Abstract
Key message
P deposition can alleviate P limitation in subtropical forests and P deposited in conjunction with high quantities of N does not enhance P limitation.
Abstract
Increasing nitrogen (N) and phosphorus (P) deposition could influence plant growth and survival to varying extents. N and P deposition is essential for subtropical forests where plant growth is limited by lower soil P availability in highly weathered soils. However, whether N and P deposition can increase leaf P concentration and alleviate P limitation is unclear. We investigated changes in N and P concentrations, N:P ratios, and resorption for six dominant species (two tree species and four understory species), following 2 years of N and P additions in a subtropical forest in southern China. P addition either alone or together with the addition of N increased green leaf P concentrations (except in Schima superba) and senesced leaf P and decreased N:P ratios (except in Pinus massoniana), but had no influence on P resorption. N addition had no apparent influence on leaf N concentrations, N:P ratios, or N resorption in all species. The considerable influence of P addition can be explained by rising soil P availability. Our results suggest that subtropical forests are P limited and that increasing P deposition can moderately alleviate the limitation of P. Furthermore, P deposited in conjunction with high quantities of N does not enhance P limitation, as it is insensitive to elevated N input.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) and phosphorus (P) are common elements that are necessary for plant growth in terrestrial ecosystems. N is needed for the synthesis of amino acids, proteins, and the enzymes that catalyze photosynthesis. P is the primary constituent of ATP and is an important element in nucleic acids, phospholipids, and other cellular metabolites (Elser et al. 1996). The supply of soil N and P determines plant productivity to a large extent. To explore which of these vital elements limit primary productivity, many experiments studying N and P fertilization have been conducted over the last few decades (Elser et al. 2007; Li et al. 2016), where the findings of such studies have helped elucidate the influence of increasing N and P deposition on the structure and function of terrestrial ecosystems (Bennett et al. 2001; Elser and Bennett 2011; Galloway et al. 2004).
The most important index of N or P limitation for plant growth is leaf N or P concentration. Generally, the positive relationships between leaf N or P and plant productivity have been widely observed in most N- and P-limited ecosystems (Gusewell 2004; Li et al. 2016). Furthermore, with the exception of external soil conditions (available N and P), leaf N and P concentration can be influenced by the internal resorption of N and P by plants (Aerts 1996; Killingbeck 1996). A common strategy for plants is to resorb N and P from senescing leaves prior to abscission, where it is estimated that approximately 50% of leaf N and P are recycled via this method of resorption (Yuan and Chen 2009a, b), thereby increasing the leaf N and P use efficiency. Therefore, exploring the influence of N and P deposition on leaf N and P concentrations and leaf resorption can improve our understanding of N and P limitation in terrestrial ecosystems.
Subtropical forests can be used as a model to study the effect of P limitation. With the high temperatures and quantities of precipitation common to subtropical forests, soils are depleted in P as a result of substantial weathering and high fractions of recalcitrant P (Walker and Syers 1976). Coincidently, this is also the region with the highest rate of N deposition (20–30 kg N hm−2 year−1) (Zhu et al. 2015). Furthermore, we have previously observed that P deposition occurs at a very low rate (0.34 kg hm−2 year−1) in subtropical forest ecosystems (Zhu et al. 2016). This imbalance in atmospheric N and P deposition could alter nutrient availability and further increase P limitation in these regions (Li et al. 2016; Peñuelas et al. 2013).
Given the aforementioned scenarios of P limitation in subtropical forests, we hypothesize that elevated P deposition will directly enhance soil P availability and subsequently increase leaf P concentration. To date, while the potential effect of increasing P deposition has been tested in other P-limited ecosystems, such as tropical forests (Lawrence 2001; Mayor et al. 2014; Tanner et al. 1990) and N-saturated grasslands (Huang et al. 2016; Long et al. 2016), it has not been tested in subtropical forests to the best of our knowledge. Furthermore, while it is possible that N deposition indirectly influences plant P concentration via enhanced P uptake by stimulating phosphatase activity and subsequently organic P mineralization (Marklein and Houlton 2012; Rodrı́guez and; Fraga 1999), N deposition could also decrease P uptake by roots by increasing the toxicity of soil cations such as Fe2+, Mn2+, Al3+ after soil acidification (Tian and Niu 2015). Thus, given the higher soil N availability and lower labile P substrates in subtropical forests, we hypothesized that the influence of N deposition on plant P concentration would be minimal.
Leaf N and P concentrations are influenced by leaf N and P resorption to a certain degree. Species with low P concentrations (< 0.5 mg g−1) in senesced leaves were regarded to have a high P resorption efficiency (Killingbeck 1996), while leaf P concentrations in many species from subtropical forests are approximately 0.5 mg g−1, suggesting high P resorption efficiencies. We hypothesize that leaf P resorption is relatively stable under conditions of N and P addition owing to the constrained flexibility of leaf P resorption in long-term low P soils (Harrington et al. 2001; Treseder and Vitousek 2001). Leaf N resorption should have no significant response to N and P addition, as subtropical forests typically have optimal green leaf N concentrations above 7.0 mg g−1 (Killingbeck 1996). However, the aforementioned hypotheses have not been well tested in subtropical forests (Chen et al. 2015).
Here, we conducted an experiment with N and P addition in a secondary subtropical forest in southern China. N and P, individually and in combination, were applied on a monthly basis. In practice, we sampled six dominant plant species—including dominant trees and understory shrubs and herbs—and investigated the effects of N and P addition on N and P concentrations, N:P ratios, and resorption efficiencies in a field-based experiment. The main objectives were to (1) investigate the response of leaf N and P concentration and resorption in subtropical forests, and (2) to test the aforementioned hypotheses.
Materials and methods
Study site
The experiment was carried in a subtropical mixed pine and broadleaf forest (23°9′41″N, 112°32′36″E, 50 m elevation) in the Dinghu Mountain region of southern China. This area is characterized by a typical subtropical monsoon and humid climate. The mean annual temperature is approximately 21.5 °C, where the mean monthly temperature ranges from 12.6 °C in January to 28.0 °C in July (Liu et al. 2013). The mean annual precipitation is approximately 1927 mm, approximately 75% of which falls during March–August (Liu et al. 2012). Zonal vegetation was evergreen broad-leaved forests, but it was heavily destroyed due to land conversion for agricultural production. The vegetation was restored during 1930–1950 by planting Pinus massoniana (PM). The forests were naturally invaded and colonized by broadleaf species, Schima superba (SS). Hence, it is a transitional forest from pine to monsoon evergreen broadleaf forest.
The soil is lateritic red earth developed from sandstone, with a depth of 30–60 cm. The mean bulk density of the top soil (0–10 cm) is 1.4 g cm−3, while the average soil pH is 4.1. The averages of soil total carbon, total N, and total P concentrations for the top 10 cm of soil are 13.6, 0.79, and 0.14 g kg−1, respectively. Precipitation-based inorganic N deposition in 2013 was 22.8 kg N ha−1 year−1, with an NH4 +-N to NO3 −-N ratio of 1.4:1, while P deposition was 0.34 kg N ha−1 year−1 (Zhu et al. 2016).
Experimental design and sampling
Our research site was established at the bottom of a hill with a slope around 30°. Stand density was ca. 150 stems ha−1 for PM and ca. 960 stems ha−1 for SS. The average of volume was about 0.18 m3 tree−1 for PM (DBH, 22.7 cm; height, 11.0 m) and 0.06 m3 tree−1 for SS (DBH, 11.2 cm; height, 9.5 m). Topographic position and slope were considered to ensure uniformity among plots. The experimental design included four treatments with four replicates (total of 16 plots): control (CK, no N and P addition), N addition (N, 100 kg N ha−1 year−1 NH4NO3), P addition (P, 100 kg P ha−1 year−1 NaH2PO4), and N plus P addition (NP, 100 kg N ha−1 year−1 and 100 kg P ha−1 year−1). Each plot was 20 m × 20 m, and was separated from neighboring plots by a 10-m buffer. All fertilizers were dissolved in 50 L of water and sprayed using a backpack sprayer, and were applied monthly from June 2013 to June 2015. In lieu of a fertilizer solution, control groups were sprayed with water (equivalent to 0.5 mm of rainfall).
Young green leaves from six species were collected in June 2015 for green leaf nutrient measurement. The present study included two tree species (SS and PM), and four dominant understory species [two shrubs: Psychotria rubra (PR) and Ixora chinensis (IC), and two herbs: Dicranopteris dichotoma (DD) and Lophatherum gracile (LG)]. Three healthy mature trees were randomly selected in each plot in June 2015. One twig from sun-exposed branch within the upper two-third of the crown was collected using a tall tree trimmer with a bamboo pole. The current-year fully expanded leaves were detached from each twig and were bulked to yield one sample. For four understory species, mature leaves from 3 to 5 healthy individuals per plot were collected by hand and mixed to one sample. Two 1 × 1 m nylon mesh traps (about 0.5 m high from the ground) were used to collect senesced tree leaves in each plot. Senesced tree leaves fallen in December 2015 with no signs of decay were pooled per plot and evenly mixed to yield one sample for senesced leaf nutrient measurement. Senesced leaves with no signs of decay from PR were collected and measured the nutrient content of senesced leaves, while the quantities of senesced leaves from the other three species were not sufficient to be collected and analyzed. All samples were dried at 65 °C for 48 h and ground to homogeneity with a ball mill for chemical analyses. Five soil cores (5 cm diameter) were collected from each plot at 0–10 cm depth and bulked into one sample. Soil samples for ammonium (NH4 +) and nitrate (NO3 −) measurement were obtained every 2 months from August 2013 to June 2015. Soil P content was measured only in June 2015. Soil NH4 + and NO3 − concentrations were determined by an extraction using a solution of 2 M KCl, followed by a colorimetric analysis on an AutoAnalyzer (SEAL AA3, Norderstedt, Germany). Soil inorganic N was calculated as the sum of NH4 + and NO3 − concentrations. Soil available P was extracted using an NH4F–HCl solution as described by Bray (1945).
Chemical analyses
Leaf N concentrations were measured using the micro-Kjeldahl method (Sparks 1996), while leaf P concentrations were analyzed by the ammonium molybdate method after persulfate oxidation (Sparks 1996). Leaf N resorption efficiency (NRE) and leaf P resorption efficiency (PRE) were calculated as the proportion of N and P recovered from a senesced leaf (Killingbeck 1996; Yuan and Chen 2009b):
where Ngreen and Pgreen were the N and P concentrations of green leaves, and Nsenesced and Psenesced were the N and P concentration of senesced leaves.
Statistical analyses
A two-way ANOVA was used to test the impacts of N, P, and NP addition on green leaf and senesced leaf N and P concentrations, green leaf N:P ratio, and leaf N and P resorption efficiencies. Dunnett’s test was conducted to determine the significant differences between different experimental treatments. Pearson correlations were used to investigate the relationship between green leaf N and P concentrations and soil available N and P concentrations. All statistical analyses were performed using the SPSS 18.0 software package (SPSS, Chicago, IL, USA). The significance level was set at P = 0.05 for all calculations.
Results
Changes in leaf N, P, and related resorption efficiencies
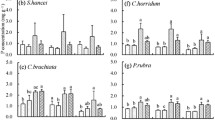
Neither N or P addition alone, nor the addition of NP combined, changed green leaf N concentrations in any species in the present study (Fig. 1a). P addition increased green leaf P concentration by an average of 41% (Fig. 1b). No interactions of N and P addition were detected. More specifically, P addition increased green leaf P concentrations in P. rubra, I. chinensis, and L. gracile. NP addition enhanced green leaf P concentrations in all species with the exception of S. superba. The increase in green leaf P concentration by P addition was observed to be higher in understory species than in tree species.
Effects of nitrogen (N) and phosphorus (P) addition (CK: control, N: N addition, P: P addition, NP: both N and P addition) on green leaf N and P concentrations in a subtropical forest. Data represent mean and standard error (n = 4). Asterisks indicate significant differences from the control treatment (P < 0.05). SS, Schima superba; PM, Pinus massoniana; PR, Psychotria rubra; IC, Ixora chinensis; DD, Dicranopteris dichotoma; LG, Lophatherum gracile
Similarly, senesced leaf N concentrations in S. superba, P. massoniana, and P. rubra exhibited no changes in N in response to N or P addition, either individually or in combination (Fig. 2a). P and NP addition increased senesced green leaf P concentrations in both S. superba and P. rubra (Fig. 2b). The positive effect of P addition was stronger in P. rubra than in S. superba.
Effects of nitrogen (N) and phosphorus (P) addition (CK: control, N: N addition, P: P addition, NP: both N and P addition) on senesced leaf N and P concentrations in a subtropical forest. Data represent mean and standard error (n = 4). Asterisks indicate significant differences from the control treatment (P < 0.05). SS, Schima superba; PM, Pinus massoniana; PR, Psychotria rubra
N addition exerted no effect on green leaf N:P ratio in any of the species tested (Fig. 3). S. superba, P. rubra, I. chinensis, D. dichotoma, and L. gracile exhibited significantly lower N:P ratios in the P treatments, and I. chinensis, D. dichotoma and L. gracile exhibited significantly lower N:P ratios in the NP treatments in comparison to CK. The two understory herbs—D. dichotoma and L. gracile—displayed the lowest N:P ratios of all six species. In the tree species, the N:P ratio was lower in P. massoniana than in S. superba.
Effects of nitrogen (N) and phosphorus (P) addition (CK: control, N: N addition, P: P addition, NP: both N and P addition) on green leaf N:P ratios in a subtropical forest. Data represent mean and standard error (n = 4). Asterisks indicate significant differences from the control treatment (P < 0.05). SS, Schima superba; PM, Pinus massoniana; PR, Psychotria rubra; IC, Ixora chinensis; DD, Dicranopteris dichotoma; LG, Lophatherum gracile
Leaf NRE and PRE in S. superba, P. massoniana, and P. rubra did not change with both when added individually and in combination (Fig. 4). On average, NRE and PRE in the three plant species tested were 32.3% (Fig. 4a) and 50.3% (Fig. 4b), respectively, although PRE in P. massoniana was higher than that of S. superba.
Effects of nitrogen (N) and phosphorus (P) addition (CK: control, N: N addition, P: P addition, NP: both N and P addition) on leaf N resorption efficiency (NRE) and leaf P resorption efficiency (PRE) in the subtropical forest. Data represent mean and standard error (n = 4). Asterisks indicate significant differences from the control treatment (P < 0.05). SS, Schima superba; PM, Pinus massoniana; PR, Psychotria rubra
Soil available N and P concentrations
N addition significantly increased soil NH4 + and NO3 − concentrations (Table 1). Soil available P concentration was eight times higher in P and NP treatments (24.26 and 27.97 mg kg−1, respectively on average) than in the CK group (2.73 mg kg−1), while the addition of N (2.82 mg kg−1) did not change it. There were no interactions of N and P addition on soil properties. The average pH value in the control soil was 3.96, while N addition significantly decreased soil pH to 3.90, and P addition increased pH to 4.04.
Relationships of N and P concentrations between plants and soil
Leaf N:P stoichiometry was sensitive to soil available P rather than soil available N. More specifically, leaf P concentrations in all species were significantly and positively correlated with soil available P, while leaf N concentrations had no apparent relationship to soil available N and P, except in P. massoniana (Table 2). As a result, leaf N:P ratios were negatively correlated with soil available P in nearly all species, and exhibited no relationship with soil available N. In addition, plant N and P were not correlated with soil pH values.
Discussion
In a subtropical coniferous and broadleaved mixed forest in southern China, P addition generally increased leaf P concentrations and decreased N:P ratios, while N addition did not alter leaf N or P concentrations. Therefore, our results confirm the hypothesis that the subtropical forest is indeed P-limited and not N-limited. Our findings also support the hypothesis that N and P addition exerted no effects on leaf N and P resorption in the subtropical forest ecosystem in southern China.
P-acquiring ability, rather than P resorption, influenced leaf P concentration
The 41% increase in green leaf P after P addition observed in the present study was consistent with the average range (30–60%) of previously reported increases in green leaf P in tropical forest ecosystems under conditions of P addition (Li et al. 2016; Yuan and Chen 2015). Our findings demonstrate that while P addition increased leaf P concentrations, it did not influence leaf P resorption (Figs. 1, 4). This suggests that leaf P was regulated directly by an external supply and not by the internal remobilization of P. These findings are consistent with the meta-analysis of Yuan and Chen (2015), who found that P addition increased both green and senesced leaf P concentrations in conifers and evergreen angiosperms, while having no influence on PRE. The underlying reasons for the directly proportional increases in P concentration in both green and senesced leaves under conditions of P addition are poorly understood. Generally, it has been shown that nutrient use efficiency would decrease with increasing nutrient availability, both in the field (Yuan and Chen 2009a; Yuan et al. 2011) and in fertilization experiments (Yuan and Chen 2015). Whether the effects of P addition on PRE vary with the length of the fertilization period requires further investigation. With regard to specific types of forest ecosystems, previously published information regarding the effect of P addition on PRE in subtropical forests is lacking (Yuan and Chen 2015).
Furthermore, the ability to uptake soil available P by leaf P accumulation is an important survival strategy for many plant species. Previous studies have reported that some plant species growing in P-poor environments may not downregulate P uptake under conditions where a higher supply of P is available (Lambers and Shane 2007; Standish et al. 2007). However, in such cases, the addition of P is not a common condition that plants are likely to experience in the wild. It is possible that high leaf P concentration under conditions of P addition may not necessarily be correlated with high physiological demand, or that leaf P concentrations reach a level of saturation. In addition, to paint a more complete picture of the effects of P addition on leaf P concentration in subtropical forest, and ultimately on plant productivity, longer-term studies will be necessary.
The influence of P addition on leaf P concentration was independent of soil N availability, as no significant differences between P and NP treatments were observed in the present study (Fig. 1). Our findings were consistent with the results of previous studies performed on different growth types of plants or in P limited tropical forests (Li et al. 2016; Yuan and Chen 2015). For instance, Mayor et al. (2014) reported that there was no interaction between N and P fertilization in four common tree species in a lowland tropical forest in Panama following 13 years of N, P, and NP addition. Additionally, based on a meta-analysis, Yuan and Chen (2015) observed no significant differences in the P effect on leaf P concentrations in evergreen angiosperms. The most plausible reason for these observations is that there was no effect of N addition on soil P supply (Table 1). In soils, the supply of P originates from two processes: from organic P mineralization or from P addition. Soil pH is another important factor that influences organic P mineralization, as lower soil pH will decrease soil phosphatase activity. Based on our observations, soils under conditions of P addition exhibited significantly higher pH values (mean = 4.04 ± 0.15 SD during 2013–2015) than soils under conditions of NP addition (mean = 3.93 ± 0.14 SD during 2013–2015). This was likely because N addition intensifies soil acidification to some extent. These findings suggest that soil P mineralization rates should be lower under conditions of NP addition. However, the amount of 100 kg P ha−1 year−1 from externally available P addition was much higher than the quantity of mineralized P owing to the lower amounts P substrates of available in subtropical soils. Hence, the differences in soil P mineralization under conditions of varying levels of acidity can be ignored. These findings also highlight the fact that high ratios of atmospheric N and P deposition in the future could have the potential to increase leaf P concentrations.
Plant responses to P addition were species specific, signifying that shifts in community composition could occur in response to P availability in subtropical forests. Forests in southern China are typically broad-leaved evergreen forests. The forest investigated in the present study was a mixed coniferous and broad-leaved secondary forest developing from a P. massoniana plantation. P. massoniana, which is being replaced by S. superba, exhibited a stronger response to P addition in comparison to the nutrient-conserving S. superba. In comparison to S. superba, the higher P resorption efficiency of P. massoniana and lower green leaf N:P indicate a higher P use efficiency. These characteristics of P. massoniana suggest that P addition would be more favorable for P. massoniana than it would be for S. superba. Therefore, P addition could have potential to delay the succession of subtropical forests in this region, but this effect should be less because the amount of ambient P deposition was low (0.34 kg hm−2 year−1) in this region (Zhu et al. 2016) although P was one of the key factors contributing to forest succession. In addition, the lower P concentration in S. superba contributed to the higher leaf N:P in S. superba than in P. massoniana, which were consistent with the result of these two species in Han et al. (2005). However, when scaling up to the level of functional group, we should notice that there was no significant difference between the N:P values of broadleaf and coniferous species in China, even only for tropical and subtropical regions (Han et al. 2005).
Responses of plant P limitation to external N and P inputs
Plant productivity in most tropical and subtropical forests is limited by P availability, which have been confirmed by previous fertilization experiments (Cleveland et al. 2002; Mayor et al. 2014; Townsend et al. 2007) and meta-analyses (Elser et al. 2007; Li et al. 2016). This is also true in the present study, as indicated by the response of leaf P concentration to P treatments. Meanwhile, the significant positive effect of P addition on the growth rate of small tree (< 15 cm DBH) showed in Li et al. (2017) based on this experiment also supported P addition can alleviate P limitation in subtropical forests. Furthermore, Olde Venterink (2016) highlighted “Chemical facilitation” which was named as a non-added nutrient can be enhanced by one added nutrient through changing soil chemical conditions (e.g., soil acidity). Our study provides new evidence of no chemical facilitation via N and P addition.
Liebig’s Law of the minimum, a theory that applies to the effects of nutrient limitation on plant growth, developed from the idea that growth is governed not by the availability of all resources, but by the availability of the most limiting resource (single-resource limitation). Expanding on this idea, Harpole et al. (2011) reported four types of nutrient limitations on plant growth: single limitation, simultaneous co-limitation, independent co-limitation, and serial limitation; while Olde Venterink (2016) argued that chemical facilitation between nutrients is common. For example, N addition has been shown to decrease soil pH (Tian and Niu 2015), and subsequently increase P availability for plants through P desorption (Jin et al. 2014; Wang et al. 2011). In the present study, soil acidity induced by the addition of NH4NO3 exerted no influence on soil P availability (Table 1; Fig. 1), as P is adsorbed to Fe or Al oxides and hydroxides in acidic soils (Hinsinger 2001). The rise in soil pH under conditions of P addition (Table 1) likely exerted a positive effect on soil P availability; however, this increase of soil available P caused by high soil pH was much less in comparison to the amount of P addition. Our findings demonstrate that the subtropical coniferous and broadleaved mixed forest is solely limited by P availability, and that the possibility of chemical facilitation between nutrients related to soil acidity is unlikely.
Unexpectedly, our findings are not consistent with the perspective that atmospheric deposition with a high N:P ratio, owing to high N deposition and low P deposition, would exacerbate P limitation in subtropical forests (Li et al. 2016; Zhu et al. 2016). The general view of enhanced P limitation under conditions of N addition, based on stimulated plant production by N addition, and then plant P demand based on N:P homeostasis, mostly occurs in N and P co-limited temperate forests (Braun et al. 2010; Gress et al. 2007). In the present case, this is not true because leaf N and P are insensitive to N addition. Hence, at the very least, the influence of high atmospheric N:P deposition would be overestimated for this type of forest.
Under normal conditions, plants are generally able to downregulate nutrient uptake under varied natural soil conditions. However, when the soil environment shifts from conditions of high P limitation to conditions of no P limitation in a short period of time, an excessive P uptake by plants may occur, even to saturated or toxic levels (Ostertag 2010). In this type of situation, leaf P concentration or N:P ratio may not reflect the actual demands of plant growth under conditions of P addition. Thus, the findings of this 2-year study could be further elucidated and verified by the long-term applications of potentially limiting nutrients to subtropical forest ecosystems.
Author contribution statement
HY conceived the idea, conducted the analysis and wrote the manuscript.
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Bennett EM, Carpenter SR, Caraco NF (2001) Human impact on erodable phosphorus and eutrophication: a global perspective. Bioscience 51:227–234
Braun S, Thomas VFD, Quiring R, Flückiger W (2010) Does nitrogen deposition increase forest production? The role of phosphorus. Environ Pollut 158:2043–2052
Bray RH (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Chen FS, Niklas KJ, Liu Y, Fang XM, Wan SZ, Wang, Hm (2015) Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol 35:1106–1117
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:0680–0691
Elser J, Bennett E (2011) Phosphorus cycle: A broken biogeochemical cycle. Nature 478:29–31
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N:P stoichiometry. Bioscience 46:674–684
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gress SE, Nichols TD, Northcraft CC, Peterjohn WT (2007) Nutrient limitation in soils exhibiting differing nitrogen availabilities: what lies beyond nitrogen saturation? Ecology 88:119–130
Gusewell S (2004) N : P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Han WX, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken MES, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
Harrington RA, Fownes JH, Vitousek PM (2001) Production and resource use efficiencies in N- and P-limited tropical forests: a comparison of responses to long-term fertilization. Ecosystems 4:646–657
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Huang J, Yu H, Lin H, Zhang Y, Searle EB, Yuan Z (2016) Phosphorus amendment mitigates nitrogen addition-induced phosphorus limitation in two plant species in a desert steppe, China. Plant Soil 399:221–232
Jin J, Tang C, Hogarth TW, Armstrong R, Sale P (2014) Nitrogen form but not elevated CO2 alters plant phosphorus acquisition from sparingly soluble phosphorus sources. Plant Soil 374:109–119
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Lambers H, Shane MW (2007) Phosphorus nutrition of Australian proteaceae and cyperaceae: a strategy on old landscapes with prolonged oceanically buffered climates. S Afr J Bot 73:274–275
Lawrence D (2001) Nitrogen and phosphorus enhance growth and luxury consumption of four secondary forest tree species in Borneo. J Trop Ecol 17:859–869
Li Y, Niu SL, Yu GR (2016) Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta-analysis. Glob Change Biol 22:934–943
Li Y, Tian DS, Yang H, Niu SL (2017) Size-dependent nutrient limitation of tree growth from subtropical to cold temperate forests. Funct Ecol 00:1–11
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Liu JX, Huang WJ, Zhou GY, Zhang DQ, Liu SZ, Li YY (2013) Nitrogen to phosphorus ratios of tree species in response to elevated carbon dioxide and nitrogen addition in subtropical forests. Glob Change Biol 19:208–216
Long M, Wu HH, Smith MD, La Pierre KJ, Lü XT, Zhang H-Y, Han XG, Yu Q (2016) Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 408:475–484
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Mayor JR, Wright SJ, Turner BL (2014) Species-specific responses of foliar nutrients to longterm nitrogen and phosphorus additions in a lowland tropical forest. J Ecol 102:36–44
Olde Venterink H (2016) Productivity increase upon supply of multiple nutrients in fertilization experiments: co-limitation or chemical facilitation? Plant Soil 408:515–518
Ostertag R (2010) Foliar nitrogen and phosphorus accumulation responses after fertilization: an example from nutrient-limited Hawaiian forests. Plant Soil 334:85–98
Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA (2013) Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sparks D (1996) Methods of soil analysis. SSSA and ASA, Madison
Standish RJ, Stokes BA, Tibbett M, Hobbs RJ (2007) Seedling response to phosphate addition and inoculation with arbuscular mycorrhizas and the implications for old-field restoration in Western Australia. Environ Exp Bot 61:58–65
Tanner EVJ, Kapos V, Freskos S, Healey JR, Theobald AM (1990) Nitrogen and phosphorus fertilization of Jamaican montane forest trees. J Trop Ecol 6:231–238
Tian DS, Niu SL (2015) A global analysis of soil acidification caused by nitrogen addition. Environ Res Lett 10:024019
Townsend AR, Cleveland CC, Asner GP, Bustamante MMC (2007) Controls over foliar N:P ratios in tropical rain forests. Ecology 88:107–118
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wang X, Guppy CN, Watson L, Sale PWG, Tang C (2011) Availability of sparingly soluble phosphorus sources to cotton (Gossypium hirsutum L.), wheat (Triticum aestivum L.) and white lupin (Lupinus albus L.) with different forms of nitrogen as evaluated by a 32P isotopic dilution technique. Plant Soil 348:85
Yuan ZY, Chen HYH (2009a) Global trends in senesced-leaf nitrogen and phosphorus. Glob Ecol Biogeogr 18:532–542
Yuan ZY, Chen HYH (2009b) Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob Ecol Biogeogr 18:11–18
Yuan ZY, Chen HYH (2015) Negative effects of fertilization on plant nutrient resorption. Ecology 96:373–380
Yuan ZY, Chen HYH, Reich PB (2011) Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat Commun 2:344
Zhu J, He N, Wang Q, Yuan G, Wen D, Yu G, Jia Y (2015) The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci Total Environ 511:777–785
Zhu J, Wang Q, He N, Smith MD, Elser JJ, Du J, Yuan G, Yu G, Yu Q (2016) Imbalanced atmospheric nitrogen and phosphorus depositions in China: implications for nutrient limitation. J Geophys Res Biogeosci 121:1605–1616
Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (31290221) and the National Key Research and Development Program (2016YFC0503706).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that she has no conflict of interest.
Additional information
Communicated by T. Koike.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, H. Effects of nitrogen and phosphorus addition on leaf nutrient characteristics in a subtropical forest. Trees 32, 383–391 (2018). https://doi.org/10.1007/s00468-017-1636-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1636-1