Abstract
An efficient and improved process for the synthesis of novel 1-indanones derivatives has been developed. In the first step of the presented new process, 4-bromobenzaldehyde reacts with 5, 6-dimethoxy-2, 3-dihydro-1H-inden-1-one by N-arylation of corresponding compounds with a variety of aliphatic and aromatic amines to produce a novel category of 1-indanones as the final step. Comparison of this newly designed method with conventional methods show that our method is much more efficient in producing a higher total yield. This innovative synthesis methodology offers several advantages, among which the consumption of inexpensive reagents and the resulting higher total yields of final products are of its remarkable points.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1-Indanones are important chemical, pharmaceutical, and agrochemical intermediates [1]. On the other hand, various 1-indanone structures are also commonly found in numerous bioactive natural products [2, 3]. Many indanone structures have demonstrated various biological activities, including antiproliferative activity, as well as acetylcholinesterase inhibition as therapeutics [4], such as donepezil (Aricept), for the treatment of Alzheimer’s disease, etc. [5]. Alzheimer's disease (AD) is the most common form of dementia, accounting for about 50–60 % of the overall cases of dementia among persons over 65 years of age [6, 7].
However, while many other compounds have anti-Alzheimer's effects with different structures from that of donepezil [8–23], donepezil remains the most important drug for the treatment of AD [24, 25]. Its unique chemical structure makes it more specific in targeting Alzheimer's disease much more efficiently compared to similar drug classes. There are many processes for producing donepezil [26–29], but the prior procedures for its preparation of have certain disadvantages, such as multiple reduction steps, expensive catalysts, side-product formation, and low yield results [28, 29]. Due to the mentioned disadvantages, development of novel, simple, and efficient routes for the synthesis of donepezil and donepezil-like compounds continues to attract a great deal of interest. The crystal structure of donepezil in its crystal state and in solution clearly shows three main parts: a 1-indanone moiety, a linker moiety, and a benzyl piperidine moiety (Fig. 1) [27]. As shown in Fig. 1, the principle difference between the presented donepezil-like compounds and donepezil is in both the linker and basic components. Otherwise, all of them are similar in the indanone part and in the position of the basic part at C-2. On the other hand, more of the synthesized donepezil-like compounds are of the kind bearing amine moieties in their structure, such that the synthesis of these compounds possessing aliphatic and aromatic amines are scarcely reported in the literature. Moreover, it is well-known that the combination of the indanone moiety with an aromatic ring via an aldol condensation enhances the activity [30, 31]. It is notable that recently some new donepezil like compounds have been reported that are different in the indanone moiety [32–35].
A literature survey shows that two methods for the synthesis of donepezil-like compounds have been developed. The most popular method is the use of commercially available aldehydes with various functional groups in the para position [36]. Another approach is the consumption of 4-fluorobenzaldehyde and a subsequent arylation reaction in the specific conditions [37]. Due to weak points of each method, the development of novel, simple, and efficient routes for the synthesis of donepezil-like compounds have attracted growing interest.
These observations prompted us to design a novel method for the synthesis of an indanone-based library of new small molecules represented by V (Fig. 1), in which the 5,6-dimethoxy-2,3-dihydro-1H-inden-1-one ring was retained and new side-chains with various amine groups linked to the para position of benzylidene group were added at C-2. Because of structural similarity of V with IV [38], III [39], II [36] and I, we anticipated that molecules based on V might show biological activities.
Results and discussion
As the starting point, we designed and synthesized compound (8) by two methods (Scheme 1) to investigate the feasibility of two strategies, and furthermore, to optimize the reaction conditions. Comparing the final results introduced the most efficient method.
For this purpose, in method A 4-halobenzaldehyde 3 reacted with 5, 6-dimethoxy-2, 3-dihydro-1H-inden-1-one 4 and compound 5 was produced. To optimize the reaction conditions, a variety of solvents (EtOH, MeOH) and bases (KOH, NaOH) were tested. The results are summarized in Table 1.
To avoid superfluous byproducts produced during the reaction procedure in high temperatures and reflux, the reaction must be performed at room temperature. As Table 1 shows, the best result (86 % yield) was obtained when the reaction was performed using EtOH as a solvent and 10 mol % of NaOH as a base (entry 4). With the precursor 5 in hand, we focused on the feasibility of the following CuI catalyzed intermolecular N-arylation reaction with imidazole 6a. As shown in Table 2, to identify the best operative system for N-arylation of imidazole 6a with 5, the coupling reaction was surveyed by a variety of solvents, bases, ligands, and halobenzaldehyde in different temperatures. Among various solvents and bases screened at 80 °C and in the presence of 2-picolonic acid as ligands, the optimal result was obtained when DMSO was used as a solvent and Cs2CO3 was employed as the base (Table 2, entry 5).
It was also observed that l-proline was better than other ligands (Fig. 2) in producing considerable reaction yields (Table 2, entries 5 and 8–11). As it is presented in the following, the reaction was studied at different temperatures (Table 2, entries 8 and 12–14), which in conclusion led to the selection of 100 °C as the optimal temperature (Table 2, entry 13). Thus, a catalyst system consisting of 10 mol % CuI, 20 mol % l-proline, and 2.0 equiv Cs2CO3 in DMSO at 100 °C was employed for the reaction under study, which produces 86 % of the desired product 8a as a successful result. In continuance, we chose a variety of substituted forms of compound 5 to compare. The results show that among different types of halobenzaldehyde used (Table 2, entries 13, 15, 16), the yield of bromo derivative 5c is much greater than that of the corresponding flouro and chloro analogues.
It is notable that use of 4-bromobenzaldehyde 5c as starting material is economic, due to its reasonable price and availability in comparison to other halobenzaldehydes.
In continuance, we investigated method B, which is the most popular method for the preparation of indanone derivatives in the literature [38] (Scheme 1, path B). As the starting point for this method, the reaction of 4-bromobenzaldehyde 3c with imidazole 6a was chosen as our model reaction to investigate. For this purpose, we used an optimized catalytic system which was obtained from the arylation step of path A.
Initially we synthesized 4-imidazolobenzaldehyde 7a via the Ullman reaction of 4-bromobenzaldehyde 3c with imidazole 6a (Scheme 2) using dimethylsulfoxid (DMSO) as the solvent and CuI/l-proline as catalyst. The reaction was performed at 100 °C for 24 h. 7a reacted with 5, 6-dimethoxy-2, 3-diydro-1H-inden-1-one 5, using ethanol as a solvent and NaOH 10 % as a base at room temperature for 24 h to afford the 2-(4-imidazolobenzilidine)-5,6-dimethoxy-2, 3-diydro-1H-inden-1-one 8a in good yields (Scheme 3).
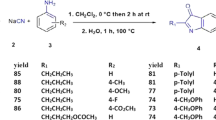
Comparison of the total yields of methods A and B shows that the results of method A is more satisfactory than that of method B. To explore the scope and generality of method A, we extended this method to the synthesis of the corresponding indanone derivatives 8a–g via the N-arylation of compound 5c with a variety of aliphatic and aromatic amines 6a–g (Fig. 3) under the optimal condition.
As shown in Scheme 4, the reactions were carried out efficiently and the desired products were produced in good yields (79–86 %).
Conclusion
In summary, we have demonstrated a simple, efficient, and novel route for the synthesis of a drug-like indanone scaffold (8a–g). Two different methods were examined and a novel series of 2-(4-dialkylaminobenzilidine)-5, 6-dimethoxy-2, 3-dihydro-1H-inden-1-one was synthesized in two steps. In the first step, condensation of aldehyde with 6-dimethoxy-2, 3-dihydro-1H-inden-1-one produced intermediate compound 5. In the second step, N-arylation of compound 5 with a variety of aliphatic and aromatic amines synthesized the corresponding final products. Ready availability of 4-bromobenzaldehyde as a starting material, the use of cheaper reagents, and higher total yields of the final products are some advantages of this method over previously reported processes. This provides a convenient synthetic route to a variety of substituted indene derivatives with good yields. Studies of possible practical applications of these new drug-based materials are also being actively pursued. The results of these and related studies will be reported in due course.
Experimental
General methods
Melting points were determined on a MEL-TEMP model 1202D and are uncorrected. FT-IR spectra were recorded on a Bruker Tensor 27 spectrometer as KBr disks. The 1H NMR spectra were recorded on a Bruker Spectrospin Avance 400 spectrometer using CDCl3 as solvent. 13C NMR spectra were determined on the same instrument at 100 MHz. All chemical shifts were reported as δ (ppm) and coupling constants (J) were given in Hz. Elementary analyses (C, H, N) were performed on a Vario EL III analyzer. Column chromatography was done using silica gel (MerkKieselgel 60 HF254, Art. 7739). The chemical reagents used in synthesis were purchased from Merck and Sigma-Aldrich.
General procedure for the synthesis of compound 5c
To a stirred solution of 4-bromobenzaldehyde (1 mmol) and indanone (1 mmol) in EtOH (10 cc) the aqueous solution of NaOH (10 %) was added dropwise. The reaction mixture was stirred overnight at room temperature. The obtained solid was collected by filtration and purified by recrystallization from EtOH to give 5c as a pure solid.
2-(4-bromobenzilidine)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one (5c)
Pale yellow solid; Yield 86 %; mp: 178–180 °C; FT-IR (KBr) ν 2961, 1687, 1630, 1497, 1304, 1252, 1087 cm−1; 1HNMR (400 MHz, CDCl3): δ 3.90 (2H, s, -inden-CH2), 3.94 (3H, s, O–CH3), 3.99 (3H, s, O–CH3), 6.96 (1H, s, Ar–H), 7.31 (1H,s, Ar–H), 7.47–7.50 (2H, d, J = 8.4 Hz, Ar–H), 7.50 (1H, s, methine-H), 7.55–7.57 (2H, d, J = 8.4, Ar–H).
General procedure for the synthesis of compounds 8a–g by method A
To a solution of compound 5c (2 mmol) in DMSO (3 mL), Cs2CO3 (2 eq), CuI (10 mol %), and l-proline (20 mol %) and compounds 6a–g were added, and the reaction mixture was heated at 100 °C for 24 h (monitored by TLC). After cooling, the reaction mixture was poured into water and extracted with EtOAc. The organic layer was washed with brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by silica gel preparative layer chromatography (EtOAc/n-hexane, 1:9) to give the desired products 8a–g.
2-(4-imidazolobenzilidine)-5, 6-dimethoxy-2,3-dihydro-1H-inden-1-one 8a
Yellow solid; Yield 86 %; mp: 223–225 °C; FT-IR (KBr) ν 2935, 2838, 1688, 1635, 1497, 1303, 1250, 1093 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.92 (2H, s, inden-CH2), 3.93 (3H, s, O–CH3), 3.98 (3H, s, O–CH3), 6.96 (1H, s, Ar–H), 7.22 (1H, s, Ar–H), 7.31 (1H, s, Ar–H), 7.32 (1H, s, Ar–H), 7.44–7.46 (2H, d, J = 8.2 Hz, Ar–H), 7.55 (1H, s, Ar–H), 7.71–7.73 (2H, d, J = 8.2 Hz, Ar–H), 7.91 (1H, s, methine-H) ppm; 13CNMR (100 MHz, CDCl3): δ 31.0, 55.1, 55.2, 104.0, 106.1, 116.7, 120.3, 126.0, 129.5, 129.8, 129.9, 130.8, 133.7, 134.3, 135.1, 136.4, 143.6, 148.7, 154.5, 191.7 ppm. Anal. Calcd. For C21H18N2O3: C, 72.89; H, 5.25; N, 8.09; Found: C, 70.51; H, 5.08; N, 7.83 %.
2-(4-pyrrolidinobenzilidine)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one 8b
Auburn solid; Yield 84 %; mp: 170–172 °C; FT-IR (KBr) ν 2960, 1672, 1516, 1385, 1300, 1178, 1303, cm−1; 1H NMR (400 MHz, CDCl3): δ 1.97–2.02 (4H,m, pyrrolidino-CH2-3,4), 3.33–3.37 (4H,m,pyrrolidino-CH2-2,5),3.90 (2H, s, inden-CH2), 3.94 (3H, s, O–CH3), 3.99 (3H, s, O–CH3), 6.55–6.57 (2H, d, J = 8.2 Hz, Ar–H), 6.94 (1H, s, Ar–H), 7.31 (1H,s, Ar–H), 7.52–7.54 (2H, d, J = 8.2 Hz, Ar–H), 7.54 (1H, s, methine-H) ppm; 13CNMR (100 MHz, CDCl3): δ 24.4, 31.4, 46.5, 55.1, 55.2, 103.9, 106.1, 110.8, 121.7, 129.0, 130.7, 131.6, 132.7, 143.2, 147.5, 155.5, 191.9 ppm. Anal. Calcd. For C22H23NO3: C, 75.70; H, 6.65; N, 4.01; Found: C, 73.42; H, 6.41; N, 3.86 %.
2-(4-morpholinobenzilidine)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one 8c
Yellow solid; Yield 84 %; mp: 210–212 °C; FT-IR (KBr) ν 2967, 2833, 1677, 1594, 1504, 1223, 1301, cm−1; 1H NMR (400 MHz, CDCl3): δ 3.21–3.23 (4H,t, J = 8 Hz, Morpholino-CH2-2,-6), 3.81–3.83 (4H,t, J = 8 Hz, Morpholino-CH2-3,-5,), 3.83 (2H, s, inden-CH2), 3.89 (3H, s, O–CH3), 3.94 (3H, s, O–CH3), 6.86–6.88 (2H, d, J = 8.7 Hz, Ar–H), 6.92 (1H, s, Ar–H), 7.27 (1H, s, Ar–H), 7.47 (1H, s, methine-H), 7.51–7.53 (2H, d, J = 8.7 Hz, Ar–H), ppm; 13CNMR (100 MHz, CDCl3): δ 31.0, 46.7, 54.8, 55.0, 65.4, 103.6, 105.9, 113.4, 125.2, 130.1, 130.9, 131.0, 131.2, 143.3, 148.2, 150.3, 153.7, 191.9 ppm. Anal. Calcd. For C22H23NO4: C, 72.39; H, 6.36; N, 3.83; Found: C, 70.18; H, 6.14; N, 3.83 %.
2-(4-piperidinobenzilidine)-5, 6-dimethoxy-2, 3-dihydro-1H-inden-1-one 8d
Brown solid; Yield 85 %; mp: 204–206 °C; FT-IR (KBr) ν 2927, 2849, 1680, 1587, 1507, 1232, 1301 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.62–1.68 (6H,m, piperidino-CH2-3, 4, 5), 3.29–3.31 (4H,t, J = 10.3 Hz, piperidino-CH-2, 6), 3.89 (2H, s, inden-CH2), 3.94 (3H, s, O–CH3), 3.97 (3H, s, O–CH3), 6.90–6.92 (2H, d, J = 8.8 Hz, Ar–H), 6.96 (1H, s, Ar–H), 7.32 (1H, s, Ar–H), 7.53–7.55 (2H, d, J = 9.0 Hz, Ar–H), 7.55 (1H, s, methine-H) ppm; 13CNMR (100 MHz, CDCl3): δ 23.2, 24.4, 31.2, 48.0, 55.1, 55.1, 103.9, 105.7, 106.1, 113.0, 113.8, 124.0, 130.3, 130.5, 131.3, 132.0, 132.2, 143.4, 148.4, 151.0, 153.8, 192.3 ppm. Anal. Calcd. For C23H25NO3: C, 76.10; H, 6.95; N, 3.85; Found: C, 73.09; H, 6.73; N, 3.73 %.
2-[4-(N-methylpiperazinobenzilidine)]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one. 8e
Brown solid; Yield 79 %; mp: 220–222 °C; FT-IR (KBr) ν 2925, 2862, 1684, 1610, 1510, 1312, 1127, 1093 cm−1; 1H NMR (400 MHz, CDCl3): δ 2.39 (3H, s, CH3), 2.62–2.64 (4H,t, J = 8 Hz, piperazino-CH), 3.35–3.37 (4H, t, J = 8 Hz, piperazino-CH),3.87 (2H, s, inden-CH2), 3.94 (3H, s, O–CH3), 3.98 (3H, s, O–CH3), 6.86–6.88 (2H, d, J = 8.7 Hz, Ar–H), 6.92 (1H, s, Ar–H), 7.27 (1H, s, Ar–H), 7.47 (1H, s, methine-H), 7.51–7.53 (2H, d, J = 8.7 Hz, Ar–H), ppm; 13CNMR (100 MHz, CDCl3): δ 32.0, 46.7, 52.0, 54.8, 55.0, 57.0, 104.6, 105.9, 114.4, 124.2, 130.1, 130.9, 131.0, 132.2, 143.3, 149.2, 150.3, 152.7, 192.9 ppm. Anal. Calcd. For C23H26N2O3: C, 73.07; H, 6.94; N, 7.41; Found: C, 70.84; H, 6.68; N, 7.12 %.
2-(4-benzimidazolobenzilidine)-5, 6-dimethoxy-2, 3-dihydro-1H-inden-1-one. 8f
Yellow solid; Yield 82 %; mp: 238–240 °C; FT-IR (KBr) ν 3002, 2964, 1679, 1494, 1457, 1302, 1254, 1220, 1120 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.94 (3H, s, O–CH3), 4.00 (3H, s, O–CH3), 4.00 (2H, s, inden-CH2), 6.99 (1H, s, Ar–H), 7.33–7.38 (3H, m, Ar–H), 7.60 (1H, s, Ar–H), 7.59–7.61 (2H, d, J = 8.4 Hz, Ar–H), 7.61 (1H, s, methine-H), 7.82–7.84 (2H, d, J = 8.4 Hz, Ar–H), 7.88–7.91 (1H,m, Ar–H), 8.16 (1H,s, Ar–H) ppm; 13CNMR (100 MHz, CDCl3): δ 31.0, 55.1, 55.2, 103.9, 106.0, 109.4, 119.7, 122.0, 122.8, 129.5, 129.8, 130.9, 132.2, 134.1, 135.3, 135.6, 140.9, 143.1, 143.6, 148.6, 154.5, 191.7 ppm. Anal. Calcd. For C25H20N2O3: C, 75.82; H, 5.10; N, 7.07; Found: C, 72.96.24; H, 4.92; N, 6.83 %.
2-(4-pyrazolobenzilidine)-5, 6 dimethoxy-2, 3-dihydro-1H-inden-1-one 8g
Yellow solid; Yield 81 %; mp: 220–222 °C; FT-IR (KBr) ν 3115, 2926, 1685, 1598, 1501, 1308, 1121, 1090 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.98 (2H, s, inden-CH2), 3.94 (3H, s, O–CH3), 4.00 (3H, s, O–CH3), 7.00 (1H, s, Ar–H), 7.34 (1H, s, Ar–H), 7.59 (1H, s, Ar–H), 7.72–7.98 (5H, m, Ar–H), 7.99 (1H, s, Ar–H), ppm; 13CNMR (100 MHz, CDCl3): δ 31.1, 55.1, 55.2, 104.0, 106.1, 107.1, 118.0, 125.6, 130.0, 130.2, 130.6, 132.5, 134.4, 139.3, 140.6, 143.7, 148.6, 154.4, 191.9 ppm. Anal. Calcd. For C21H18N2O3: C, 72.89; H, 5.25; N, 8.09; Found: C, 70.48; H, 5.07; N, 7.79 %.
Supplementary material
Experimental details, 1H NMR, 13C NMR, FT-IR and elemental analysis of new compounds are available.
References
C.D. Duarte, E.J. Barreiro, C.A. Fraga, Mini Rev. Med. Chem. 7, 1108 (2007)
W. Steglich, B. Fugmann, S. Lang-Fugmann, Rompp Encyclopedia of Natural Products (Thieme, New York, 2000)
A. Saito, M. Umakoshi, N. Yagyu, Y. Hanzawa, Org. Lett. 10, 1783 (2008)
F. Zemek, L. Drtinova, E. Nepovimova, V. Sepsova, J. Korabecny, J. Klimes, K. Kuca, Expert Opin. Drug Saf. 13, 759 (2014)
Y.S. Huang, J.Q. Liu, L.J. Zhang, H.L. Lu, Ind. Eng. Chem. Res. 51, 1105 (2012)
P. Maresova, H. Mohelska, J. Dolejs, K. Kuca, Curr. Alzheimer Res. 12, 903 (2015)
P. Maresova, H. Mohelska, K. Kuca, Appl. Econ. 48, 1936 (2016)
C. Costagli, A. Galli, Biochem. Pharmacol. 55, 1733 (1998)
Z. Omran, T. Cailly, E. Lescot, J. Sopkova-de Oliveira Santos, J.H. Agondanou, V. Lisowski, F. Fabis, A.M. Godard, S. Stiebing, G. Le Flem, M. Boulouard, F. Dauphin, P. Dallemagne, S. Rault, Eur. J. Med. Chem. 40, 1222 (2005)
K.K. Roy, A. Dixit, A.K. Saxena, J. Mol. Graph. Model. 27, 197 (2008)
L. Rafael, C.D.L. Ríos, J. Marco-Contelles, O. Huertas, X. Barril, F.J. Luque, M.G. López, A.G. García, M. Villarroya, Bioorg. Med. Chem. 16, 7759 (2008)
X. Zhou, X.B. Wang, T. Wang, L.Y. Kong, Bioorg. Med. Chem. 16, 8011 (2008)
Y. Zhu, K. Xiao, L. Ma, B. Xiong, Y. Fu, H. Yu, W. Wang, X. Wang, D. Hu, H. Peng, J. Li, Q. Gong, Q. Chai, X. Tang, L.J. Zhang, J. Shen, Bioorg. Med. Chem. 17, 1600 (2009)
A.A.N. De Paula, J.B.L. Martins, M.L. Santos, L. De, C. Nascente, L.A.S. Romeiro, T.F.M.A. Areas, K.S.T. Vieira, N.F. Gamboa, N.G. Castro, R. Gargano, Eur. J. Med. Chem. 44, 3754 (2009)
B. Kaboudin, S. Emadi, A. Hadizadeh, Bioorg. Chem. 37, 101 (2009)
V. Alptüzün, M. Prinz, V. Hörr, J. Scheiber, K. Radacki, A. Fallarero, P. Vuorela, B. Engels, H. Braunschweig, E. Erciyas, U. Holzgrabe, Bioorg. Med. Chem. 18, 2049 (2010)
S. Gupta, A. Fallarero, P. Järvinen, D. Karlsson, M.S. Johnson, P.M. Vuorela, C.G. Mohan, Bioorg. Med. Chem. 21, 1105 (2011)
Y.S. Cho, S.K. Kim, C.B. Ahn, J.Y. Je, Carbohydr. Polym. 84, 690 (2011)
M.A. Ali, R. Ismail, T.S. Choon, R.S. Kumar, H. Osman, N. Arumugam, A.I. Almansour, K. Elumalai, A. Singh, Bioorg. Med. Chem. Lett. 22, 508 (2012)
T. Mohamed, W. Osman, G. Tin, P.P. Rao, Bioorg. Med. Chem. Lett. 23, 4336 (2013)
M. Catto, L. Pisani, F. Leonetti, O. Nicolotti, P. Pesce, A. Stefanachi, S. Cellamare, A. Carotti, Bioorg. Med. Chem. 21, 146 (2013)
M. Singh, M. Kaur, H. Kukreja, R. Chugh, O. Silakari, D. Singh, Eur. J. Med. Chem. 70, 165 (2013)
L. Yurttaş, Z.A. Kaplancıklı, Y. Özkay, J. Enzyme Inhib. Med. Chem. 28, 1040 (2013)
H. Sugimoto, Y. Limura, Y. Yamanishi, K. Yamatsu, Bioorg. Med. Chem. 2, 871 (1992)
F. De Vos, P. Santens, H. Vermeirsch, I. Dewolf, F. Dumont, G. Slegers, R.A. Dierckx, J. De Reuck, Nucl. Med. Biol. 27, 745 (2000)
H. Sugimoto, Y. Iimura, Y. Yamanishi, K. Yamatsu, J. Med. Chem. 38, 4821 (1995)
H. Sugimoto, Y. Yamanish, Y. Iimura, Y. Kawakami, Curr. Med. Chem. 7, 303 (2000)
C.R. Elati, N. Kolla, S.R. Chalamala, P.J. Vankawala, V. Sundaram, H. Vurimidi, V.T. Mathad, Synth. Commun. 36, 169 (2006)
Y. Kagoshima, M. Mori, E. Suzuki, N. Kobayashi, T. Shibayama, M. Kubota, Y. Kamai, T. Konosu, Chem. Pharm. Bull. 58, 1157 (2010)
F.C. Meng, F. Mao, W.J. Shan, F. Qin, X.S. Li, L. Huang, Bioorg. Med. Chem. Lett. 22, 4462 (2012)
G. Kryger, I. Silman, J.L. Sussman, Structure 7, 297 (1999)
J. Korabecny, R. Dolezal, P. Cabelova, A. Horova, E. Hruba, J. Ricny, L. Sedlacek, E. Nepovimova, K. Spilovska, M. Andrs, K. Musilek, Eur. J. Med. Chem. 82, 426 (2014)
V. Sepsova, J.Z. Karasova, G. Tobin, D. Jun, J. Korabecny, P. Cabelova, K. Janska, J. Krusek, K. Skrenkova, K. Kuca, M. Valko, Gen. Physiol. Biophys. 34, 200 (2015)
K.O. Yerdelen, M. Koca, B. Anil, H. Sevindik, Z. Kasap, Z. Halici, K. Turkaydin, G. Gunesacar, Bioorg. Med. Chem. Lett. 25, 5576 (2015)
M.Y. Wu, G. Esteban, S. Brogi, M. Shionoya, L. Wang, G. Campiani, M. Unzeta, T. Inokuchi, S. Butini, J. Marco-Contelles, Eur. J. Med. Chem. (2016). doi:10.1016/j.ejmech.2015.10.001
J.P. Qiao, C.S. Gan, C.W. Wang, J.F. Ge, D.D. Nan, J. Pan, J.N. Zhou, ChemBioChem 13, 1652 (2012)
L. Huang, C. Lu, Y. Sun, F. Mao, Z. Luo, T. Su, H. Jiang, W. Shan, X. Li, J. Med. Chem. 55, 8483 (2012)
R. Sheng, X. Lin, J. Li, Y. Jiang, Z. Shang, Y. Hu, Bioorg. Med. Chem. Let. 15, 3834 (2005)
Y. Shen, R. Sheng, J. Zhang, Q. He, B. Yang, Y. Hu, Bioorg. Med. Chem. 16, 7646 (2008)
Acknowledgments
We thank the Research Affairs of the University of Tabriz for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teimuri-Mofrad, R., Nikbakht, R. & Gholamhosseini-Nazari, M. A convenient and efficient method for the synthesis of new 2-(4-amino substituted benzilidine) indanone derivatives. Res Chem Intermed 42, 7501–7511 (2016). https://doi.org/10.1007/s11164-016-2549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2549-0