Abstract
A plausible origin and function of dynamic chaos in the cerebellar networks along with the role of electrical synapses between cells of inferior olives (IO) are critically revised. First we demonstrate that behaviour of cerebellar module model critically depends on microchanges in the system parameters. Second we observe chaos dynamics in low dimensional system despite it has been hypothesized earlier as a consequence of multidimensionality of the system. That means the chaos in inferior olives emerges independently of electrical connections between IO cells. Third we show that the electrical synapses between IO cells can substantially reduce unnecessary synchronicity of these cells. Fourth we demonstrate that the results of measurements of electrical connections between IO cells in presence of activation of GABA receptors can be explained by electrical scheme with constant values of intercellular resistance. It is proposed that the role of GABAergic cerebellar nuclear cells is an invertor of inhibitory Purkinje cell output into excitatory action of the Purkinje cells to the IO cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In two previous papers of this series [1, 2], we have demonstrated the emergence of dynamic chaos phenomenae in the model of the cerebellar module and existence of some basic features of these phenomenae in work of the live cerebellum. Further we have shown that when different Climbing Fiber Cells (ClFCs) perform extracerebellar function equalization using the same Granule Cells inputs, they have very low level of cross-correlation. Along with that we have demonstrated that chaotic behaviour of the cerebellar modules is immanent to the structure of the cerebellar loop of each single ClFC - Purkinje cells (PCs) - cerebellar nuclear cells (NuCs) - back to the ClFC and does not need the electrical synapses between climbing fiber cells. It is present in single ClFC cerebellar module.
So the existing arguments on the role of electrical connections between ClFCs for dynamical chaos [3, 4] should be a subject for further analysis. In connection with this fact, we tried to find out what electrical connections between the ClFCs can be used for in the cerebellar modules.
In the current article we come to the hypothesis that electrical connections can reduce the synchronization between ClFCs. In computational experiments below we further explore the emergence of chaotic behaviour in the cerebellar module and demonstrate the principal possibility of the proposed ClFC desynchronization mechanism.
Dynamic chaos in ClFC cerebellar modules
In [1, 2] we have demonstrated emergence of chaos in a separate single ClFC cerebellar loop module. We have not yet performed detailed analysis of mathematical mechanisms of chaos emergence in our model. Such a detailed model should be performed in future research. In this work, we restrict ourselves with obtaining evidence that the phenomenae observed in the system are manifestations of dynamic chaos. To establish beyond the doubts the fact of presence of the chaotic phenomenae in our computational model and to evaluate its functional role we have performed two sets of computational experiments which are described in this Section.
Small perturbations of ClFC threshold
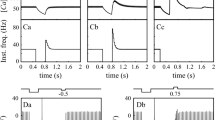
To explore which features of the model give rise to chaotic behaviour we tried to estimate how small perturbations in system parameters affect the behaviour of the cerebellar model which has been extensively explored in[1, 2]. Figure 1 presents results of the corresponding computational experiment. Here comparison of interspike intervals generated in the experiment shown at Fig.3d-f of [2] with the modified version of the same experiment is shown. The modification was that at definite moment (marked with an arrow above the curve) a small perturbation is made to one of important system parameters, the threshold of the ClFC. The impulse sequence vector at each time moment consists of 100 numbers of successive ClFC interspike intervals durations starting with the current interval. The ordinate shows the cosine similarity between these impulse sequence vectors for the original model and for the model in which ClFC threshold was given a small perturbation. Figure 1 demonstrates that when the perturbation is of 1e-7 value of the base threshold and more, the system trajectory dramatically changes as judged by the data on impulse sequence vectors. This data obviously demonstrates the critical instability of the cerebellar module behaviour on small perturbation of its clue parameters.
Small perturbations of ClFC threshold influence the ClFC output impulse sequences. In the model of periodic signal equalization[1] the equalization process was completed at 3000 s since the start of the simulation. The moment of perturbation is denoted by arrow. The perturbation values were 0.01 (extreme right), 0.05, 0.1, 0.3, 1.0, 5.0. They were added to the base value of the threshold (Th = 400 000.0, in arbitrary units)
Dynamic chaos in the simplest model of the cerebellar-type module
Up to this point we considered a cerebellar module with large number of basic elements of the model (granule cells synapses, n>=300). Here we describe the simplest case of the same type of construction with just two granule cells which fire alternatively with the period of embedding input signals of the system \(T_{S}\)=530ms.
The \(T_{equ}\) for the Mauk equations[1, 2] is considered to be 1000 ms. The external input to the climbing fiber cell was kept constant. The first GrC was active within the first half of the embedding period (0–265 ms), while the second GrC was active within the second half (266–530 ms). We start the computational session with random values of synaptic weights from the GrCs to the PC. As in our previous experiments, the total input to the ClFC becomes practically constant (and oscillates with a small amplitude) due to synaptic plasticity in our model. The average interspike interval of the ClFC impulses becomes equal to 1 s (i.e. equal to \(T_{equ}\)). Figure 2ab shows Poincare plots for the ClFC interspike intervals; they are taken from the data in the intervals 13250–21200 s and 21200–29150 s of modeling time respectively. Importantly, to human observer the difference between Poincare plots of Fig. 2a and Fig. 2b obtained at different times seems insignificant. Also, it can be seen from Fig.2ab that the pattern of activation of the ClFC is chaotic. Figure 2c presents the histogram of the ClFC interspike intervals. The variation coefficient for this histogram is 0.48, which is close to the values of that coefficient of the PC complex spikes[5].
Figure 2d-f shows the results of the experiment which differs from the previous one only in that the first GrC was active within the first quarter of the embedding period (0–132.5 ms) and the second GrC was active within the last three quarters (132.5–530 ms). The difference between Poincare plots of Fig. 2d and Fig. 2e obtained at different times is also insignificant. The variation coefficient for the histogram of Fig. 2f is 0.27. We can see the stability of the chaotic pattern of the ClFC activity. The patterns of Fig. 2ab and Fig. 2de differ from each other and are chaotic.
So the dynamic chaos emerges in the cerebellar-type model even in the simplest case of two granule cells.
To the role of electrical synapses between the climbing fiber cells
Interactions between ClFCs
Many authors report seemingly random and statistically stationary activity of ClFCs [6,7,8]. Due to these facts the presence of strong electrical connections ([9], and many later works) between ClFCs is quite surprising.
Along with the mostly independent of ClFC firing there have been found rare but statistically significant almost synchronous pairwise activations of ClFCs[10]. Here we propose a mechanism which might explain both the mostly independent firing and the cause of weak synchronization of ClFCs, i.e. rare synchronous events in ClFC firing.
We demonstrate that desynchronization might be supported by electrical connections between ClFCs. While synchronous events are bound to the interaction between ClFCs provided by special type of interaction by the mechanism of signal propagation in the three-synaptic link: ClFC => PC => NuC => ClFC as described below.
In this section we take into consideration the fact that in cerebellum the each ClFC gives climbing fiber synapses to several (in average, 10 [11, 12]) PCs. The PCs act on GABAergic NuCs. The NuCs give inhibitory connections back to ClFCs. NuCs act on those ClFCs which act on the PCs which act on these NuCs,Footnote 1 see also[13]. The described connections pattern is illustrated at the Fig. 3. For simplicity, in this case we show two ClFCs each of which controls its own two PCs and these PCs act on twelve NuCs which act back to the ClFCs. It is convenient to "cut" each ClFC on the output part (at the left side of the Fig. 3) and the input part (right side of the Fig. 3). At the Fig. 3 some NuCs are connected only to PC which belong to the same ClFC while there are NuCs which get synapses from PC belonging to both ClFCs. At the Fig. 3 the neural connections for the "mixed" representation of both ClFCs by single NuCs are shown in red. And this "mixed representations" (more complicated with the larger number than two ClFCs) cause the issues which we discuss below. The latter mentioned issues are also connected with the pattern of output of PC to the impulses of ClFC. Complex spikes (with total duration about 10–20 ms) of PCs present reaction of the whole body of PC to one impulse of ClFC. At the PCs axons the complex spikes yield just one regular shaped impulse generated at the time of onset of the complex spike. These impulses in the axons of all PCs which are controlled by one ClFC are practically synchronous with the precision of less than 1 ms.
The GABAergic NuCs are spontaneously active, they have no excitatory synaptic inputs. Synapses from PCs exert inhibitory actions on the activity of NuCs. The synchronous activations of PCs imposed by excitation of ClFC will have strong and synchronous inhibitory action on all postsynaptic NuCs. That in fact will act postsynaptically on ClFC as the kind of excitatory postsynaptic potential. This possibility has not yet been identified in the analysis of the cerebellar circuitry.
Scheme for backward action of impulses of inferior olive cells through Purkinje cells and inhibitory NuCs Notations: ClFCi(o) and ClFCj(i) are outputs and inputs of ClFC parts; PCij (i,j = 1, 2) are Purkinje cells belonging to corresponding ClFCs; In the middle there are twelve nuclear cells (NuCs), three for each Purkinje cell; NCIOI are non-cerebellum inferior olive inputs; GJ - gap junctions, electrical synapses between ClFCs. The relative sizes of the network elements are chosen to visualize the features of the system and do not correspond to the actual physical dimensions of these elements, other details are in text
Our evaluation of the role of electrical synapses between ClFCs in cerebellar information processing is based on three types of arguments:
-
1.
Possible interactions between ClFCs via shared NuCs.
-
2.
The inevitability of short-term synchronous break of inhibition from NuCs to the targeted ClFC caused by PC complex spikes caused by that ClFC impulses.
-
3.
Possible existence of asymmetric electrical synapses between ClFCs. The asymmetry might include two factors. First, the synapses might connect soma of one cell with dendrite of another cell. Second, the electrical asymmetry (direct current rectification) of synapses might exist so that inhibition might be transferred from soma to dendrite while excitation transfer is blocked. Each of these three types of arguments is described further in this Section.
Interaction between ClFCs via NuCs
As stated before we suppose that each ClFC gets synapses from all NuCs which get synapses from PCs belonging to this ClFC. As illustrated at Fig. 3 each ClFC besides the synapses of NuCs which are controlled by the PCs which in turn are controlled by the same ClFCs might get synapses from the NuCs which are controlled by other ClFCs.
Our approach in next sections is based on the hypothesis that NuCs with contacts with PCs belonging to different ClFCs are present in cerebellum in significant quantities.
The role of PC complex spikes in ClFC interaction
ClFC impulse causes complex spikes in all PCs which this ClFC controls with its climbing fibers[14, 15]. At the output of PC the ClFC impulse produces one short latency impulse followed by a pause 10–50 ms in duration. All PCs connected to one ClFC send inhibitory impulses to NuCs which send inhibitory connections to the ClFC PCs of which act on the NuCs. The input of PCs to NuCs caused by complex spikes is synchronous in all synapses of all NuCs which provide feedback of ClFC impulse to the ClFC.
Inhibitory impulses from PC to NuCs cause a short-term stop of activity of NuCs. In turn the pause of activity of NuCs causes a kind of excitatory postsynaptic potential on all ClFCs which get synapses from NuCs which receive impulses from PCs controlled by the ClFC. The excitatory potential has the maximum amplitude on the ClFC which has generated the original impulse. However, this excitation arrives to that ClFC in the refractory period caused by generation of the impulse and therefore is ineffective. Along with that, action of the impulse of a given ClFC might generate "excitatory postsynaptic potential" in other ClFCs and might provoke activation of other ClFCs almost synchronously with the original ClFC. It is possible that the proposed mechanism is responsible for synchronous activation of different ClFCs which has first been reported by [10]. Below we describe how this non-necessary excitation might be mostly blocked with electrical synapses between ClFCs.
Evaluation of the role of electrical synapses between ClFCs
Our hypothesis of the role of asymmetric electrical synapses between ClFCs substantially uses two ideas:
-
A.
In many cases electrical synapses are structurally asymmetric and we use asymmetry of electrical synapses between dendrites and somas of neurons[16].
-
B.
We hypothesize that post-impulse hyperpolarization might inhibit neurons which have electrically asymmetric electrical connections with the given neuron. In this respect the postimpulse hyperpolarization of ClFCs is of particular importance. The presence of this type of hyperpolarization has been demonstrated in rat inferior olive neurons (Fig.1C4 in[5]).
The consequences of structural asymmetry between dendrites and soma can be evaluated using electrical scheme of such contacts at Fig. 4. Here we have the cell compartment of one ClFC (c1) and dendrite and cell compartments of the second ClFC (c2). The dendrite of c2 is electrically connected with the soma compartment of c1. The diode d in the scheme is described later.
The relations between parameters and variables shown at Fig. 4 are described with the following equations given as \(U_{c1}\) and \(U_{d2}\):
where
\(R_z^2 = R_d(R_c+R_g) + (R_c+R_g)(R_{dc2}+R_c) + R_d(R_{dc2}+R_c)\)
In physiological conditions \(R_c \ll R_{d2}, R_{dc2}, R_g\),
the \(R_{d2}, R_{dc2}, R_g\) are of the same order of values.
From the Fig. 4 and equations it follows that the change in dendrite potential \(U_{d2}\) has a small influence on \(U_{c1}\)
If this electrical connection is electrically asymmetric at the site of contact then this scheme shows that there will be only inhibition from the soma of the just excited ClFC on dendrites of those ClFCs which have dendritic electrical synapses with the soma of c1. The electrical asymmetry is denoted at the scheme at Fig. 4 as zero inner conductance diode which shows that synapse in fact conducts only strong inhibitory signals from c1 to the dendrite of c2. In case of electrical asymmetry there will be no interaction between c1 and c2 of Fig. 4 besides the short periods of postimpulse hyperpolarization of c1 and c2. So this effectively removes undesirable excitation of ClFC which otherwise would be caused by positive feedback loop from other ClFCs which share common NuCs with the considered ClFC.
Further, synapses from those NuCs which get synapses from PCs of say two different ClFCs should be located on those dendrites which have electrical connections with somas of the "partner" ClFC. It would be beneficial if these synapses besides being structurally asymmetric will be electrically asymmetric (i.e. DC rectifying) as well as shown at Fig. 4.
The circuit diagram for two electrical connections between ClFCs. G is responsible for the coupling strength (G=inf means cells are uncoupled). Index 2 means second cell. Index d means dendrite compartment. Rc, Ec1 - first IO cell soma resistance and EMF. Rc, Ec2 - second IO cell soma resistance and EMF. Rd2, Ed2 - second IO cell dendrite compartment resistance and EMF. G - coupling strength between 1st and 2nd IO cells. Rdc2 - somatodendritic resistance of the 2nd cell. Capacitance is ignored
In the next section we illustrate the potential workability of the proposed mechanisms of ClFC desynchronization with electrical synapses with the help of computational model.
Computational model of ClFCs interaction
We consider a very simplified model of the processes in the cerebellar loop. The model includes 20 ClFCs. Each ClFC acts on 5 PCs, each PC is randomly connected to five NuCs. Each NuC is then connected back to all ClFCs PCs of which have synapses on this NuC are acting on all ClFCs, from which they get synapses via the corresponding PCs. In these computation experiments we follow only the consequences of impulses of the ClFCs in presence and absence of the electrical synapses. We looked at the membrane potentials of the ClFCs taking into consideration only consequences of the complex spikes of the PCs. Based on the data of [5] we take the duration of afterhyperpolarization after ClFC impulse to be 5ms. “Excitatory postsynaptic potentials” (see above)
The properties of our computational model are following.
-
1.
We ignore the details of regular action of almost all synapses on the ClFCs. Instead, we consider that all ClFCs are firing randomly and independently from each other with the average interspike interval of 1 s (the mechanism of generation of ClFC impulses is replaced by random generation in our model). Further we suppose that the membrane potential of each ClFC is almost constant except for some features described below.
-
2.
We neglect the immediate effect of the ClFC action potential via the electrical synapses on other ClFCs on the following reasons. First, when the soma acts on the dendrite, the action potential is filtered out by the low-pass capacitance filter of the dendrite. Second, dendrite influence on the soma is negligible because of high output impedance of dendrites so most of potential difference falls on dendrite. Third, the postulated rectifying property (shown as diode at Fig. 4) blocks transfer of positive potential from soma to dendrite.
-
3.
We consider only events which follow excitation of each single ClFC.
As stated earlier, each impulse of ClFC immediately generates synchronous spikes in all the PCs to which it sends its climbing fibers. The impulses of different PCs belonging to the given ClFC impose synchronous inhibition on all NuCs to which they send synapses. This inhibition synchronously interrupts inhibitory action of NuCs on ClFCs. As stated above, the change of NuCs action on ClFC caused by synchronous excitation of PCs acts in fact as a kind of an excitatory postsynaptic potential from PCs to their ClFC. For the ClFC which has just excited, this excitatory potential will not be effective (see above). However, for an action from the current ClFC to those ClFCs, with which it shares common NuCs, the effect might be substantial. It might cause undesirable firing of some of those ClFCs a few milliseconds after the firing of the current ClFC.
To consider the processes in the system in detail, we performed computational experiments illustrated on Fig. 5. The figure shows the superposed tracks of the membrane potential of the neurons #7 and #11
In this figure, the action potentials of the neurons are not shown. Instead, only post-impulse hyperpolarizations connected with action potentials are displayed. On Fig. 5a we switched on and off electrical connections between ClFCs. It could be seen that the amplitude of positive peaks in ClFC membrane potential caused by NuCs is substantially reduced when the electrical synapses are switched on. Without electrical connections some of those peaks are high and might cause erroneous excitation of ClFCs.
Figure 5bc shows at smaller time scale membrane potential tracks of neurons #7 and #11 with presence (Fig. 5b) and absence (Fig. 5c) of electrical coupling between cells. The timing of events for Fig. 5b and Fig. 5c strictly corresponds to each other. The simulations with switched on and switched off electrical synapses were performed with exactly the same sequence of random activations of ClFCs. At Fig. 5c one can see a strong postimpulse hyperpolarization for neuron #7 and one for neuron #11. These hyperpolarizations have large amplitudes and last for five milliseconds after the moment of ClFC impulse generation. In case of coupling turned on, the hyperpolarisation of neuron 7 reduces the amplitude of action of this neuron on neuron 1 via NuCs and vice versa, the hyperpolarization after action potential of neuron 11 reduces action of neuron 7 on neuron 11. The smaller amplitudes 11 on neuron 7 of positive deflections of membrane potentials in the figures are connected with actions of four other neurons on neurons 7 and 11.
So in this section we have demonstrated that electrical connections between two ClFCs can reduce and potentially abolish the undesirable excitatory action of ClFCs on each other.
On measurements of electrical coupling between ClFCs with or without activation of NuCs
The well-known hypothesis states that the main effect of action of GABAergic NuCs on ClFCs causes diminishing of coupling between ClFCs[17, 18].
One of the main evidence of the action of NuCs excitation on the coupling between ClFCs is based on direct measurement of electrical coupling between ClFCs in in vitro experiment[19].
In this subsection we try to estimate effects of switching on activity of NuCs on measurements of electrical connections between ClFCs. Based on the arguments given in above for this estimation we used the model where electrical contacts are connecting dendrites of one cell with the soma of another cell. For simplicity, we have considered case of two ClFCs where this electrical connection is mutual and symmetric. Also we considered that the contacts are rectifying, i.e. electrically asymmetric. In this case the electrical scheme of the communicating ClFCs is presented at Fig. 6. For that scheme we have calculated the dependence of amplitude of the shifts of potential from the resting value in the ClFC to which we inject the test current (the master cell) and in the ClFC which is electrically connected to the first ClFC (the slave cell). The plot is presented at Fig. 6. In other words, we simulate the procedure of measurements of electrical coupling of neurons as it is presented at Fig.5 of [19]. The scheme contains the identical components of two cells: cell bodies, dendrites and two contacts between the cells. As stated in section 3.4, we considered that connections are directed from the cell body of one cell to the dendrite of another cell. We calculate the data for the plots in two conditions: first, GABAergic synapses are not activated; second, GABAergic synapses are activated. The plot clearly shows that the ratio of shifts of slave cell potential to master cell potential depends on the presence of GABA. In absolute values for the parameters chosen at Fig. 6 with diodes, the coupling coefficient æ\(_{GABA+}\)=\(dU_{c2}\)/\(dU_{c1}\)= 0.049, æ\(_{GABA-}\)=\(dU_{c2}\)/\(dU_{c1}\)= 0.096. So the ratio of coupling coefficients with and without GABA is æ\(_{GABA-}\)/æ\(_{GABA+}\)= 1.95. That ratio for the scheme with no diodes is 1.86.
Left: The circuit diagram for two electrical connections between ClFCs. G is responsible for the coupling strength (G=inf means cells are uncoupled). Index 2 means second cell. Index d means dendrite compartment. Rc, Ec1 - first IO cell soma resistance and electromotive force (EMF). Rc, Ec2 - second IO cell soma resistance and EMF. Rd2, Ed2 - second IO cell dendrite compartment resistance and EMF. G - coupling strength between 1st and 2nd IO cells. Rdc2 - somatodendritic resistance of the 2nd cell. Capacitance is ignored. All resistor face values were 100 M except Rc1=Rc2=20 M, Rd1=Rd2=30 M in presence of GABA and Rc1=Rc2=20 M, Rd1=Rd2=100 M in absence of GABA. Ec1=Ec2=-60mV, Ed1=Ed2=-80mV. The diodes are ideal with bias of 0mV. Right: For scheme on the left side of the Figure, dependence of membrane potential of slave cell c2 on membrane potential of master cell c1 with injection of constant current into the master cell, in presence (blue) and absence (black) of GABA
However in calculations we didn’t change connections between cells, i.e. we didn’t change values of the resistors \(R_{g12}\) and \(R_{g21}\). In other words, the real experiments of [19] and our calculations show the same results. It should be noted that in our calculations the coupling resistors (usually considered to characterize the electrical connection strength) have the same values for both calculated conditions.
Discussion
Sources of chaotic phenomenae in cerebellum
In this paper it is demonstrated that the loop ClFC - PCs - NuCs - ClFC provides chaotic behaviour of ClFC firing and the chaos in this system does not need interaction between different ClFCs. So the chaotic phenomenae revealed in our work do not coincide with the chaotic phenomenae explored in [3].
As for the mechanism for the dynamic chaos in our system, its plausible source might be connected with biphasic plasticity (synaptic depression followed by synaptic potentiation) of the GrCs to PC synapses. It might induce dynamic chaos in accordance with the scenario of processes in dissipative structures[20]. Thus, the electrical synapses between ClFCs of the inferior olives are not needed for chaotic behaviour of ClFCs.
The possible functional role of dynamic chaos that can be inferred from our computational modeling is clear for signals which we used in our modeling. In this case finally the GrC to PC synapses learn to make the total input to the ClFC constant. The external signal to the ClFC was a fixed function (sine function, in our case). For representation of that function the homogeneous sampling mechanism of all the phases of the equalized ClFC signal is needed. In principle this homogeneity can be achieved either by regular sampling at small fixed intervals or by multiple samplings homogeneously distributed over the period of the signal which is equalized. The first option actually is not available in neural structures while the homogeneous distribution of sampling points might be achieved either by adding noise to the system or with the help of chaotic dynamics[21]. The noise option is energetically costing and brings in non-necessary informational disturbance, so chaotic sampling might be optimal for the system functioning as has been noted beforehand in [3] although for the different source of chaos in ClFCs.
The results obtained in our work are in fact contradicting the current view [3, 4] on the origin of chaotic phenomenae in the cerebellum. The chaos which has been revealed might be referred to as cerebellar structural chaos (CSC) as opposed to multiple inferior olive cells interaction chaos (MIOCIC). The role of chaos in both CSC and MIOCIC is similar. It serves for sampling of all phases of the recorded signals at the GrCs to PCs synapses. However, it can be argued that CSC provides much more efficient use of recording informational capacity of each GrC to PC synapse. In principle, it can be resolved experimentally which of the models better suits the properties of real cerebellum. That can be made by evaluation of the amount of information capacity needed to perform concrete functions of the cerebellum. However this method of comparison is far from the reach of modern physiological knowledge of cerebellar participation in implementation of physiological functions. This argument might be used to argue that CSC is more likely to work in live cerebellum because of the higher amount of informational capacity which it might provide to the cerebellum[22].
Role of electrical synapses
In Section 3 we have demonstrated that electrical synapses between ClFCs can reduce synchronicity of firing of different ClFCs. This conclusion seems to contradict the common sense general idea that electrical connections between cells might cause synchronization of these cells activities. Meanwhile Tokuda et.al.[3] claim desynchronization of ClFCs with the help of electrical synapses between them by mechanism of multicell dynamic chaos. It should be noted that both Tokuda et.al. and we are talking about the chaos that ClFC is involved in. However in our case the sources of chaos in all ClFCs are independent from each other while in the case of Tokuda et.al. the chaos is generated by the interaction of different ClFCs.
The mechanism which we have proposed uses structural asymmetry of electrical synapses. We suppose that most of electrical connections between ClFCs are formed between soma of one cell and dendrite of another cell. Also we suppose that there is electrical asymmetry (i.e. such electrical synapses which conduct current only in one direction) so that the coupling between cells works only in case when when the soma is more negative than dendrite. This hypothesis might be a subject for experimental verification.
The use of many elementary memorizing devices provides advantages only in those cases when these devices in practice might be used independently of each other[22]. This general consideration should be true for such elementary memorizing devices as synapses and in particular for synapses between granule cells and PCs of the cerebellum. The latter constitute about the half of all synapses in human central nervous system. From this point of view the independent work of individual ClFCs is beneficial for the whole system functionality. The presence of the experimentally established fact that ClFCs have electrical synapses at first glance contradicts to independence of ClFCs. Dielectrical connections between neurons are generally considered as a mean to facilitate positive interaction, synchronization between them. This is definitely true for symmetric electrical synapses. There is no precise knowledge on whether electrical synapses between ClFCs are symmetric or non-symmetric. The non-symmetric connection might be established between soma of one cell and distal dendrite of another cell.
On electrical measurements of couplings between ClFCs
We have demonstrated that the standard action of inhibitory NuCs on the targeted cells might result in apparent change of coupling between ClFCs if measured by standard electrical measurements procedure.Footnote 2
Our calculations did not use change in electrical conductivity of contacts between the cells (\(R_{g12}\) and \(R_{g21}\) at Fig. 6). So the change in measured coupling between the cells might be referred to as apparent and "not real". In other words, it could be stated that activation of NuCs does not affect coupling between ClFCs.
These conditions should be further explored in large-scale modeling[24] and in physiologically natural experimental conditions[25] as well as for cerebellar modules application to cognitive tasks[26]. In any case, our calculations demonstrate that the observed phenomenae do not mean that the presence of GABA changes electrical conductance of contacts between ClFCs. It should be also noted that measurements of electrical coupling are performed for that range of the membrane potential which does not correspond to the range of membrane potential variations which provides input signal integration in ClFCs.
Thus, it is natural to consider that the action of NuCs is a standard action of inhibitory cells on their target objects, and is not unique to cerebellar nuclear to inferior olives special type of interaction as it has been proposed in [17, 18].
It should be noted that in [1, 2, 27] and in the current paper we use the model in which PCs directly act on the ClFCs. Of course in reality this action is mediated by NuCs. However as the NuCs apparently get excitatory drive autonomous for each cell[28], this feature of the model might not be a meaningful distortion of a functional role of PC on the ClFCs. Further, this looks plausible as in our model action of PCs on ClFCs is excitatory and in reality action of PCs on ClFCs implemented with the help of NuCs results in excitatory action.
Complex spikes action
Complex spikes present reaction of the PC to the incoming impulses from ClFCs. They yield initial spike of a complex form in the axon of PC usually followed by a silence of up to 50 ms in duration [6]. The action of this output pattern is different on (1) the cerebellar nuclei output glutamatergic cells and on (2) GABAergic NuCs in the olivocerebellar loop. As to the former, the PCs belonging to the same climbing fiber give synapses to different glutamatergic cerebellar NuCs [29]. Obviously each output NuC gets input from PCs which are controlled by different ClFCs[29]. A complex spike from PC to concrete NuC caused by input from ClFC and followed by a small pause can not have significant impact as ClFCs fire generally asynchronous.
The opposite situation happens in the network of olivo-cerebellar loop. Each excitation impulse of ClFC almost simultaneously causes complex spikes in all PCs to which it sends climbing fibers. The PCs in turn act on NuCs which act back on the ClFC which generated the impulse which caused PCs complex spikes.
Importantly, there is a notable intersection between the sets of NuCs which act on different ClFCs. With help of a skeleton model we have demonstrated that the electrical synapses between ClFCs can abolish synchronous firing of ClFCs which might be produced due to the fact that different ClFCs can share inputs from NuCs. Our demonstration in fact presents a hypothesis which should be verified both experimentally and on the large-scale computational model of the cerebellum. Contrary to our skeleton model the large-scale model should include a functionally meaningful set of ClFCs. The model should involve description of mechanisms for impulse generation by ClFCs including all the neurons outside the cerebellum and of the cerebellum circuitry connected to the selected set of ClFCs.
Conclusion
In three papers of this series ([1, 2] and the current paper) we have demonstrated that:
-
1.
It is highly probable that the ClFC impulses are inner signals of the olivo-cerebellar loop and are not the signals of organism’s behaviour errors.
-
2.
The base function of ClFC is to equalize inferior olive membrane potential which is implemented with the architecture of the olivo-cerebellar-olivary loop and with known features of the GrCs to PCs synaptic plasticity (other points of view are given in [30]). The plasticity of granule cells to PCs synapses provides equalization of the total input to the ClFCs and the activity of ClFCs reflects only discrepancy of the latter from constant values.
-
3.
The olivocerebellar loop with bidirectional plasticity of GrCs to PCs synapses is inherently chaotic independently for each ClFC module. That property does not need interaction between climbing fiber cells via the electrical synapses as has been hypothesized earlier[18].
-
4.
The main role of electrical connections between ClFCs is diminishing probability of synchronous firing of ClFCs thus providing almost maximum possible independence of different ClFCs firing. Our calculations based on electrical scheme of intercellular connections clearly demonstrate that synapses from GABAergic NuCs to ClFCs do not affect the conductivity of intercellular contacts between ClFCs thus providing explanation of the results of experiments of measuring electrical connections between ClFCs with the help of optogenetically-induced release of GABA[19].
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to the institutional policy.
Notes
Enrico Mugnaini, personal communication with W.L.D-B. at SFN Annual Meeting, Miami, 1999.
Measurements of electrical couplings between cells of different tissues in many details are described in [23]
References
Shakirov, V., Altunina, O., Shaposhnikov, D., Podladchikova, L., Dorofeev, V., Dunin-Barkowski, W.L.: Cerebellar plasticity-based equalization of total input to inferior olive cells in time domain: comparison of computational and physiological experimental data. Submitted to Neuroscience and Behavioral Physiology (2022)
Shakirov, V., Dorofeev, V., Dunin-Barkowski, W.L.: Cerebellar plasticity-based equalization of total input to inferior olive cells in time domain: Properties of the model dynamics. Submitted to Neuroscience and Behavioral Physiology(2022)
T. Tokuda, I., E. Han, C., Aihara, K., Kawato, M., Schweighofer, N.: The role of chaotic resonance in cerebellar learning. Neural Networks 23, 836–842 (2010)
Kawato M, Ohmae S, Hoang H, Sanger T (2021) 50 years since the Marr, Ito, and Albus models of the cerebellum. Neuroscience 462:151–174. https://doi.org/10.1016/j.neuroscience.2020.06.019. In Memoriam: Masao Ito-A Visionary Neuroscientist with a Passion for the Cerebellum
Khosrovani, S., Van Der Giessen, R.S., De Zeeuw, C.I., De Jeu, M.T.: In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc Natl Acad Sci U S A 104(40), 15911–15916 (2007)
Itō, M.:The Cerebellum: Brain for an Implicit Self. FT press, ??? (2012)
Gibsons, A.R., Horn, K.M., Pong, M.: Activation of climbing fibers. The Cerebellum 3(4), 212–221 (2004). https://doi.org/10.1080/14734220410018995
Gellman, R., Houk, J.C., Gibson, A.R.: Somatosensory properties of the inferior olive of the cat. The Journal of Comparative Neurology 215(2), 228–243 (1983).https://doi.org/10.1002/cne.902150210
Llinas, R., Baker, R., Sotelo, C.: Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol 37(3), 560–571 (1974)
Llinás, R., Sasaki, K.: The Functional Organization of the Olivo-Cerebellar System as Examined by Multiple Purkinje Cell Recordings. Eur J Neurosci 1(6), 587–602 (1989)
Sugihara, I., Wu, H.S., Shinoda, Y.: The entire trajectories of single olivocerebellar axons in the cerebellar cortex and their contribution to Cerebellar compartmentalization. J Neurosci 21(19), 7715–7723 (2001)
Smolyaninov, V.V.: About some features of the organization of the cerebellar cortex. In: Tsetlin, M.L., Fomin, S.V. (eds.) Models of Structural and Functional Organization of Some Biological Systems (in Russian), pp. 203–262. Nauka, Moscow (1966)
Kenyon, G.T., Medina, J.F., Mauk, M.D.: A mathematical model of the cerebellar-olivary system i: Self-regulating equilibrium of climbing fiber activity. Journal of Computational Neuroscience 5(1),17–33(1998). https://doi.org/10.1023/A:1008874209991
Eccles, J.C., Ito, M., Szentágothai, J.:The Cerebellum as a Neuronal Machine. Springer, ??? (1967). https://doi.org/10.1007/978-3-662-13147-3
Ito, M.: The Cerebellum and Neural Control. Raven Press, ??? (1984)
Berkinblit, M.B.: Neural Networks (in Russian). M.: Miros, ??? (1993)
Lang, E.J., Apps, R., Bengtsson, F., Cerminara, N.L., Zeeuw, C.I.D., Ebner, T.J., Heck, D.H., Jaeger, D., Jörntell, H., Kawato, M., Otis, T.S., Ozyildirim, O., Popa, L.S., Reeves, A.M.B., Schweighofer, N., Sugihara, I., Xiao, J.: The roles of the olivocerebellar pathway in motor learning and motor control. a consensus paper. The Cerebellum 16(1),230–252(2016).https://doi.org/10.1007/s12311-016-0787-8
Hoang, H., Lang, E.J., Hirata, Y., Tokuda, I.T., Aihara, K., Toyama, K., Kawato, M., Schweighofer, N.: Electrical coupling controls dimensionality and chaotic firing of inferior olive neurons. PLOS Computational Biology 16(7),1008075(2020). https://doi.org/10.1371/journal.pcbi.1008075
Lefler, Y., Yarom, Y., Uusisaari, M.Y.: Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron 81(6), 1389–1400 (2014).https://doi.org/10.1016/j.neuron.2014.02.032
Mori, H., Kuramoto, Y.: Dissipative Structures and Chaos-Springer, (1998).https://doi.org/10.1007/978-3-642-80376-5
Andreyev, Y.V., Belsky, Y.L., Dmitriev, A.S., Kuminov, D.A.: Information processing using dynamical chaos: neural networks implementation. IEEE Transactions on Neural Networks 7(2), 290–299 (1996).https://doi.org/10.1109/72.485632
Borisyuk, G., Borisyuk, R., Dunin-Barkowski, W., Kovalenko, E.: Estimation of information capacity of purkinje cells. Locally Interacting Systems and their application in Biology 653, 72–90 (1978)
Berkinblit, M., et al.: Highpermiability Membrane Contacts and Their Role in Intercellular Interactions (in Russian). Nauka, ??? (1981)
Yamazaki, T., Igarashi, J., Yamaura, H.:Human-scale brain simulation via supercomputer: A case study on the cerebellum. Neuroscience 462, 235–246 (2021).https://doi.org/10.1016/j.neuroscience.2021.01.014
Fernandez-Iriondo, I., Jimenez-Marin, A., Diez, I., Bonifazi, P., Swinnen, S.P., Muñoz, M.A., Cortes, J.M.: Small variation in dynamic functional connectivity in cerebellar networks. Neurocomputing 461,751–761 (2021).https://doi.org/10.1016/j.neucom.2020.09.092
Teddy, S.D., Lai, E.M.-K., Quek, C.: A cerebellar associative memory approach to option pricing and arbitrage trading. Neurocomputing 71(16-18), 3303–3315 (2008). https://doi.org/10.1016/j.neucom.2008.04.039
Dunin-Barkowski WL (2002) Analysis of output of all purkinje cells controlled by one climbing fiber cell. Neurocomputing 44–46:391–400. https://doi.org/10.1016/S0925-2312(02)00386-7. Computational Neuroscience Trends in Research 2002
Uusisaari, M., Obata, K., Knöpfel, T.: Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Journal of Neurophysiology 97(1),901–911 (2007).https://doi.org/10.1152/jn.00974.2006
Apps, R., Hawkes, R., Aoki, S., Bengtsson, F., Brown, A.M., Chen, G., Ebner, T.J., Isope, P., Jörntell, H., Lackey, E.P., Lawrenson, C., Lumb, B., Schonewille, M., Sillitoe, R.V., Spaeth, L., Sugihara, I., Valera, A., Voogd, J., Wylie, D.R., Ruigrok, T.J.H.: Cerebellar modules and their role as operational cerebellar processing units. The Cerebellum 17(5), 654–682 (2018).https://doi.org/10.1007/s12311-018-0952-3
Streng, M.L., Popa, L.S., Ebner, T.J.: Complex spike wars: a new hope. The Cerebellum 17(6),735–746 (2018).https://doi.org/10.1007/s12311-018-0960-3
Acknowledgements
The work is financially supported by State Program of SRISA RAS No.FNEF-2022-0003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.s
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shakirov, V., Dorofeev, V., Lebedev, A. et al. Dynamic chaos in cerebellum and electrical synapses between climbing fiber cells of inferior olives. Neurosci Behav Physi 53, 717–728 (2023). https://doi.org/10.1007/s11055-023-01420-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01420-w