Abstract
We present a novel biologically plausible model of cerebellar learning. The study includes three substantial points. First, we show that the effective model of cerebellum does not need any explicit error signals of organism actions to perform learning tasks. In our model the synapses weights from granule cells to Purkinje cells change so that the latter learn to reconstruct extracerebellar input to its climbing fiber cells of inferior olives (ClFCs). The second point is that we demonstrate the emergence of chaotic behaviour in our model which does not depend on electrical synapses between the cells of inferior olives. Third, we compare climbing fiber cells activity in the model with the Purkinje cells complex spikes sequences in guinea pig cerebellum using ordinal analysis method and three other independent statistical properties (variation coefficients, autocorrelation functions and intervalograms). We conclude that all examined theoretical and experimental properties are in good accordance with each other. The plausible importance of the revealed phenomenae for cerebellar function is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The theories of cerebellar learning expressed in [1,2,3,4,5,6,7] have produced deep impact on the studies of the cerebellar function. Some consequences of those theories were revealed in many computational (e.g. [8]) and physiological experiments [9,10,11]; for review see collection of papers published in the memory of Masao Ito [12]. Experiments also demonstrated that original theories need substantial changes [13]. There were two important experimental findings in cerebellar studies which affected the further progress of studies of the cerebellar neurons and networks mechanics. The first was discovery of both LTD [10] and LTP [14] in granule cells (GrCs) to Purkinje cells (PC) synapses. The second important finding was the anatomical discovery of the feedback from the Purkinje cells to the cells of inferior olives [15]. The first interpretation of meaning of these two findings has been proposed by Mauk and Medina. According to their hypothesis, the inferior olive (IO) cerebellar cortex loop discovered by Fredette and Mugnaini [15] provides homeostasis of the firing frequency of inferior olive cells [16]. This general idea has been explored in models of cerebellum [17,18,19,20,21,22]. On the other hand it has been known for years that impulse activity of the inferior olivary cells very often looks practically random in spite of salient modulation of the Purkinje cell simple spikes activity in many functional circumstances (for review, see [23]). The simplest interpretation of these facts is that total afferent inflow to the cells of inferior olives in those cases most often is constant in time with random fluctuations. Such state of the system might be achieved when input to the inferior olive cells from the external (extracerebellar) sources is exactly complemented with the feedback from Purkinje cells. In earlier publications we have demonstrated that such precise matching can be achieved in cerebellar-like construction which makes use of plasticity of granule cells to Purkinje cells synapses [18, 21]. Here we present more modeling results and use more physiological observations for comparison of computational and experimental data. We present description of features of impulse activity of inferior olive cellsFootnote 1 and demonstrate that they practically coincide with the corresponding features of our model data.
In particular we have found resemblance of the series of complex spike generation moments in physiological records and in the model using ordinal analysis of sequences [24]. Besides it has been found that the variation coefficients of the interspike intervals of the ClFCs for the model data lie close to the range of these values for complex spike activity of the guinea pig Purkinje cells. The comparison enables formulation of the hypothesis that the main neuronal operation of the cerebellum is provision of constancy in time of the total input to the inferior olive cells. Below we formulate this hypothesis in detail and discuss its experimental consequences and potential functions. Preliminary data have been published elsewhere [25,26,27].
Computational and biological experimental results
Equalization of the total input to the ClFC
In [21] we have proposed a simple model of the cerebellum circuitry, in which one can observe the equalization of the total input to inferior olive cells due to the plasticity of granular cell synapses to Purkinje cells. The same approach is applied in the current paper. The model is based on the system of equations which we call Mauk equations (equations 1-4 in [21]).
The model includes one ClFC, one PC, and hundreds of GrCs. In the model we use a direct excitatory action of PC on a ClFC, while in real cerebellum the excitatory connection from PC to ClFC is provided with two sequential inhibitory synapses [15]. The total input to ClFC is the sum of extracerebellar signal to ClFC and feedback signal from Purkinje cell.
The plasticity of synapses from granule cells to Purkinje cell is controlled by the function \(\chi \) in Mauk equations. The time derivatives of \(\sigma _i\) (synaptic weight from i-th granule cell to PC synapse (i=1..n)) are proportional to the three terms: a constant synaptic learning rate \(\epsilon \), the ClFC activity dependent plasticity factor \(\chi (t)\), and eligibility factor \(e_i\) [17, 28] (for i-th granule cell). The function \(\chi (t)\) is defined as follows: \(\chi (t) = \chi (t - t^*(t))\), where \(t^*(t)\) is the last moment of activation of the ClFC before the moment t.
where \(A>0, a>0.\)
We use A which is significantly larger than a.
We denote the value \(T_{equ} = \Delta (1 + A/a)\) as equilibrium ClFC interval. In wide range of model parameters the average firing intervals of ClFC in stationary conditions is equal to \(T_{equ}\). It is known that climbing fiber cells of inferior olives of all animal species are firing with the constant average rate which is different for different cells but for the most of animal species lies in the interval of 0.4 - 1.2 seconds [2, 5, 29,30,31]. Most of the time (at least 90% of total time) the firing of ClFCs looks as if random with an average rate mentioned above. To connect the model data to physiology we consider that \(T_{equ}\) is equal to 1 second so all the model time dependent parameters below are described based on this correspondence.
In this paper (as well as in our previous and following papers on this subject [21, 32, 33]) we are dealing with excitation of granule cells not considering how this activity emerges from mossy fiber activation patterns.
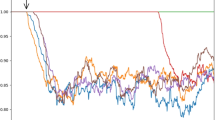
Below the model and the obtained results are presented in more detail. Following [21], the cerebellar module is embedded in a periodic environment. That is, granule cell activity vector in n-dimensional space is a fixed periodic vector function of time and external signal to the ClFC is a fixed periodic scalar function of time with the same period as granule cells periodic vector function. In all time moments there were exactly the same number of active granule cells and each granule cell had equal number of firing episodes over the period of activity. In our computational experiments in this article the number of granule cells was 300. Example of granule cell activity pattern for one period of the embedding signal with the postulated properties is shown at the Fig. 1. The pattern has been generated with the help of pseudo-random number generator. Computational experiments with the model are started with random values of synaptic weights from granule cells to the Purkinje cell. The external signal to the ClFC was a sinusoidal function with a period of 400 milliseconds.
Computational experiments show that the adaptation of the model in time makes the sum of (a) Purkinje cell output signal and (b) extracerebellar signal to the climbing fiber cell almost constant. At the beginning of the computational experiment the output of the PC is almost constant. So the sum of the PC’s activity and external signal yields practically ideal sine wave. The complex spikes in the beginning of the adaptation are synchronous with the phases of the external signal and look random at the steady state (cf Fig.2c in [21]). We refer to the state of the model described above as the model steady (stationary) state. In that state, the input from PC to ClFC yields almost exact negative copy of the input to the ClFC from the extracerebellar sources. Figure 2 shows three stages of equalization progress in the model. The stage of Fig. 2c demonstrates the steady state of signal equalization. These computational results in essential details repeat our data of [21].
Comparison of statistical properties of ClFC firing for computational model and guinea pig Purkinje cell complex interspike intervals
In this section we compare our computational data with data obtained in experiments with live guinea pig cerebellum. The latter were taken from the experiments performed earlier [6, 34].
In the steady state of the model, the sequence of climbing fiber cell impulses looks seemingly random. We used Poincare plots to characterize properties of these random looking activities in different conditions.
Figure 3 demonstrates Poincare plots for successive intervals of ClFC impulses in the stationary state. The most salient property of these plots is presence of horizontal, vertical and diagonal white strips which are free of experimental points. The origin of these strips is obvious: in our computational experiments, the pattern of granule cell firing is strictly periodic with period of the embedding periodic signal. Due to properties of \(\chi \)-function the synaptic weights of granule cells which are active at the moment of generation of ClFC impulse become reduced for time duration of about \(\Delta \).
After one period of external signal the granule cells activity becomes the same as it has been integer number of periods from the moment of generation of previous ClFC impulse. So the amplitude of sum of actions of granule cells on the Purkinje cell at that moment and for the duration of \(\Delta \) will be lower than usual for time between t and t+Delta, and between t+T and t+delta+T (where T is the period of GrC activity) and between t+2T and t+Delta+2T etc. As the total input to ClFC would be lower than usual, ClFC potential would not cross threshold in these periods hence no intervals with length from T to T+delta and (less strictly) with length from from 2T to 2T+delta etc. That reasoning explains horizontal and vertical strips. The same is true for the sum of two sequential intervals of ClFC excitation which explains diagonal strips at the figures at the Poincare plots. The obvious stochasticity of activation patterns of ClFC depends on the learning rate of granule cells to Purkinje cell synapses. This stochasticity is in fact the consequence of dynamic chaos which emerges in the work of the cerebellum module (the details will be discussed elsewhere [33]).
The data on Fig. 3 are obtained for different values of model learning rate. Changing the learning rate for five orders of value does not remove apparent stochasticity of ClFC firing. However, zeroing learning rate in stationary conditions of the system makes firing pattern "non-ergodic": firing might be totally abolished or might run through very short cycles of fixed interval values (in these cases plots contained less than 10 discrete points each).
An important and frequently used parameter of neural activity in neurophysiological literature is variation coefficient, a ratio of the standard deviation of interspike intervals to their mean value.
Table 1 shows that variation coefficient \(\nu \) in model neurons depends on learning rate \(\varepsilon \), decreasing linearly with exponential decrease of learning rate. At large learning rates it is within the range of the values of the variation coefficient for real ClFCs. Notably, the average interspike interval of ClFC firing in stationary conditions remains equal to the \(T_{equ}\), when learning rate is non-zero.
In principle the comparison of variation coefficient in the model and in live cerebellum might be used for estimation of the learning rate in real cerebellum. Such procedure should be made in independent physiological experiments jointly with computational modeling. The detailed studies in this direction present tasks for further investigation in future works. For crude estimation of learning rate of GrCs to PCs in cerebellum see the pioneer works in that field [35]. The complete rewiring of GrCs to PC synapses can be obtained in 15 minutes of its work which includes about one thousand impulses of the ClFCs.
As mentioned above, here we use data on complex spikes of Purkinje cells activity in guinea pig cerebellum from [6, 34]. The data were also used in the paper [6], more details on data preparation is given there. The data are referred to as CS data below.
To compare the obtained model results with guinea pigs data we analyse statistics of interspike intervals of the CS data. We analyze their variation coefficient \(\nu \) which is defined as standard deviation divided by mean. The values of \(\nu \) for all neurons turned out to be equal (in ascending order):
\(\nu \) = 0.61; 0.64; 0.64; 0.66; 0.66; 0.67; 0.72; 0.75; 0.75; 0.76. They are consistent with other published data [36]. The corresponding numbers of impulses were 847; 1079; 713; 747; 915; 908; 753; 1255; 1275; 694. We note here that in our computational experiments this quantity lies in the range: 0.67 - 0.92 [25]. We can conclude that there is a reasonable coincidence of the model and live cerebellar data on the values of variation coefficient of complex spikes.
Interspike ordinal analysis in model and in guinea pigs data
Using method proposed by Masoller [24], we have collected and analyzed the statistics of successive interspike intervals durations. In our notation, "2 0 1" means that among three consecutive interspike intervals (numbered 0, 1, 2), the longest interval was the second, zeroth interval was less than the second one, and the first interval was less than zeroth one. Similarly five other possible combinations of the interval duration relations are defined.
On Fig. 4 the data are presented as 10 equidistant (arbitrarily chosen) points of abscissa for convenience of visual evaluation of each experimentally obtained set of data for individual neurons and their relation to the average values of the analysed parameters. For these 10 neurons one can see that population looks statistically homogeneous (Fig. 5). One can see that the order "2 1 0" has the smallest average probability, the opposite one ("0 1 2") has the nearest larger average probability, and the rest of the others have larger probabilities. The difference between probabilities of "2 1 0" and "0 1 2" is not statistically significant while the differences between any of them with the others are statistically significant (\(p < 0.01\)). For biological neurons the data are on Fig. 4, for the model neurons the data are on the Fig. 6.
So in comparison of computational experiments with the biological data we can see that (1) both the model ClFC sequences and biological CS firing look random and the variation coefficient of the biologically observed intervals corresponds to the variation coefficient of ClFC intervals with non-small values of learning rate; (2) for the computational experiments, the sequences "0 1 2", "2 1 0" have smaller counts than other sequences for large enough values of learning rate (Fig. 6). For biological data we also see that sequences "0 1 2" and "2 1 0" have less counts than other combinations of interval durations; (3) Intervalograms of ClFC impulse sequences and guinea pig complex spikes sequences data have visually obvious similarity (Fig. 7).
Visual evaluation of similarity of interspike interval sequences for model ClFC and live cerebellar complex spikes
At Fig. 7, the plots of 21 sequences of 700 consecutive interspike intervals each are presented. The ordinates range of all plots are the same in terms of average interspike interval. Fig. 7abdeijlmpu shows plots for biological sequences and Fig. 7cfghnopqrst shows plots for computational sequences. The plots of biological and computational data on the Fig. 7 are randomly intermixed with each other. The Fig. 7k (in the middle) is a sequence of successive intervals of a guinea pig cortical neuron. Our statement is that for human observer it’s obvious that the pattern of Fig. 7k substantially differs from the patterns of the other twenty sequences. At the first glance the visual evaluation of the interval sequences plots does not yield clues to determine the source of the plot (model vs. biological). More attentive visual analysis enables observer to see difference between model and biological sequences.
Discussion
Summary of computational experiments results
The presented above results of computational experiments and their comparison to the properties of the cerebellum of a particular animal model (guinea pig) can be summarized as follows:
-
1)
In the process of model cerebellar learning implemented by biphasic plasticity of GrCs to PC synapses the total input to the ClFCs becomes equalized in time domain.
-
2)
Switching on learning leads to emergence of stochastic dynamic properties of ClFC impulse activity. The variation coefficient of interspike intervals monotonically increases with learning rate.
-
3)
Monotonically increasing and monotonically decreasing triples of intervals both in real neurons and in the model have lower probabilities than other triples.
-
4)
Intervalograms show salient resemblance of the impulses in real cerebellum and in the model.
Below we discuss specific possible mechanisms responsible for our results and their functional role.
-
1)
In steady-state conditions there is absence or weak correlation of the activity of ClFCs with the temporal patterns of dynamic physiological processes, in which specific parts of the cerebellum are clearly involved (e.g. [37]). On the contrary, there is salient modulation of simple spike activity of Purkinje cells controlled by those ClFCs with the mentioned temporal patterns in the same conditions. This discrepancy has long been considered one of the main mysteries of the functioning of the cerebellum [5]. The equalization of the total input to the ClFCs which is finally provided by tuning of the synapses of GrCs to PCs revealed in our computational experiment yields straightforward explanation of these enigmatic features of activity of cerebellar neurons. The weak correlation of ClFCs activity with many physiologically meaningful events in the organism which is often observed [13, 23] does not contradict our statement. The explanation which we propose here states that impulses of ClFC are the signals belonging to functioning of olivocerebellar loop and they only partly might be connected to the organism behaviour controlling mechanisms. In favour of our explanation of ClFC activity is extreme complexity of attempts to obtain casual connection between the error signals and moments of ClFC impulse generation [38, 39]. The attempts to find exact causal connection of moments of ClFC excitation with organism’s error reactions are extremely complicated and hard to verify [38, 39], while our explanation is short and exhaustive.
-
2)
In this paper we are dealing only with single ClFC which is connected to single Purkinje cell and demonstrate the obvious chaos connected phenomenae, with chaos mechanisms dramatically different from [40]. The random looking pattern of ClFC activity in our model means that there is no need for ClFC ensemble chaotic behaviour to provide apparent randomness of sequences of climbing fiber cell activity. It can happen in each single ClFC isolated subnetwork. This fact challenges ensemble-based chaotic models of the inferior olives activity and also questions the existing interpretations of the role of the electrical synapses between ClFCs [41].
-
3)
Studies of dynamic chaos phenomenae in concrete physical systems usually are based on large sets of data. The data which we have used in this paper are small by all criteria used for chaotic phenomenae identification. As stated in Section 2.3, we have used 10 sets of sequential complex interspike intervals for different Purkinje cells recorded in live cerebellum. The number of intervals in records was between 694 and 1275. The analysis of small datasets is a hard problem in chaotic studies. Fortunately the ordinal analysis of [24] is applicable in our case.
-
4)
The final argument in favour of hypothesis that biological data are generated by the same mechanics as the model ClFC intervals is based on human visual evaluation of the experimental data (Fig. 7). The overall expression (the human answer to the question which of these 20 sequences are similar and which are not) unanimously points to similarity between all 20 sequences as opposed to the 21st (Fig. 7k) sequence which is strikingly different. These patterns of activation are not characteristic for other brain neurons (Fig. 7k). The described features can be used as arguments in favour of the hypothesis that the mechanisms of generating model ClFC intervals are similar to the mechanisms of generating live complex interspike intervals.
In the model one can see that activation of climbing fiber is caused by deflection of the total input to the ClFC from constancy. These deflections might be imposed either by extra excitation of external signal to the cerebellum or extra excitation from the feedback from PC. So the ClFC activity presents the discrepancy between the input from extracerebellar sources and Purkinje cell activity and it does not present signal of the organism’s behavior errors. Of course in cases of unpredictable changes in activity of extracerebellar inputs to ClFCs the ClFC’s impulses are generated in the moments of these changes. These impulses are not necessarily related to the errors of the organism’s behaviour. This conclusion contradicts the generally accepted understanding of the cerebellar functions [7, 13, 42, 43]. The functional role of the alignment in time of total input to ClFC is obviously important but yet unknown.
Our hypothesis means that the organism’s error signals coming to the ClFCs do not differ in their influence on the generation of ClFC impulses from other signals which are coming to the ClFC. All of them have the same role in forming the output of the ClFC.
Based on our results we can describe in other terms the main functional operation which is performed in cerebellum. The central operation unit of the cerebellum is the ClFC. The module gets two kinds of inputs from the sources outside the cerebellum. One of them is input to the IO neurons from non-cerebellar sources, another is mossy fibers input to granule cells. The extracerebellar input to IO neurons depends on the particular part of the IO in which the ClFC is located and the set of all inputs is individual for each ClFC. In many cases the input includes signals from the organism’s error detecting structures. It is not known whether this is obligatory for every ClFC. The ClFC has the sample of diverse parts of the brain activity at its extracerebellar input. The second component of the ClFC input is the input from the Purkinje cells to which each ClFC sends its climbing fibers. All inputs to the Purkinje cells come from granule cells which in turn get their inputs from the cerebellar mossy fibers. From the anatomical data, it is clear that each Purkinje cell has access to extremely large diversity of information sources via mossy fibers. As each ClFC is directly connected to a small number (3-20, average 10) of Purkinje cells, the diversity of sources of the signals which come back to ClFC from Purkinje cells is an order of value higher than diversity of signals coming to individual Purkinje cells. Our hypothesis states that cerebellar learning mechanisms make constant sum of the signals coming from external sources to the ClFC and from the PCs feedback. In other terms when the equilibrium state of the system is achieved the sum of signals from the Purkinje cells connected to one ClFC represents exact negative replica of extracerebellar signals to the same ClFC. It is worth to mention that the main result in DNA hereditary copying [44] was the demonstration of the important role of the exact replica copy of the original DNA strand.
It is clear from anatomical data that the size of set of signals that act through granular cells on Purkinje cells subserved by a particular ClFC is significantly larger than the size of set of extracerebellar signals that directly converge on that ClFC.
Thus the replica is generated mostly from the signals which are not represented in the extracerebellar signals to the climbing fibers. It is true because the number of external signals to the ClFC is several thousand fibers while the number of sources which affect activity of PCs of the ClFC model is more than hundred thousand mossy fibers (the number of granule cells which affect activity of single human Purkinje cell is about two hundred thousand and they come from diverse parts of the cerebellar cortex). How this "neuronal mechanics" can be applied for implementation of the useful functions of the cerebellum? At least one example of that kind of application has been described recently [45]. In that paper Roschin et.al. demonstrated that a robotic arm can be trained to achieve specified points at the body surface using different sources of sensory signals (vision, touch or joint angles). The critical mechanism to obtain such results in their work [45] was matching of the robotic arm endpoint position determined by joint angles with the position of body surface point which is touched by the arm endpoint. Our hypothesis proposes an alternative mechanism to achieve this matching. This example shows possible directions of use of cerebellar mechanisms for movement control tasks.
Wide range associative heteroencoder
It is reasonable at this point to discuss the properties of the proposed model of the cerebellar functioning in more general terms. First of all the main operation of the cerebellum is supposed to match signal in one source (extracerebellar input to IO cells) by taking combinations of other sources. That operation can be referred to as neural heteroencoding [46] (as opposed to the neural autoencoders [47]).
By heteroencoding we mean obtaining an approximation of the sum of signals from one set of sources by the tuned combination from the other set of the sources (different kinds of sensory, effector and associative signals). Thus according to our hypothesis the cerebellum implements heteroencoding as its main information processing operation. The heteroencoder possesses properties presence of which might be deduced from the knowledge of real cerebellum features. Among the properties of cerebellar curcuits, there are two types of associativity. The first one is associativity caused by tuning granule cells threshold by Golgi cells feedback signals. The second property is associativity connected with control of Purkinje cell activity by basket and stellar cells. Taking these points into account we can call the main cerebellar information processing operation as wide associative heteroencoding. In other words, it can be stated that the cerebellum implements wide learning in brain (in contrast to deep learning [48]). It is essential that Purkinje cell gets input from much wider set of sensory, efferent and brain internal state signals than the extracerebellar input to ClFC.
Conclusion
In this paper, first we confirmed the results of computational experiments [21] that the cerebellar type model with a periodic external input tunes the weights of synaptic connections from GrCs to PCs so that total input to ClFC becomes constant in time with small randomly looking variations. Then, we have compared sequences of impulses of the model ClFCs and sequences of complex spikes of guinea pig cerebellum. Comparing activity patterns of ClFCs in our computational experiments (later referred to as artificial data) with experimental data on guinea pig cerebellum (later referred to as biological data) we have found resemblance between these two types of sequences. We have compared three independent features which are present in both types of sequences when the learning rate of the model lies within the parameter range for biological data. First, the variation coefficient for artificial sequences lies within the limits of values of this parameter for biological data. Second, the ordinal analysis of the sequences shows that the number of monotonous sequences of triads of interspike intervals for both experimental and artificial data are reduced in comparison to that number for randomly reshuffled sequences. Third, for human observer it is diffucult to reveal the difference of the patterns of interspike intervalograms for ClFC model and complex spike biological data while patterns of activation of other neuron impulse sequences look clearly different from those two types of sequences.
Based on these results we hypothesize that biological data are generated by the mechanism which in principle coincides with the mechanism of artificial data generation in our computational experiments. Namely when the input to ClFC is equalized the sequence of ClFC impulses is looking like stationary random sequence which does not depend on the external input to the climbing fiber cells.
The stated hypothesis can explain well-known randomness of ClFCs activity in many experimental situations [23]. For example, there are PCs simple spikes which are phase modulated with phases of walking while in the same experiments ClFCs fire similarly randomly [49]. The detailed proof of our hypothesis is yet to come. However the listed arguments seem to be a worthful reason to discuss the consequences of its validity. In our model, the ClFC firing just reflects the deflection from constant of the total input to ClFC (which is a sum of signals from extracerebellar sources summed with the feedback from PCs). If the hypothesis is valid then the same is true for firing of live ClFCs. In particular that means that errors of the organism’s performance are not the leading factor of the ClFC activation. In other words, ClFC impulses are inner signals of the olivocerebellar loop and are not the signals of organism’s action errors contrary to what is stated in majority of modern publications [12]. The formulated hypothesis also raises other questions such as probable chaotic origin of the equilibrium ClFC firing pattern and the plausible role of the electrical connections between ClFCs. These subjects are treated at length in the accompanying papers [32, 33].
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the institutional policy
Notes
We used the experimentally obtained complex spike activity of guinea pig Purkinje cells. It has been shown that these sequences represent about 90% of impulses of the ClFC which sends its climbing fiber to the registered Purkinje cell [6].
References
Brindley, C.S.: The use made by the cerebellum of the information that it receives from sense organs. IBRO Bull. 3, 80 (1964)
Eccles, J.C., Ito, M., Szentágothai, J.: The Cerebellum as a Neuronal Machine. Springer, ??? (1967). https://doi.org/10.1007/978-3-662-13147-3. https://doi.org/10.1007/978-3-662-13147-3
Marr, D.: A theory of cerebellar cortex. J. Physiol. 202(2), 437–470 (1969)
Albus, J.S.: A theory of cerebellar function. Mathematical Biosciences 10 (1), 25–61 (1971). https://doi.org/10.1016/0025-5564(71)90051-4
Ito, M.: The Cerebellum: The Cerebellum and Neural Control. Raven, New York, ??? (1984)
Dunin-Barkowski, W.L., Markin, S.N., Podladchikova, L.N., Wunsch, D.C.: Activity of the climbing fiber innervating various Purkinje cells. Biofizika 44(6), 1094–1100 (1999)
Kawato, M., Ohmae, S., Hoang, H., Sanger, T.: 50 years since the Marr, Ito, and Albus models of the cerebellum. Neuroscience 462, 151–174 (2021). https://doi.org/10.1016/j.neuroscience.2020.06.019. In Memoriam: Masao Ito — A Visionary Neuroscientist with a Passion for the Cerebellum
Prieto, A., Prieto, B., Ortigosa, E.M., Ros, E., Pelayo, F., Ortega, J., Rojas, I.: Neural networks: An overview of early research, current frameworks and new challenges. Neurocomputing 214, 242–268 (2016). https://doi.org/10.1016/j.neucom.2016.06.014
Gilbert, P.F.C., Thach, W.T.: Purkinje cell activity during motor learning. Brain Research 128(2), 309–328 (1977). https://doi.org/10.1016/0006-8993(77)90997-0
Ito, M., Sakurai, M., Tongroach, P.: Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar purkinje cells. J. Physiol. 324(1), 113–134 (1982)
Dunin-Barkowski, W.L., Zhukowska, N.M., Larionova, N.P., Chajlakhian, L.M., Chudakov, L.I.: Parallel fibers - purkinje cell synaptic effectiveness increases after simultaneous stimulation of climbing and parallel fibers. Nejrofiziologia 19(2), 156–164 (1987). in Russian
In memoriam: Masao Ito—a visionary neuroscientist with a passion for the cerebellum. Neuroscience 462(2021)
Streng, M.L., Popa, L.S., Ebner, T.J.: Complex spike wars: a new hope. The Cerebellum 17(6), 735–746 (2018). https://doi.org/10.1007/s12311-018-0960-3
Sakurai, M.: “Depression and potentiation of parallel fiber - Purkinje cell transmission in in vitro cerebellar slices”. In: The Olivocerebellar System in Motor Control, vol. 17, pp. 221–230. Springer, ??? (1989)
Fredette, B.J., Mugnaini, E.: The GABAergic cerebello-olivary projection in the rat. Anatomy and Embryology 184(3), 225–243 (1991)
Kenyon, G.T., Medina, J.F., Mauk, M.D.: A mathematical model of the cerebellar-olivary system i: Self-regulating equilibrium of climbing fiber activity. Journal of Computational Neuroscience 5(1), 17–33 (1998). https://doi.org/10.1023/A:1008874209991
Schweighofer, N., Arbib, M.A., Kawato, M.: Role of the cerebellum in reaching movements in humans. i. distributed inverse dynamics control. Eur J Neurosci 10(1), 86–94 (1998)
Dunin-Barkowski, W.L., Shishkin, S.L., Wunsch, D.C.: Stability properties of cerebellar neural networks: The purkinje cell — climbing fiber dynamic module. Neural Processing Letters 9, 97–106 (1999)
Dunin-Barkowski, W.L., Wunsch, D.C.: Phase-based storage of information in the cerebellum. Neurocomputing 26-27, 677–685 (1999)
Dunin-Barkowski, W.L., Wunsch, D.C.: Phase-based cerebellar learning of dynamic signals. Neurocomputing 32-33, 709–725 (2000)
Dunin-Barkowski, W.L.: Analysis of output of all purkinje cells controlled by one climbing fiber cell. Neurocomputing 44-46, 391–400 (2002). https://doi.org/10.1016/S0925-2312(02)00386-7. Computational Neuroscience Trends in Research 2002
Dunin-Barkowski, W.L., Flerov, Y.A., Vyshinsky, L.L.: Prognosis of dynamical systems behavior based on cerebellar-type neural technologies. Optical Memory and Neural Networks 20(1), 43–58 (2011). https://doi.org/10.3103/S1060992X11010073
Itō, M.: The Cerebellum: Brain for an Implicit Self. FT press, ??? (2012)
Aragoneses, A., Perrone, S., Sorrentino, T., Torrent, M.C., Masoller, C.: Unveiling the complex organization of recurrent patterns in spiking dynamical systems. Scientific Reports 4(1), 4696 (2014)
Dunin-Barkowski, W.L., Shakirov, V.V.: Multidimensional dynamic chaos in cerebellum. Opera Medica et Physiologica, Issue Supplement S2, p. 86 (2016)
Dunin-Barkowski, W.L., Altunina, O.V., Karandashev, I.M., Kiselev, I.I., Shakirov, V.V., Solovyeva, K.P.: Chaos and Randomness in Neuronic Systems. Opera Medica et Physiologica Vol. 4(S1) (2018)
Altunina, O.V., Shakirov, V.V., Dunin-Barkowski, W.L.: Stochastic and chaotic resonance in cerebellum. NDES 2017, Zernes, Switzerland, Conference Program, p. 28 (2017)
Voicu, H., Mauk, M.D.: Parametric analysis of cerebellar LTD in eyelid conditioning. Neurocomputing 69(10-12), 1187–1190 (2006). https://doi.org/10.1016/j.neucom.2005.12.072
Bloedel, J.R., Ebner, T.J.: Climbing fiber function: Regulation of purkinje cell responsiveness. In: Proceedings in Life Sciences, pp. 247–259. Springer, ??? (1984). https://doi.org/10.1007/978-3-642-69980-1_17. https://doi.org/10.1007/978-3-642-69980-1_17
Ebner, T.J., Bloedel, J.R.: Role of climbing fiber afferent input in determining responsiveness of purkinje cells to mossy fiber inputs. Journal of Neurophysiology 45(5), 962–971 (1981). https://doi.org/10.1152/jn.1981.45.5.962
Ebner, T.J., Bloedel, J.R.: Temporal patterning in simple spike discharge of purkinje cells and its relationship to climbing fiber activity. Journal of Neurophysiology 45(5), 933–947 (1981). https://doi.org/10.1152/jn.1981.45.5.933
Shakirov, V., Dorofeev, V., Dunin-Barkowski, W.L.: Cerebellar plasticity-based equalization of total input to inferior olive cells in time domain: Properties of the model dynamics. Submitted to Neurocomputing (2022)
Shakirov, V., Dorofeev, V., Lebedev, A., Dunin-Barkowski, W.L.: Dynamic chaos in cerebellum and electric synapses between climbing fiber cells of inferior olives. Submitted to Neurocomputing (2022)
Dunin-Barkowski, W.L., Markin, S.N., Podladchikova, L.N.: Estimation of purkinje cells complex and simple spike contribution to local field potentials of the cerebellar cortex. Biofizika 42(2), 520 (1997)
F.Colin, J.Manil, J.C.Desclin: The olivocerebellar system. I. Delayed and slow inhibitory effects: An overlooked salient feature of cerebellar climbing fibers. Brain Research 187, 3–27 (1980)
Khosrovani, S., Van Der Giessen, R.S., De Zeeuw, C.I., De Jeu, M.T.G.: In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc Natl Acad Sci U S A 104 (40), 15911–15916 (2007)
Dunin-Barkovskii, V.L., Antsiferova, L.I., Gusev, A.G.: Cross-correlation analysis of neuron connections in the cerebellum of conscious rabbits. Neurosci Behav Physiol 28(5), 539–546 (1998)
Bouvier, G., Aljadeff, J., Clopath, C., Bimbard, C., Ranft, J., Blot, A., Nadal, J.-P., Brunel, N., Hakim, V., Barbour, B.: Cerebellar learning using perturbations. Neuroscience (2018)
Zang, Y., Schutter, E.D.: Climbing fibers provide graded error signals in cerebellar learning. Frontiers in Systems Neuroscience 13 (2019). https://doi.org/10.3389/fnsys.2019.00046
T.Tokuda, I., E.Han, C., Aihara, K., Kawato, M., Schweighofer, N.: The role of chaotic resonance in cerebellar learning. Neural Networks 23, 836–842(2010)
Hoang, H., Lang, E.J., Hirata, Y., Tokuda, I.T., Aihara, K., Toyama, K., Kawato, M., Schweighofer, N.: Electrical coupling controls dimensionality and chaotic firing of inferior olive neurons. PLOS Computational Biology 16(7), 1008075 (2020). https://doi.org/10.1371/journal.pcbi.1008075
Hoang, H., Tsutsumi, S., Matsuzaki, M., Kano, M., Kawato, M., Kitamura, K., Toyama, K.: Dynamic organization of cerebellar climbing fiber response and synchrony in multiple functional modules reduces dimensions for reinforcement learning. bioRxiv (2022). https://arxiv.org/abs/https://www.biorxiv.org/content/early/2022/12/05/2022.12.05.518634.full.pdfhttps://doi.org/10.1101/2022.12.05.518634
Lisberger, S.G.: The rules of cerebellar learning: Around the ito hypothesis. Neuroscience 462, 175–190 (2021). https://doi.org/10.1016/j.neuroscience.2020.08.026. In Memoriam: Masao Ito — A Visionary Neuroscientist with a Passion for the Cerebellum
Watson, J.D., Crick, F.H.C.: Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 171(4356), 737–738 (1953)
Roschin, V.Y., Frolov, A.A., Burnod, Y., Maier, M.A.: A Neural Network Model for the Acquisition of a Spatial Body Scheme Through Sensorimotor Interaction. Neural Computation 23(7), 1821–1834 (2011). https://arxiv.org/abs/https://direct.mit.edu/neco/article-pdf/23/7/1821/850979/neco_a_00138.pdf. https://doi.org/10.1162/NECO_a_00138
Roweis, S., Brody, C.: Linear heteroencoders. Technical report, Gatsby Computational Neuroscience Unit, Alexandra House (1999)
Goodfellow, I., Bengio, Y., Courville, A.: Deep Learning. MIT Press, ??? (2016). http://www.deeplearningbook.org
LeCun, Y., Bengio, Y., Hinton, G.: Deep learning. Nature 521(7553), 436–444 (2015)
Arshavsky, Y.I., Gelfand, I.M., Orlovsky, G.N.: Cerebellum and Rhythmical Movements. Springer, ??? (1986)
Acknowledgements
The work is financially supported by State Program of SRISA RAS No.FNEF-2022-0003 and by the Southern Federal University Strategic Academic Leadership Program (“Priority 2030”)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest
Additional information
Highlights
\(\bullet \) The cerebellum model can work without external error signal delivery to IO cells
\(\bullet \) Chaos phenomenae in cerebellum do not need climbing fiber cells interactions
\(\bullet \) The proposed model of cerebellum is in good accordance with experimental data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shakirov, V., Altunina, O., Shaposhnikov, D. et al. Cerebellar plasticity based equalization of total input to inferior olive cells. Neurosci Behav Physi 53, 739–751 (2023). https://doi.org/10.1007/s11055-023-01422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01422-8