Abstract
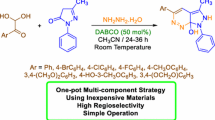
In this research, a mild, efficient, and general method has been developed to synthesize the new derivatives of 2-aryl/alkyl-3H-indol-3-ones in moderate to excellent yields. This method allowed the syntheses of these compounds via the three-component reaction of anhydride compound, sodium cyanide, and aniline derivatives using acetic anhydride as an organic catalyst in one-pot reactions. The advantages of this method include mild reaction conditions, simple procedures, and easy workup.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
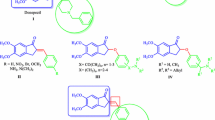
2-Aryl-3H-indol-3-ones were found as one of the most important five-membered ring nitrogen heterocyclic compounds which had a wide range of biological activities such as antimalarial [1], antiplasmodial [2], antibacterial [3, 4], and antifungal [5, 6]. Indol-3-ones were used as a molecular core in the design and synthesis of some new molecules such as imidazoloindolines [7], matemone [8], phalarine [9], difluoroalkylated indolin-3-ones [10], 2,3-dihydro-1H-imidazo[1,5-a]indol-9(9aH)-one derivatives [11], and 3-(2-isocyanoethyl)indoles [12]. Because of these important applications, several methods have been described for the preparation of indol-3-ones derivatives, including oxidation of indoles with TEMPO + BF4 [13], oxidative activation of o-alkynylanilines [14], dearomative oxyarylation, oxyallylation, and oxycyanation of indoles with TEMPO oxoammonium salt [15], dearomatization–alkoxylation of N-Boc indoles with ruthenium-catalyzed oxidative [16], cascade reaction of o-nitroalkynes from indoles with gold(I)-catalyzed [17], 2-alkynyl arylazides with palladium catalyzed [18], Michael addition of 1-acetylindolin-3-ones to β,γ-unsaturated α-ketoesters [19], and cyclizations of ortho-nitrochalcones [20]. Although these methods have been widely used in the literature, they suffer from several disadvantages, such as exclusive substrates and catalysts, relatively low performance, hazardous reagents, limited substrate scope, and inconvenient reaction conditions. Therefore, it is desirable to access indol-3-one compounds via multicomponent reactions without transition metal. In the past few years, more transition-metal-free reactions have been developed for organic transformation [21]. Herein, we described a metal-free intermolecular coupling reaction of cyanide with aniline derivatives and excess amounts of organic acid anhydride for the synthesis of 3H-indol-3-ones derivatives under mild reaction conditions. As a continuation of the research, the development of a new multicomponent methodology was applied for the preparation of some 3H-indol-3-ones (4) from excess anhydride acetic derivatives (1), sodium cyanide (2), and aniline derivatives (3) with high yields, which are shown in Scheme 1. The applicability of the present method to a large-scale process was examined with 60 mmol sodium cyanide, 50 mmol aniline, and 105 mmol of acetic anhydride, which gave 2-methyl-3H-indol-3-one in 85% yield.
Results and discussion
The reaction of anhydride derivatives (1), sodium cyanide (2), and aniline derivatives (3) in CH2Cl2 afforded the corresponding 2-aryl/alkyl-3H-indol-3-ones (4) by a one-pot procedure in high yields (Table 2).
In our initial study, the reaction of acetic anhydride (1), sodium cyanide (2), and aniline (3) was chosen as a model reaction to optimize the reaction condition including the ratio of substrates, solvent, temperature, and time by applying the same portion of substrates (acetic anhydride, sodium cyanide, and aniline) (Table 1). Trace amount of product was obtained at room temperature and under reflux conditions (Table 1, entries 1 and 2). It is noted that the yield of the product was increased from 60 to 80% by increasing the molar ratio of acetic anhydride from 1.5 to 2.1, respectively (Table 1, entries 4–5). No significant change to yield of product was shown in the molar ratio of acetic anhydride to 2.5 and excess (Table 1, entry 6). The results in Table 1 (entries 7–8) show that the use of 1.2 molar ratio cyanide is sufficient to promote the reaction. In addition, chloroform, n-hexane, benzene, carbon tetrachloride, and methanol were also tested as a solvent. In these cases, product was formed in slightly lower yields (Table 1, entries 13–16). As a result of performed experiments, CH2Cl2 (10 mL), and the ratio of acetic anhydride/sodium cyanide/aniline (1:1.2:2.1) at room temperature was chosen as the optimal condition for further studies (Table 1, entry 10).
Under the optimized reaction conditions, various amines reacted with acetic anhydride and cyanide to give 2-aryl/alkyl-3H-indol-3-ones in high yields within 2 h, and the results are shown in Table 2. The use of various aryl amines reveals that aryl amines containing electron-donating groups exhibited a slightly better effect than those with electron-withdrawing substituents. Investigations on some anhydrides with aliphatic and aromatic groups were done by the reaction of amines with aliphatic and aromatic anhydrides. The results showed that even in the presence of aromatic anhydrides, the formation of 3H-indol-3-ones is favorable under the reaction conditions (Table 2, entries 3 m, 3p).

The proposed mechanism of the reaction is presented in Scheme 2. Firstly, the cyanide ion reacted with the carbonyl group of acetic anhydride. Acetic acid acetoxy-cyano-methyl-methyl ester intermediate was formed and reacted with aniline. After the cyclization reaction of aniline with alkyl nitrile in the presence of excess acetic anhydride, aromatization was performed and finally with the addition of water the desirable product formed.
Conclusion
In conclusion, a new and efficient method with an easy procedure was described for the synthesis of 3H-indol-3-ones for some novel products provided with moderate to good yields. We have developed a one-pot synthesis of 2-aryl/alkyl-3H-indol-3-ones via a three-component reaction between an anhydride compound, sodium cyanide, and aniline derivatives. The structure of all products was confirmed by FT-IR spectroscopy, mass spectrometer, 1H NMR, and 13C NMR spectra.
Experimental
Materials
All chemicals and reagents were obtained from Sigma–Aldrich and used without further purification.
Instruments
The products were characterized by various analyses such as nuclear magnetic resonance (NMR) spectroscopy, mass spectrometer using electrospray ionization (ESI) in positive-ion and Fourier transform infrared (FT-IR) spectroscopy. 1H NMR and 13C NMR spectra were obtained on a Bruker Avance 400 MHz and 100 MHz, respectively, and chemical shifts are expressed in ppm using TMS as an internal standard. FT-IR spectra were obtained on a Bruker, Equinox 55 spectrometer, and mass spectra were obtained using a commercial apparatus (Agilent Technologies, CA, USA).
Procedure for the synthesis of 2-methyl-3H-indol-3-one (Table 1, entry 11)
Briefly, 0.62 g of sodium cyanide 95.0% (12 mmol), 0.9 ml of aniline (10 mmol), and 10 mL of anhydrous CH2Cl2 were added to a round-bottomed flask (50 ml). The reaction mixture was cooled to 0 °C in an ice bath and placed under a nitrogen atmosphere. Then, 2.0 ml acetic anhydride (21 mmol) was added dropwise to the reaction mixture at 0 °C, allowed to warm up to room temperature, and stirred for another 2 h. The solvent was removed, and then, 10 mL water was added, stirred, and refluxed for 1 h. The reaction was monitored with TLC. After completing the reaction, the reaction mixture was cooled to room temperature, 20 mL of cooled water was added and the product was extracted with CH2Cl2 (3 × 10 mL). The eluting organic solvent was evaporated with a vacuum evaporator. The product was dried and purified by recrystallization from ethanol.
2-Methyl-3H-indol-3-one (3a)
FT-IR ν(cm−1): 1757, 1724, 1539, 835, 760; 1H NMR (400 MHz, CDCl3): δ 2.34 (s, 3H), 3.11 (s, 3H), 7.24 (dd, J = 7.88, 1 Hz, 1H), 7.37 (d, J = 7.88 Hz, 1H), 7.69 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 13.77, 21.10, 98.05, 116.40, 124.67, 133.39, 134.51, 138.66, 149.93, 195.92; HRMS m/z (ESI): calculated for C10H9NO [M + H]+ 159.0679, found 159.0677.
2,5-Dimethyl -3H-indol-3-one (3b)
FT-IR ν(cm−1): 1757, 1724, 1539, 835, 760; 1H NMR (400 MHz, CDCl3): δ 2.34 (s, 3H), 3.11 (s, 3H), 7.24 (dd, J = 7.88, 1 Hz, 1H), 7.37 (d, J = 7.88 Hz, 1H), 7.69 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 13.77, 21.10, 98.05, 116.40, 124.67, 133.39, 134.51, 138.66, 149.93, 195.92; HRMS m/z (ESI): calculated for C10H9NO [M + H]+ 159.0679, found 159.0677.
5-Methoxy-2-methyl-3H-indol-3-one (3c)
FT-IR ν(cm−1): 1758, 1723, 1539, 835, 763; 1H NMR (400 MHz, CDCl3): δ 3.03 (s, 3H), 3.79 (s, 3H), 6.98 (dd, J = 8.76, 2.76 Hz, 1H), 7.36–7.42 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.59, 58.47, 105.49, 116.45, 123.97, 128.20, 157.87, 158.52, 162.29, 191.38; HRMS m/z (ESI): calculated for C10H9NO2 [M + H]+ 175.0628, found 175.0627.
5-Fluoro-2-methyl-3H-indol-3-one (3d)
FT-IR ν(cm−1): 1759, 1724, 1537, 837, 764; 1H NMR (400 MHz, CDCl3): δ 3.02 (s, 3H), 7.15–7.22 (m, 1H), 7.50 (d, J = 8.76 Hz, 1H), 7.63 (dd, J = 8.28, 2.64 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.74, 99.17, 112.01, 112.28, 115.68, 115.72, 120.18, 120.40, 136.28, 136.36, 159.45, 161.97, 190.08; HRMS m/z (ESI): calculated for C9H6FNO [M + H]+ 163.0428, found 163.0431.
2-Ethyl-3H-indol-3-one (3e)
FT-IR ν(cm−1): 1759, 1726, 1541, 837, 762; 1H NMR (400 MHz, CDCl3): δ 1.24 (t, J = 7.36 Hz,3H), 3.14 (q, J = 7.4 Hz,2H), 7.16–7.22 (m, 1H), 7.27–7.36 (m, 1H), 7.65 (d, J = 9.12,1H), 7.97(d, J = 8.76,1H); 13C NMR (100 MHz, CDCl3): δ 14.81, 25.37, 115.93, 119.09, 121.25, 128.44, 131.29, 157.54, 159.67, 190.79; HRMS m/z (ESI): calculated for C10H9NO [M + H]+ 159.0679, found 159.0678.
2-Propyl-3H-indol-3-one (3f)
FT-IR ν(cm−1): 1757, 1721, 1537, 835, 760; 1H NMR (400 MHz, CDCl3): δ 0.97 (t, J = 7.52 Hz, 3H), 2.28–2.39 (m, 2H), 3.06 (t, J = 7.36 Hz, 2H), 7.15–7.21 (m, 1H), 7.29–7.36 (m, 1H), 7.65(d, J = 9 Hz, 1H), 7.99(d, J = 8.88 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 9.70, 14.91, 22.24, 113.79, 121.38, 123.20, 130.99, 134.36, 140.09, 147.47, 186.73; HRMS m/z (ESI): calculated for C10H9NO2 [M + H]+ 173.0835, found 173.0831.
5-Methyl-2-propyl-3H-indol-3-one (3 g)
FT-IR ν(cm−1): 1764, 1724, 1538, 770, 692; 1H NMR (400 MHz, CDCl3): δ 0.97 (t, J = 7.4 Hz,3H),1.72–1.82 (m, 2H), 2.36 (s, 3H), 3.06 (t, J = 7.36 Hz, 2H), 7.02 (d, J = 8.88 Hz,1H), 7.36 (s, 1H), 7.84 (d, J = 8.88 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 13.78, 17.12, 22.43, 25.37, 113.33, 118.01, 120.59, 131.96, 141.86, 158.21, 159.32, 190.42; HRMS m/z (ESI): calculated for. [M + H]+ 187.0992, found 187.0990.
5-Methoxy-2-propyl-3H-indol-3-one (3 h)
FT-IR ν(cm−1): 1762, 1721,1546, 1249, 1043, 780, 761, 757; 1H NMR (400 MHz, CDCl3): δ 0.97 (t, J = 7.25 Hz, 3H), 1.71–1.81 (m, 2H), 3.03 (t, J = 7.24 Hz, 2H), 3.82 (s, 3H), 6.71 (sd, J = 1.36 Hz 1H), 6.85 (dd, J = 9.4, 2 Hz, 1), 7.82 (dd, J = 9.24, 0.88 Hz, 1); 13C NMR (100 MHz, CDCl3): δ 13.75, 17.09, 22.65, 55.56, 90.16, 116.13, 121.93, 125.59, 158,92, 158.96, 161.77, 190.49; HRMS m/z (ESI): calculated for C12H13NO2. [M + H]+ 203.0941, found 203.0940.
5-Fluoro-2-propyl-3H-indol-3-one (3i)
FT-IR ν(cm−1): 1755, 1724, 1537, 1265, 1221, 834, 762; 1H NMR (400 MHz, CDCl3): δ 0.98 (t, J = 7.36 Hz, 3H), 1.70–1.85 (m, 2H), 3.06 (t, J = 7.12 Hz, 2H), 6.99 (td, J = 9.36, 2 Hz, 1H), 7.17–7.21(m, 1H), 7.97–8.02(m,1H); 13C NMR (100 MHz, CDCl3): δ 13.71, 17.03, 25.36, 98.19, 98.44, 116.76, 121.40, 121.70, 123.88, 123.98, 157.76, 157.89, 160.03, 160.05, 162.52, 190.26; HRMS m/z (ESI): calculated for C11H10FNO. [M + H]+ 191.074194, found 191.0739.
2-propyl-3H-indol-3-one-5-Carboxylic acid methyl ester (3j)
FT-IR ν(cm−1): 1760, 1751, 1726, 1537, 1283, 1115, 833, 755; 1H NMR (400 MHz, CDCl3): δ 0.99 (t, J = 7.52 Hz, 3H), 1.74–1.85 (m, 2H), 3.10 (t, J = 7.24 Hz, 2H), 3.92 (s, 3H), 7.76 (d, J = 9.12 Hz,1H), 8.04 (d, J = 9.12,1H), 8.44 (s,1H); 13C NMR (100 MHz, CDCl3): δ 13.75, 17.05, 22.29, 52.83, 119.66, 119.92, 121.70, 127.56, 133.32, 157.31, 160.43, 165.66, 190.16; HRMS m/z (ESI): calculated for. [M + H]+ 231.0890, found 231.0889.
2-Propyl-3H-indole-3-one-5-carbonitrile (3 k)
FT-IR ν(cm−1): 2237, 1755, 1722, 1537, 1437, 967, 835, 760; 1H NMR (400 MHz, CDCl3): δ 0.99 (t, J = 7.36 Hz, 3H), 1.74–1.85 (m, 2H), 3.11 (t, J = 7.24 Hz, 2H), 7.27 (d, J = 9.12 Hz, 1H), 8.11–8.17 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 13.71, 16.97, 22.64, 115.67, 117.43, 118.85, 123.68, 123.71, 127.72, 155.88, 161.18, 189.98; HRMS m/z (ESI): calculated for C12H10N2O; [M + H]+ 198.0788, found 198.0790.
2-p-Tolyl-3H-indol-3-one (3 l)
FT-IR ν(cm−1): 1759, 1722, 1535, 1273, 874, 755; 1H NMR (400 MHz, CDCl3): δ 2.38 (s, 3H), 7.19 (d, J = 8.08 Hz, 2H), 7.22–7.32 (m, 2H), 7.39 (d, J = 8.04, 2H), 7.47 (t, J = 7.48 Hz,1H), 7.56 (d, J = 6.96 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.16, 123.66, 124.55, 125.40, 126.65, 127.51, 129.23, 130.7, 138.10, 140.85, 154.20, 167.88, 193.28; HRMS m/z (ESI): calculated for C15H11NO. [M + H]+ 221.0835, found 221.0838.
5-Methyl-2-p-tolyl-3H-indol-3-one (3 m)
FT-IR ν(cm−1): 1758, 1723, 1536, 1272, 873, 756; 1H NMR (400 MHz, CDCl3): δ 2.38 (s, 6H), 7.14 (d, J = 8.2 Hz,1H), 7.19 (d, J = 8.08 Hz, 2H), 7.25–7.30 (m, 1H), 7.39 (d, J = 8.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 21.00, 21.60, 123.53, 125.67, 126.32, 128.36, 131.10, 133.96, 135.38, 136.04, 136.90, 148.17, 156.62, 193.27; HRMS m/z (ESI): calculated for C16H13NO. [M + H]+ 235.0992, found 235.0995.
5-Bromo-2-p-tolyl-3H-indol-3-one (3n)
FT-IR ν(cm−1): 1760, 1722, 1536, 1514, 1383, 1250, 831, 757, 650; 1H NMR (400 MHz, CDCl3): δ 2.39 (s, 3H), 7.12 (d, J = 8.56 Hz,2H), 7.20 (d, J = 8.04 Hz, 2H), 7.38 (d, J = 8.04 Hz,2H), 7.57 (d, J = 8.32 Hz, 1H), 7.68 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.20, 118.49, 123.51, 126.11, 126.62, 129.30, 130.34, 131.65, 138.28, 139.89, 153.84, 166.52, 193.38; HRMS m/z (ESI): calculated for C15H10BrNO. [M + H]+ 297.9862, found 297.9870.
2-(4-Methoxy-phenyl)-3H-indol-3-one (3o)
FT-IR ν(cm−1): 1763, 1725, 1539, 1257, 1045, 833, 758; 1H NMR (400 MHz, CDCl3): δ 3.84(s,3H), 6.91(d, J = 8.92 Hz, 2H), 7.23–7.29 (m, 2H), 7.38–7.61 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 55.36, 113.92, 122.89, 124.55, 125.39, 127.42, 128.01, 131.18, 133.43, 154.20, 159.69, 167.94, 193.99; HRMS m/z (ESI): calculated for C15H11NO2. [M + H]+ 237.0784, found 237.0781.
2-(4-Methoxy-phenyl)-5-methyl-3H-indol-3-one (3p)
FT-IR ν(cm−1): 1761, 1723, 1537, 1255, 1043, 830, 759; 1H NMR (400 MHz, CDCl3): δ 2.38 (s, 3H), 3.84 (s, 3H), 6.91 (d, J = 8.29 Hz, 2H), 7.13 (d, J = 8.16, 2H), 7.24–7.29 (m, 1H), 7.37 (s, 1H) 7.42 (d, J = 8.68, 2H); 13C NMR (100 MHz, CDCl3): δ 20.77, 55.35, 113.91, 123.03, 124.51, 127.88, 128.02, 129.81, 131.80, 133.50, 138.30, 159.69, 167.98, 193.27; HRMS m/z (ESI): calculated for C16H13NO2. [M + H]+ 251.0941, found 251.0940.
5-Bromo-2-(4-methoxy-phenyl)-3H-indol-3-one (3q)
FT-IR ν(cm−1): 1762, 1724, 1538, 1515, 1384,1253, 1043, 831, 756, 652; 1H NMR (400 MHz, CDCl3): δ 3.84 (S, 3H), 6.91 (d, J = 8.8 Hz, 2H) 7.12 (d, J = 8.56 Hz, 1H), 7.42 (d, J = 8.68 Hz, 2H), 7.57 (d, J = 7.84 Hz, 1H), 7.67 (s, 1H); 13C NMR (100 MHz, CDCl3): δ, 55.35, 114.00, 122.77, 126.13, 127.96, 130.32, 131.70, 133.20, 134.06, 153.85, 159.82, 166.48, 193.33; HRMS m/z (ESI): calculated for C15H10BrNO2. [M + H]+ 314.9895, found 314.9891.
Acetic acid 3-(3H-indol-3-one-2-yl)-propyl ester (3r)
FT-IR ν(cm−1):1758, 1752, 1720, 1539,1251, 833, 761; 1H NMR (400 MHz, CDCl3): δ 1.96 (s, 3H), 2.08–2.15 (m, 2H), 3.22 (t, J = 7.24 Hz, 2H), 4.14 (t, J = 6.64 Hz, 2H), 7.18–7.24 (m, 1H), 7.31–7.37 (m,1H), 7.66 (d, J = 9.12 Hz, 1H), 7.97 (d, J = 8.76 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 20.90, 22.52, 23.25, 63.40, 116.01, 119.21, 121.13, 128.71, 131.39, 157.59, 159.50, 171.03, 189.10; HRMS m/z (ESI): calculated for C13H13NO3 [M + H]+ 231.0890, found 231.0888.
References
Rakotoarivelo NV, Perio P, Najahi E, Nepveu F (2014) Interaction between antimalarial 2-Aryl-3H-indol-3-one derivatives and human serum albumin. J Phys Chem B 118:13477–13485
Najahi E, Valentin A, Fabre P-L, Reybier K, Nepveu F (2014) 2-Aryl-3H-indol-3-ones: synthesis, electrochemical behaviour and antiplasmodial activities. Eur J Med Chem 78:269–274
Sridhar SK, Saravanan M, Ramesh A (2001) Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Med Chem 36:615–625
Yousif EI, Ahmed RM, Hasan HA, Al-Fahdawi AS, Al-Jeboori MJ (2017) Metal complexes of heterocyclic hydrazone Schiff-bases: preparation, spectral characterisation and biological study. Iran J Sci Technol Trans A Sci 41:103–109
Wollein U, Bracher F (2011) The gramine route to pyrido[4,3-b]indol-3-ones - identification of a new cytotoxic lead. Sci Pharm 79:59–68
Kutschy P, Suchý M, Dzurilla M, Takasugi M, Kováčik V (2000) A new approach to imidazo[1,5-a]indole derivatives. Collect Czechoslov Chem Commun 65:1163–1172
Li P, Zhu B, Xu Y, Zhou Z, Hu G, Yang F, Xu S, Zhang X (2020) Palladium-catalyzed one-pot cycloaddition reactions of thioureas with 3H-indol-3-ones generated in situ from 2-alkynyl arylazides: rapid and efficient access to imidazoloindolines. Org Chem Front 7:3480–3485
Wen S-S, Zhou Z-F, Xiao J-A, Li J, Xiang H, Yang H (2017) Facile oxidative cyclization to access C2-quaternary 2-hydroxy-indolin-3-ones: synthetic studies towards matemone. New J Chem 41:11503–11506
Xia Z, Hu J, Gao Y-Q, Yao Q, Xie W (2017) Facile access to 2,2-disubstituted indolin-3-ones via a cascade Fischer indolization/Claisen rearrangement reaction. Chem Commun 53:7485–7488
Li J-S, Liu Y-J, Zhang G-W, Ma J-A (2017) Catalytic asymmetric Mukaiyama-Mannich reaction of cyclic C-acylimines with difluoroenoxysilanes: access to difluoroalkylated Indolin-3-ones. Org lett 19:6364–6367
Yuan X, Wu X, Zhang P, Peng F, Liu C, Yang H, Zhu C, Fu H (2019) Axially chiral cyclic phosphoric acid enabled enantioselective sequential additions. Org Lett 21:2498–2503
Liu Y-L, Mao X-Y, Lin X-T, Chen G-S (2018) A Zn(OTf)2 catalyzed Ugi-type reaction of 3-(2-isocyanoethyl)indoles with indole-derived ketimines: rapid access to hexacyclic spiroindolines. Org Chem Front 5:2303–2307
Yan X, Tang Y-D, Jiang C-S, Liu X, Zhang H (2020) Oxidative dearomative cross-dehydrogenative coupling of indoles with diverse CH nucleophiles: efficient approach to 2, 2-disubstituted indolin-3-ones. Molecules 25:419
Dhandabani GK, Mutra MR, Wang J-J (2019) FeCl3-Promoted ring size-dictating diversity-oriented synthesis (DOS) of N-heterocycles using in situ-generated cyclic imines and enamines. Chem Commun 55:7542–7545
Liu X, Yan X, Tang Y, Jiang C-S, Yu J-H, Wang K, Zhang H (2019) Direct oxidative dearomatization of indoles: access to structurally diverse 2,2-disubstituted indolin-3-ones. Chem Commun 55:6535–6538
Zhou X-Y, Chen X, Lei Y-Z (2021) Ru-catalyzed oxidative dearomatization-alkoxylation of N-Boc indoles. Synth Commun 51:943–951
Zhou S, Liu Q, Bao M, Huang J, Wang J, Hu W, Xu X (2021) Gold(i)-catalyzed redox transformation of o-nitroalkynes with indoles for the synthesis of 2,3′-biindole derivatives. Org Chem Front 8:1808–1816
Li P, Yang F, Hu G, Zhang X (2021) Palladium-catalyzed one-pot synthesis of pyrroloindolines from 2-Alkynyl arylazides and thioacetamides. J Org Chem. https://doi.org/10.1021/acs.joc.1c01058
Chen S, Wang Y, Zhou Z (2016) Organocatalyzed asymmetric michael addition of 1-acetylindolin-3-ones to β, γ-unsaturated α-ketoesters: an access to chiral indolin-3-ones with two adjacent tertiary stereogenic centers. J Org Chem 81:11432–11438
Aksenov NA, Aksenov DA, Arutiunov NA, Aksenova DS, Aksenov AV, Rubin M (2020) Unexpected cyclization of ortho-nitrochalcones into 2-alkylideneindolin-3-ones. RSC Adv 10:18440–18450
Mi X, Wang C, Huang M, Wu Y, Wu Y (2015) Preparation of 3-Acyl-4-arylcoumarins via metal-free tandem oxidative acylation/cyclization between alkynoates with aldehydes. J Org Chem 80:148–155
Acknowledgements
We are grateful for the financial support provided by the University of Jiroft for this research, which leads to our exceptional success.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akbari, A., Seyedi, N. & Faryabi, M.S. Design of a new method for the synthesis of novel 2-aryl/alkyl-3H-indol-3-ones. Mol Divers 28, 3–9 (2024). https://doi.org/10.1007/s11030-022-10464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10464-y