, we did not find significant effects of P fertilization on photosynthesis at the leaf level (Fig. 5a). On the other hand, pioneers species had a higher potential for carbon capture due to the increased stomatal conductance under P fertilization (Fig. 5b). Although P is not directly linked to stomatal regulation, greater availability of P in the soil positively influences osmotic regulation, thus affecting the stomatal conductance of plants (Firmano et al. 2009; Chaturvedi et al. 2011; Cunha et al. 2016). Potassium has been suggested to relieve the effects of high-light stress on crop plants under fertilization (Waraich et al. 2012; Hasanuzzaman et al. 2018). Considering that the seedlings in our experiment grew under full sunlight conditions, the higher K content in the leaves may have been important to maintain carbon assimilation under low P availability in the soil. Supporting this statement, we did not find any differences in the photochemical performance of either species type between contrasting P conditions (Table 2). Consequently, we observed a slight increasing tendency in the values of P use-efficiency, especially for nonpioneers species, in P-limited soil (Fig. 7b). Thus, we believe that the absence of nonstomatal limitations may have been important for the photosynthetic performance of trees under contrasting P conditions in the soil.
Contrary to our expectation, despite the higher N/P ratios indicating P limitation under the no P fertilization (−P) treatment for both successional statuses, the photosynthetic capacity of the trees did not appear to be affected, especially those of the nonpioneers species. The age of trees may be a determinant factor modulating the responses of native species to fertilization (Resende et al. 2005), reinforcing the importance of medium- and long-term monitoring efforts. Although our study was performed on a young plantation, we believe that our findings have important implications for the establishment of Amazonian tree species in P-limited soils. We showed that the effects of the P supply on survival and growth are species-specific, suggesting the need for more detailed studies of forest nutrition related to each priority species and not only to successional status. The best growth responses of C. fissilis, C. alliodora and G. ulmifolia to fertilization suggest the importance of the P supply to the planting of these species, and the high short-term mortality of E. fusca, a fast-growing leguminous species, may indicate that it is not sustainable to plant leguminous trees in P-limited soils. Finally, future research should assess the interspecific differences on the above- and belowground biomass due to P fertilization.
Conclusions
The short-term effects of P fertilization in a mixed plantation of Amazonian tree species are represented by increase in the survival, growth and nutritional status of plants based on successional status and species identity.
References
Alvarado A (2015) Plant nutrition in tropical forestry. Trop For Handb 2:1113–1202. https://doi.org/10.1007/978-3-642-41554-8_105-2
Araújo AC, Nobre AD, Kruijt B, Elbers JA, Dallarosa R, Stefani P, Valentini R (2002) Comparative measurements of carbon dioxide fluxes from two nearby towers in a central Amazonian rainforest: The Manaus LBA site. J Geophys Res Atmos 107(D20), LBA-58. https://doi.org/10.1029/2001JD000676, 2002
Barrow NJ (1977) Phosphorus uptake and utilization by tree seedlings. Aust J Bot 25(6):571–584. https://doi.org/10.1071/bt9770571
Bremner JM (1996) Nitrogênio total. Métodos de análise de solo: Parte 3 Métodos químicos, 5:1085–1121. https://doi.org/10.2136/sssabookser5.3.c37
Bugbee BG (1996) Growth analyses and yield components. In: Salisbury FB (ed) Units, symbols and terminology for plant physiology. Oxford University Press, pp 115–119
Campoe OC, Iannelli C, Stape JL, Cook RL, Mendes JCT, Vivian R (2014) Atlantic forest tree species responses to silvicultural practices in a degraded pasture restoration plantation: from leaf physiology to survival and initial growth. For Ecol Manag 313:233–242. https://doi.org/10.1016/j.foreco.2013.11.016
Chauvel A (1982) Os latossolos amarelos, álicos, argilosos dentro dos ecossistemas das bacias experimentais do INPA e da região vizinha. Acta Amazonica 12:47–60
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12(4):351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Chaturvedi RK, Raghubanshi AS, Singh JS (2011) Leaf attributes and tree growth in a tropical dry forest. J Veg Sci 22(5):917–931. 10.1111 / j.1654-1103.2011.01299.x
Cunha HFV, de Carvalho Gonçalves JF, Dos Santos UM, Ferreira MJ, Peixoto PHP (2016) Biomassa, trocas gasosas e aspectos nutricionais de plantas jovens de pau de balsa (Ochroma pyramidale (Cav. Ex Lamb.) Urb.) submetidas à fertilização fosfatada em ambientes contrastantes de irradiância. Scientia Forestalis 44:215–230. https://doi.org/10.18671/scifor.v44n109.21
dos Santos UM Jr, de Carvalho Gonçalves JF, Feldpausch TR (2006) Growth, leaf nutrient concentration and photosynthetic nutrient use efficiency in tropical tree species planted in degraded areas in central Amazonia. For Ecol Manag 226(1–3):299–309. https://doi.org/10.1016/j.foreco.2006.01.042
Driessche RVD (1980) Effects of nitrogen and phosphorus fertilization on Douglas-fir nursery growth and survival after outplanting. Can J For Res 10(1):65–70. https://doi.org/10.1139/x80-011
Evans J, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24(8):755–767. https://doi.org/10.1046/j.1365-3040.2001.00724.x
Falcão NP, de Silva S, JRA da (2004) Características de adsorção de fósforo em alguns solos da Amazônia Central. Acta Amazonica 34(3):337–342. https://doi.org/10.1590/S0044-59672004000300001
Ferreira MJ, Gonçalves JFC, Ferraz JBS, Corrêa VM (2015) Características nutricionais de plantas jovens de Bertholletia excelsa Bonpl. Sob tratamentos de fertilização em área degradada na Amazônia. Scientia Forestalis 43(108):863–872. https://doi.org/10.18671/scifor.v43n108.11
Field C, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. University Press, Cambridge, pp 25–55
Firmano RS, Kuwahara FA, Souza GM (2009) Relação entre adubação fosfatada e deficiência hídrica em soja. Ciência Rural 39:1967–1973
Fonseca e Cruz CA, Paiva HND, Cunha ACMCMD, Neves JCL (2011) Resposta de mudas de Senna macranthera cultivadas em Argissolo Vermelho-Amarelo a macronutrientes. Ciência Florestal 21:63–76
Fontes AG, Rodrigues ACG, Rodrigues EFG (2013) Eficiência nutricional de espécies arbóreas em função da fertilização fosfatada. Pesquisa florestal brasileira 33(73):09–18. https://doi.org/10.4336/2013.pfb.33.73.392
Furtini neto AE, Siqueira JO, Curi N, Moreira FM (2000) Fertilização em reflorestamento com espécies nativas. Nutrição e fertilização florestal. IPEF, Piracicaba, pp 351–383
Groves RH, Keraitis K (1976) Survival and growth of seedlings of three sclerophyll species at high levels of phosphorus and nitrogen. Aust J Bot 24(6):681–690. https://doi.org/10.1071/BT9760681
Guillaumet JL, Kahn F (1982) Structure et dynamisme de la forêt. Acta Amazonica 12:61–77. https://doi.org/10.1590/1809-43921982123S061
Guimarães ZTM, Dos Santos VAHF, Nogueira WLP, de Almeida Martins NO, Ferreira MJ (2018) Leaf traits explaining the growth of tree species planted in a Central Amazonian disturbed area. For Ecol Manag 430:618–628. https://doi.org/10.1016/j.foreco.2018.08.048
Haynes RJ (1982) Effects of liming on phosphate availability in acid soils. Plant Soil 68(3):289–308. https://doi.org/10.1007/BF02197935
Hasanuzzaman M, Bhuyan MHM, Nahar K, Hossain M, Mahmud JA, Hossen M, Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8(3):31. https://doi.org/10.3390/agronomy8030031
Hidaka A, Kitayama K (2009) Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen‐use efficiency of tree leaves along nutrient‐availability gradients. J Ecol 97(5):984–991
Hidaka A, Kitayama K (2013) Relationship between photosynthetic phosphorus-use efficiency and foliar phosphorus fractions in tropical tree species. Ecology and evolution 3(15):4872–4880. https://doi.org/10.1111/j.1365-2745.2009.01540.x
Hou E, Luo Y, Kuang Y, Chen C, Lu X, Jiang L, Wen D (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Commun 11(1):1–9. https://doi.org/10.1038/s41467-020-14492-w
Huante P, Rincón E, Acosta I (1995) Nutrient availability and growth rate of 34 woody species from a tropical deciduous forest in Mexico. Funct Ecol 849–858. 10.2307/2389982
Hunt R (1990) Basic Plant Growth Analysis. Unwin, Hyman, London
Instituto Nacional de Meteorologia (2019) BDMEP - Banco de Dados Meteorologicos para Ensino e Pesquisa. http://www.inpe.br/cra/projetos_pesquisas/arquivos/TerraClass_2014_v3.pdf
Ketterings QM, van Noordwijk M, Bigham JM (2002) Soil phosphorus availability after slash-and-burn fires of different intensities in rubber agroforests in Sumatra, Indonesia. Agric Ecosyst Environ 92(1):37–48. https://doi.org/10.1016/S0167-8809(01)00287-0
Luo Y, Liu J, Tan S, Cadotte MW, Xu K, Gao L, Li D (2016) Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, Southwest China. Plant Div 38(6):303–311. https://doi.org/10.1016/j.pld.2016.11.002
Luvison M (2018) Estabelecimento Inicial De Espécies Florestais De Contrastantes Grupos Sucessionais Submetidas À Fertilização Fosfatada. Dissertação (mestrado) - INPA, Manaus
Martínez-Garza C, Bongers F, Poorter L (2013) Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? For Ecol Manag 303:35–45. https://doi.org/10.1016/j.foreco.2013.03.046
McGrath DA, Smith CK, Gholz HL, de Assis Oliveira F (2001) Effects of land-use change on soil nutrient dynamics in Amazonia. Ecosystems 4(7):625–645. https://doi.org/10.1007/s10021-001-0033-0
McLean EO (1965) Aluminum. Methods of soil analysis: part 2 chemical and microbiological properties 9:978–998
Milberg PER, Lamont BB (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137(4):665–672. https://doi.org/10.1046/j.1469-8137.1997.00870.x
Miyazawa M, Pavan MA, Muraoka T, Carmo CAFS, Mello WJ (1999) Análise Química De Tecidos Vegetais. In: Silva FC (eds) Manual de Análise Química de Solos, Plantas e Fertilizantes. EMBRAPA, Brasília, Brasil, pp 172–223
Mo Q, Li ZA, Sayer EJ, Lambers H, Li Y, Zou B, Wang F (2019) Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct Ecol 33(3):503–513. https://doi.org/10.1111/1365-2435.13252
Moreira A, Fageria NK (2009) Soil chemical attributes of Amazonas state, Brazil. Commun Soil Sci Plant Anal 40(17–18):2912–2925. https://doi.org/10.1080/00103620903175371
Murphy JAMES, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nave AG, Rodrigues RR (2007) Combination of species into filling and diversity groups as forest restoration methodology. High Diversity Forest Restoration in Degraded Areas. Nova Science Publishers, New York, pp 103–126
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116(1–2):26–37. https://doi.org/10.1007/s004420050560
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87(7):1733–1743. https://doi.org/10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, HC, Muller-Landau (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89(7):1908–1920. https://doi.org/10.1890/07-0207.1
Quesada CA, Phillips OL, Schwarz M, Czimczik CI, Baker TR, Patiño S, Dávila EA (2012) Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 9(6):2203–2246. https://doi.org/10.5194/bg-9-2203-2012
Radomski MI, de Oliveira BT (2018) Produção de biomassa aérea e teor de nutrientes de Erythrina speciosa e Tithonia diversifolia cultivadas em Morretes, PR: resultados iniciais. Embrapa Florestas-Comunicado Técnico (INFOTECA-E)
Resende ÁVD, Furtini Neto AE, Muniz JA, Curi N, Faquin V (1999) Crescimento inicial de espécies florestais de diferentes grupos sucessionais em resposta a doses de fósforo. Pesquisa Agropecuária Brasileira 34(11):2071–2081. https://doi.org/10.1590/S0100-204X1999001100014
Resende AV, Furtini Neto AE, Curi N (2005) Mineral nutrition and fertilization of native tree species in Brazil: research progress and suggestions for management. J Sustain For 20(2):45–81. https://doi.org/10.1300/J091v20n02_03
Sanchez PA, Uehara G (1980) Management considerations for acid soils with high phosphorus fixation capacity. The role of phosphorus in agriculture. 471–514. https://doi.org/10.2134/1980.roleofphosphorus.c18
Sombroek W (2001) Spatial and temporal patterns of Amazon rainfall. AMBIO J Human Environ 30(7):388–396. https://doi.org/10.1579/0044-7447-30.7.388
Sombroek W (2001) Spatial and temporal patterns of Amazon rainfall. AMBIO J Human Environ 30(7):388–396. https://doi.org/10.1579/0044-7447-30.7.388
Sterck FJ, Poorter L, Schieving F (2006) Leaf traits determine the growth-survival trade-off across rain forest tree species. Am Nat 167(5):758–765. https://doi.org/10.1086/503056
Strasserf RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61(1):32–42. https://doi.org/10.1111/j.1751-1097.1995.tb09240.x
Strasser RJ, Srivastava A, Tsimilli-Michael M (1999) Screening the vitality and photosynthetic activity of plants by fuorescence transient. In: Behl RK, Punia MS, Lather BPS (eds) Crop improvement for food security. SSARM, Hisar, pp 72–115
Strasser RJ, Tsimilli-Michael M, Giang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant. Haberlea rhodopensis.Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1797. 1313–1326. https://doi.org/10.1016/j.bbabio.2010.03.008
Thomas GW (1996) Soil pH and soil acidity. In ‘Methods of soil analysis. Part 3. Chemical methods’. Soil Science Society of America: Madison, WI. (Ed. DL Sparks), pp 475–490
Tng DY, Janos DP, Jordan GJ, Weber E, Bowman DM (2014) Phosphorus limits Eucalyptus grandis seedling growth in an unburnt rain forest soil. Front Plant Sci 5:527. https://doi.org/10.3389/fpls.2014.00527
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In mycorrhiza (pp 679–703). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-78826-3_32
Uddin MB, Mukul SA, Khan MASA, Hossain MK (2009) Seedling response of three agroforestry tree species to phosphorous fertilizer application in Bangladesh: growth and nodulation capabilities. J For Res 20(1):45–48. https://doi.org/10.1007/s11676-009-0007-1
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15(1):1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38. https://doi.org/10.1097/00010694-193401000-00003
Waraich EA, Ahmad R, Halim A, Aziz T (2012) Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nutr 12(2):221–244
Warren CR (2011) How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol 31(7):727–739. https://doi.org/10.1093/treephys/tpr064
Whitmore TC (1989) Canopy gaps and the two major groups of forest tree. Ecology 70(3):536–538. https://doi.org/10.2307/1940195
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Flexas J (2004) The worldwide leaf economics spectrum. Nature 428(6985):821–827. https://doi.org/10.1038/nature02403
Wright SJ, Kitajima K, Kraft NJ, Reich PB, Wright IJ, Bunker DE, Engelbrecht BM (2010) Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91(12):3664–3674. https://doi.org/10.1890/09-2335.1
Zavišić A, Polle A (2018) Dynamics of phosphorus nutrition, allocation and growth of young beech (Fagus sylvatica L.) trees in P-rich and P-poor forest soil. Tree Physiol 38(1):37–51. https://doi.org/10.1093/treephys/tpx146
Zhang G, Zhang L, Wen D (2018) Photosynthesis of subtropical forest species from different successional status in relation to foliar nutrients and phosphorus fractions. Sci Rep 8(1):1–12. https://doi.org/10.1038/s41598-018-28800-4
Acknowledgements
We acknowledge the Fazenda Experimental da Universidade Federal do Amazonas and the Laboratório de Silvicultura group for their support of all fieldwork. We are grateful to Laboratório Temático de Solos e Plantas for supporting the nutrients analyses at the Instituto Nacional de Pesquisa da Amazonia (INPA).
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (Chamada Universal/MCTI/CNPq No. 01/2016, Processo No. 425260/2016-0). AOC. and AHBN. were supported by scholarships provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). MJF acknowledges the research productivity Grant—PQ provided by CNPq (process # 309870/2020-8).
Author information
Authors and Affiliations
Contributions
MJF and ML designed the research. MJF and ML installed the experiment. AOC, AHBN and VAHFS collected the physiological and growth data. ML and ZTMG collected the growth data. AOC, MJF and ZTMG performed the statistical analysis. AOC, AHBN, MJF and ZTMG wrote the first version of the manuscript. All authors contributed to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Carvalho, A., Neves, A.H.B., Luvison, M. et al. Short-term effects of phosphorus fertilization on Amazonian tree species in a mixed plantation. New Forests 53, 851–869 (2022). https://doi.org/10.1007/s11056-021-09890-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-021-09890-x