Abstract
Long non-coding RNAs (lncRNAs) have been confirmed to be involved in epilepsy development. It has been reported that lncRNA ZFAS1 plays a vital regulatory role in epilepsy progression. Therefore, the role and molecular mechanism of ZFAS1 in epilepsy progression deserve further investigation. Mice status epilepticus (SE) model was constructed, and hippocampal neurons were isolated from mice hippocampus tissues. The expression of ZFAS1, miR-15a-5p and oxidative stress responsive 1 (OXSR1) were determined by quantitative real-time PCR. ELISA assay was used to detect the concentrations of inflammation factors. Cell viability and apoptosis were examined by MTT assay, EdU staining and flow cytometry. Western blot analysis was conducted to measure protein levels, and the productions of SOD and MDA were measured to assess cell oxidative stress. Dual-luciferase reporter assay and RIP assay were employed to validate the relationship between miR-15a-5p and ZFAS1 or OXSR1. LncRNA ZFAS1 was highly expressed in SE mice and SE-stimulated hippocampal neurons. Silenced ZFAS1 promoted viability, while inhibited inflammation, apoptosis and oxidative stress in SE-induced hippocampal neurons. MiR-15a-5p could be targeted by ZFAS1, and its inhibitor also reversed the suppressive effect of ZFAS1 knockdown on SE-induced hippocampal neurons injury. In addition, OXSR1 was a target of miR-15a-5p, and its silencing also could relieve SE-induced hippocampal neurons injury. OXSR1 overexpression reversed the inhibition effect of miR-15a-5p on SE-induced hippocampal neurons injury. Moreover, ZFAS1 positively regulated OXSR1 expression by sponging miR-15a-5p, thereby activating the NF-κB pathway. LncRNA ZFAS1 might contribute to the progression of epilepsy by regulating the miR-15a-5p/OXSR1/NF-κB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy refers to a chronic disease of transient brain dysfunction caused by sudden abnormal discharge of brain neurons (Rao and Lowenstein 2015; Thijs et al. 2019). As a common neurological disease, the prevalence of epilepsy is second only to stroke, which often causes dysfunction such as motor, sensory, consciousness and behavior (Cavanna and Ali 2011; Pondal-Sordo et al. 2006). Status epilepticus (SE) is a more serious type of epilepsy, which refers to a seizure lasting more than 30 min, or the patient’s state of consciousness has not fully recovered between the 2 seizures (Jafarpour et al. 2018; Nelson and Varelas 2018). At present, the treatment of epilepsy is based on drugs, but long-term administration may cause organ injury to the patients (Klein et al. 2020; Schmidt and Schachter 2014). Therefore, exploring the pathogenesis of epilepsy is of great significance to the development of new treatment methods for epilepsy.
Long non-coding RNA (lncRNA) is a type of RNA molecule that does not encode protein and has a transcript length of more than 200 nt (Qian et al. 2019b). A large number of studies have shown that lncRNA plays an important role in many biological fields such as tumorigenesis and neuroscience, and is an important regulatory molecule in the human genome (Ghafouri-Fard et al. 2020; Wu et al. 2017). Studies have shown that abnormally regulated lncRNAs may mediate the injury of hippocampal neurons, thus participating in the progression of epilepsy. Han et al. suggested that lncRNA H19 could promote the apoptosis of SE-induced hippocampal neurons to aggravate temporal lobe epilepsy progression (Han et al. 2018). Also, lncRNA UCA1 had been discovered to hinder the activity of NF-κB pathway to repress the inflammation in rat epilepsy model and hippocampal astrocytes (Yu et al. 2020). LncRNA FTX was found to be lowly expressed in SE-induced hippocampal neurons, and its overexpression had an inhibition on neuron apoptosis (Li et al. 2019).
In the past research, lncRNA ZFAS1 had been proved to be an important regulator for epilepsy progression, and its abnormal expression was found to be related to the viability, apoptosis and inflammation of hippocampal neurons (He et al. 2021; Hu et al. 2020). Therefore, studies on ZFAS1 are needed to provide further evidence that it is a potential target for epilepsy. More importantly, the proposition of lncRNA/microRNA (miRNA)/mRNA axis provides an idea for revealing the molecular mechanism of lncRNA (Huang 2018; Wang et al. 2020). In addition to confirming the role of ZFAS1 in epilepsy, our study further elucidated the potential molecular mechanism by which ZFAS1 regulated the progression of epilepsy through the hypothesis of lncRNA/miRNA/mRNA axis.
Materials and methods
Mice SE model
All procedures of this study were supported by the Ethics Committee of School of Basic Medicine, Jiamusi University. Adult male C57BL/6 mice were obtained from Vital River (Beijing, China) and randomly divided into sham operation group (n = 9) and kainic acid (KA) group (n = 18). All mice were anesthetized by intraperitoneal injected with atropine (2 mg/kg, Selleck, Shanghai, China). After 30 min, mice were injected with KA (30 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) with reference to previous studies (Xiaoying et al. 2020). The mice behavior was monitored for 2 h, and the behavioral seizures were investigated. According to the Racine scale, the mice with stage 4–5 were judged as successful modeling (n = 9). Mice in stage 1–3 (n = 9) were not included in the functional study. For the sham group (n = 9), mice were injected with saline (10 mL/kg) as control. After 3 days, all mice were euthanized and the hippocampus tissues of SE mice and sham mice were collected for analysis.
Hippocampal neurons culture and transfection
According to the previously reported (Wen et al. 2018), the primary hippocampal neurons were isolated from the hippocampus tissues of SE mice or sham mice. The hippocampal neurons were grown in DMEM medium (Gibco, Carlsbad, CA, USA) containing 2% B27 (Gibco), 0.5 mmol/L glutamine(Gibco), 10% FBS (Gibco) and 1% penicillin/streptomycin solution (Sangon, Shanghai, China) at 37℃ with 5% CO2 incubator. After cultured for 24 h, Ara-C (10 µM, C1768, Sigma-Aldrich) was added into hippocampal neurons to inhibit the growth of fibroblasts and glia cells. Lipofectamine 3000 reagent (Invitrogen) was used to transfect the oligonucleotides (40 nM) and vectors into hippocampal neurons when cells reached 60% confluences. ZFAS1 small interfering RNA (si-ZFAS1) and pcDNA overexpression vector, miR-15a-5p mimic or inhibitor (anti-miR-15a-5p), oxidative stress responsive 1 (OXSR1) siRNA (si-OXSR1) and pcDNA overexpression vector, as well as their negative controls were purchased from RiboBio (Guangzhou, China). The transfection efficiency was evaluated by detecting the target gene expression.
Quantitative real-time PCR (qRT-PCR)
RNA was extracted by TRIzol reagent (Invitrogen), and cDNA was collected using M-MLV reverse transcriptase (Solarbio, Beijing, China). Finally, PCR amplification was carried out with SYBR Premix Ex-Taq (Takara, Dalian, China). GAPDH or U6 was used as internal reference and fold change was analyzed by 2−∆∆Ct method. Primary sequences have been shown in Table 1.
Subcellular localization assay
Cytoplasmic & Nuclear RNA Purification Kit (Norgen Biotek, Thorold, Ontario, Canada) was used to isolate the cytoplasm and nuclear RNA from the SE-induced hippocampal neurons basing on the kit instructions. QRT-PCR was carried out to detect ZFAS1, GAPDH (cytoplasm control) and U6 (nuclear control) expression.
ELISA assay
The medium of hippocampal neurons were collected and centrifuged at 500 g for 5 min. According to the instructions of Mouse IL-6 and TNF-α ELISA Kits (Beyotime, Shanghai, China), the concentrations of IL-6 and TNF-α were analyzed.
MTT assay
Hippocampal neurons were seeded into 96-well plates (5 × 103 cells/well). After 48 h, hippocampal neurons were induced by 20 µL MTT solution (Solarbio) and incubated for 4 h. Then, 100 µL DMSO (Solarbio) was added into cells and kept the plate in the dark for 15 min. Finally, the absorbance at 570 nm was determined by a microplate reader to evaluate cell viability.
EdU staining
EdU Kit (RiboBio) was used to determine EdU positive cells to assess cell viability. Briefly, hippocampal neurons were inoculated into 96-well plates (1 × 105 cells/well). 24 h later, the cells were incubated with EdU medium for 2 h. After washed with PBS, the cells were fixed and permeated, and then were stained by Apollo solution. Finally, after the cells were infiltrated again, the cell nuclei was stained with DAPI solution. Fluorescence images were captured under a fluorescence microscope and the rate of EdU positive cells was calculated.
Cell apoptosis assay
Flow cytometry was used to measure cell apoptosis by Annexin V-FITC/PI Apoptosis Detection Kit (Elabscience, Wuhan, China). Hippocampal neurons (2 × 105 cells) were collected and then were re-suspended with Annexin V Binding Buffer. After the cells were incubated with Annexin V-FITC staining solution and propidium iodide staining solution for 20 min, the cell apoptosis rate was analyzed using CytoFLEX flow cytometer with CytExpert 2.0 software.
Western blot (WB) analysis
Hippocampus tissues and hippocampal neurons were lysed by RIPA reagent (Beyotime) to extract total protein. The protein (20 µg) was separated on 10% SDS-PAGE gel followed by transferred to PVDF membrane (Invitrogen). After blocked with 5% non-fat milk, the membrane was incubated with primary antibody, including anti-Bax (1:1,000, ab32503, Abcam, Cambridge, MA, USA), anti-Bcl-2 (1:1,000, ab196495, Abcam), anti-OXSR1 (1:100, ab224248, Abcam), P65 (1:1000, ab32536, Abcam), p-P65 (1:1,000, ab76302 Abcam), IκBα (1:5,000, ab32518, Abcam), p-IκBα (1:1,000, ab133462, Abcam), or anti-GAPDH (1:1,000, ab9485, Abcam). The membrane was then incubated with secondary antibody (1:50,000, ab205718, Abcam), and then the protein band was visualized with Immobilon Western Chemilum HRP Substrate (Millipore, Billerica, MA, USA).
Determination of oxidative stress
SOD production and MDA production were determined in the medium of hippocampal neurons to assess cell oxidative stress basing on the manufacturer’s protocols of SOD Assay Kit and MDA Assay Kit (Elabscience).
Dual-luciferase reporter assay
According to the target binding sites predicted by the online software, the sequences of ZFAS1 and OXSR1 3’UTR were inserted into the pmirGLO reporter vector to generate the corresponding wild-type (WT) vector and mutant-type (MUT) vector. The vectors were then transfected into 293T cells with miR-15a-5p mimic or miR-NC. The luciferase activity was analyzed by Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
RIP assay
RIP assay was performed using EZ-Magna RIP Kit (Millipore). Hippocampal neurons were lysed in RIP lysis buffer, and then the cell lysates were mixed with magnetic beads coated with anti-Ago2 or anti-IgG. At last, qRT-PCR was used to examine the expression of ZFAS1, miR-15a-5p and OXSR1.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 6.0 software. All data were presented as mean ± SD from 3 independent experiments. The differences among groups were assessed by Student’s t-test or ANOVA. Pearson correlation analysis was used to analyze the correlation between miR-15a-5p and ZFAS1 or OXSR1. P < 0.05 indicated a significant difference.
Results
ZFAS1 was upregulated in SE mice and SE-stimulated hippocampal neurons
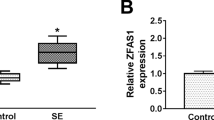
In the hippocampus tissues of SE mice, we discovered that ZFAS1 was highly expressed compared with that in sham mice (Fig. 1A). Also, the expression of ZFAS1 was significantly increased in SE-stimulated hippocampal neurons (Fig. 1B). Therefore, we speculated that ZFAS1 might play an important role in the progression of epilepsy. In addition, subcellular localization assay was used to verify the location of ZFAS1 in cell nuclear and cytoplasm. The results showed that ZFAS1 was mainly distributed in the cytoplasm (Fig. 1C), suggesting that ZFAS1 might mainly participate in post-transcriptional regulation.
ZFAS1 was upregulated in SE mice and SE-stimulated hippocampal neurons. (A) The expression of ZFAS1 in the hippocampus tissues of SE mice and sham mice was measured by qRT-PCR. (B) The ZFAS1 expression in hippocampal neurons from SE mice or sham mice was detected by qRT-PCR. (C) Subcellular localization assay was used to evaluate the distribution of ZFAS1 in cell cytoplasm and nuclear. ***P < 0.001, ****P < 0.0001
Knockdown of ZFAS1 alleviated SE-stimulated hippocampal neurons injury
To explore the role of ZFAS1 in the progression of epilepsy, we constructed si-ZFAS1 and transfected it into SE-induced hippocampal neurons. The detection results of ZFAS1 expression revealed that the increased ZFAS1 expression in the hippocampal neurons from SE mice could be decreased by si-ZFAS1 (Fig. 2A). In this, we found that knockdown of ZFAS1 inhibited the concentrations of IL-6 and TNF-α in SE-induced hippocampal neurons (Fig. 2B). MTT assay and EdU staining were used to assess the viability of hippocampal neurons, and the results showed that the viability and the EdU positive cells rate were markedly reduced in the hippocampal neurons from SE mice, while silenced ZFAS1 could enhance the viability of SE-induced hippocampal neurons (Fig. 2C-D). Through assessing cell apoptosis, we confirmed that the apoptosis rate and the apoptosis marker Bax protein expression were promoted, while anti-apoptosis marker Bcl-2 protein expression was suppressed in the hippocampal neurons from SE mice. However, ZFAS1 knockdown also inhibited the apoptosis of SE-induced hippocampal neurons (Fig. 2E-F). Meanwhile, silenced ZFAS1 significantly increased SOD production and decreased MDA production in SE-induced hippocampal neurons (Fig. 2G-H). These data showed that ZFAS1 might promote epilepsy progression.
Knockdown of ZFAS1 alleviated SE-stimulated hippocampal neurons injury. Hippocampal neurons from SE mice were transfected with si-NC or si-ZFAS1. Hippocampal neurons from sham mice were used as control. (A) The expression of ZFAS1 was measured by qRT-PCR. (B) The concentrations of IL-6 and TNF-α were determined by ELISA assay. MTT assay (C) and EdU staining (D) were used to assess cell viability. (E) Flow cytometry was performed to assess cell apoptosis rate. (F) The protein levels of Bax and Bcl-2 were detected by WB analysis. (G-H) The productions of SOD and MDA were evaluated by corresponding Assay Kits. **P < 0.01, ***P < 0.001, ****P < 0.0001
ZFAS1 directly interacted with miR-15a-5p
To search for the targeted miRNA for ZFAS1, the starbase software was used, and miR-15a-5p was found to have binding sites with ZFAS1 (Fig. 3A). After confirming that miR-15a-5p mimic could significantly increase miR-15a-5p expression (Fig. 3B), we co-transfected with miR-15a-5p mimic and the WT/MUT-ZFAS1 vectors into 293T cells. The dual-luciferase reporter assay results revealed that miR-15a-5p mimic could reduce the luciferase activity of WT-ZFAS1 vector, while had not effect on that of the MUT-ZFAS1 vector (Fig. 3C). Moreover, RIP assay was used to further confirm the interaction between ZFAS1 and miR-15a-5p and the results showed that both ZFAS1 and miR-15a-5p could be enriched in Ago2 antibody (Fig. 3D). In the hippocampus tissues of SE mice and SE-induced hippocampal neurons, we found a significant decreased miR-15a-5p expression (Fig. 3E-F). In addition, miR-15a-5p expression also was negatively correlated with ZFAS1 expression in the hippocampus tissues of SE mice (Fig. 3G). After determined that pcDNA ZFAS1 overexpression vector indeed promoted ZFAS1 expression in SE-induced hippocampal neurons (Fig. 3H), we measured miR-15a-5p expression in SE-induced hippocampal neurons transfected with si-ZFAS1 or pcDNA ZFAS1 overexpression vector. The results showed that miR-15a-5p expression could be promoted by ZFAS1 knockdown, while reduced by ZFAS1 overexpression (Fig. 3I). The above results confirmed that ZFAS1 sponged miR-15a-5p.
ZFAS1 directly interacted with miR-15a-5p. (A) The sequences of WT/MUT-ZFAS1 were shown. (B) The transfection efficiency of miR-15a-5p mimic was confirmed by detecting miR-15a-5p expression using qRT-PCR. Dual-luciferase reporter assay (C) and RIP assay (D) were used to assess the interaction between miR-15a-5p and ZFAS1. (E) The miR-15a-5p expression in the hippocampus tissues of SE mice and sham mice was determined by qRT-PCR. (F) QRT-PCR was used to detect the miR-15a-5p expression in hippocampal neurons from SE mice or sham mice. (G) Pearson correlation analysis was used to evaluate the correlation between miR-15a-5p and ZFAS1 in the hippocampus tissues of SE mice. (H) The expression of ZFAS1 was detected by qRT-PCR to assess the transfection efficiency of pcDNA ZFAS1 overexpression vector. (I) The miR-15a-5p expression was measured using qRT-PCR to determine the regulation of ZFAS1 knockdown and overexpression on miR-15a-5p expression. **P < 0.01, ***P < 0.001, ****P < 0.0001
ZFAS1 participated in the regulation of SE-induced hippocampal neurons injury via targeting miR-15a-5p
To investigate the miR-15a-5p roles in epilepsy progression, anti-miR-15a-5p were transfected into SE-induced hippocampal neurons. After transfection, we confirmed that anti-miR-15a-5p significantly decreased miR-15a-5p expression in SE-induced hippocampal neurons (Supplementary Fig. 1A). Function analysis revealed that anti-miR-15a-5p promoted the concentrations of IL-6 and TNF-α, reduced cell viability and EdU positive cells, as well as enhanced the apoptosis in SE-induced hippocampal neurons (Supplementary Fig. 1B-E). Additionally, anti-miR-15a-5p decreased SOD production and increased MDA production in SE-induced hippocampal neurons (Supplementary Fig. 1F-G). Therefore, we confirmed that miR-15a-5p could inhibit hippocampal neurons injury to alleviate epilepsy progression. To investigate whether ZFAS1 regulated epilepsy progression by sponging miR-15a-5p, si-ZFAS1 and anti-miR-15a-5p were co-transfected into SE-induced hippocampal neurons to perform the rescue experiments. We found that the enhancing effect of si-ZFAS1 on miR-15a-5p expression could be abolished by the addition of anti-miR-15a-5p (Fig. 4A). Function analysis results indicated that the suppressive effect of ZFAS1 knockdown on the IL-6 and TNF-α concentrations, as well as the promotion effect on cell viability and the EdU positive cells in SE-induced hippocampal neurons could be reversed by miR-15a-5p inhibitor (Fig. 4B-D). Also, miR-15a-5p inhibitor overturned the repressing effect of ZFAS1 knockdown on the apoptosis of SE-induced hippocampal neurons, as demonstrated by the increased apoptosis rate, the enhanced Bax protein expression, and the decreased Bcl-2 protein expression in the co-transfection group (Fig. 4E-G). Furthermore, the addition of anti-miR-15a-5p also reversed the enhancing effect of ZFAS1 knockdown on SOD production and the reducing effect on MDA production in SE-induced hippocampal neurons (Fig. 4H-I). Therefore, we confirmed that ZFAS1 sponged miR-15a-5p to regulate epilepsy progression.
ZFAS1 participates in the regulation of SE-induced hippocampal neurons injury via targeting miR-15a-5p. Hippocampal neurons from SE mice were transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-miR-NC or si-ZFAS1 + anti-miR-15a-5p. Hippocampal neurons from sham mice were used as control. (A) The miR-15a-5p expression was measured using qRT-PCR. (B) ELISA assay was used to examine the concentrations of IL-6 and TNF-α. Cell viability was determined using MTT assay (C) and EdU staining (D). (E-F) Cell apoptosis rate was assessed by flow cytometry. (G) The protein levels of Bax and Bcl-2 were determined by WB analysis. (H-I) Corresponding Assay Kits were utilized for examining the productions of SOD and MDA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
MiR-15a-5p targeted OXSR1
In addition, the target of miR-15a-5p was predicted by the starbase software, and it was found that miR-15a-5p could bind with the 3’UTR of OXSR1 (Fig. 5A). Also, our data verified that miR-15a-5p mimic only reduced the luciferase activity of WT-OXSR1 3’UTR vector (Fig. 5B), and both miR-15a-5p and OXSR1 could be enriched in Ago2 antibody (Fig. 5C). In the hippocampus tissues of SE mice, we found that OXSR1 was markedly upregulated at the mRNA level and protein level compared to the sham group mice (Fig. 5D-E). Moreover, OXSR1 protein expression also was significantly increased in SE-induced hippocampal neurons (Fig. 5F). Correlation analysis showed that OXSR1 expression was negatively correlated with miR-15a-5p expression in the hippocampus tissues of SE mice (Fig. 5G). After confirming that anti-miR-15a-5p indeed reduced miR-218-3p expression in SE-induced hippocampal neurons (Fig. 5H), we detected OXSR1 expression in SE-induced hippocampal neurons transfected with miR-15a-5p mimic or inhibitor. WB analysis data revealed that miR-15a-5p overexpression could decrease OXSR1 protein expression, while anti-miR-15a-5p had an opposite effect (Fig. 5I). All data showed that OXSR1 was a target of miR-15a-5p.
MiR-15a-5p targeted OXSR1. (A) The sequences of WT/MUT-OXSR1 3’UTR were exhibited. The interaction between miR-15a-5p and OXSR1 was measured by dual-luciferase reporter assay (B) and RIP assay (C). (D-E) The mRNA and protein expression of OXSR1 in the hippocampus tissues of SE mice and sham mice was detected by qRT-PCR and WB analysis. (F) WB analysis was performed to examine the OXSR1 protein expression in hippocampal neurons from SE mice or sham mice. (G) The correlation between miR-15a-5p and OXSR1 in the hippocampus tissues of SE mice was analyzed by Pearson correlation analysis. (H) The expression of miR-15a-5p was tested by qRT-PCR to assess the transfection efficiency of anti-miR-15a-5p. (I) The protein expression of OXSR1 was measured using WB analysis to determine the regulation of miR-15a-5p mimic or inhibitor on OXSR1 expression. **P < 0.01, ***P < 0.001, ****P < 0.0001
Downregulated OXSR1 relieved SE-stimulated hippocampal neurons injury
Then, SE-induced hippocampal neurons were transfected with si-OXSR1 to explore the role of OXSR1 in epilepsy progression. The transfection of si-OXSR1 indeed reduced OXSR1 protein expression in SE-induced hippocampal neurons (Fig. 6A). Our data suggested that OXSR1 knockdown repressed the concentrations of IL-6 and TNF-α, while enhanced cell viability and EdU positive cells in SE-induced hippocampal neurons (Fig. 6B-D). Moreover, silenced OXSR1 reduced the apoptosis rate and Bax protein expression, while promoted Bcl-2 protein expression in SE-induced hippocampal neurons (Fig. 6E-F). In addition, knockdown of OXSR1 accelerated SOD production and suppressed MDA production in SE-induced hippocampal neurons (Fig. 6G-H). These data suggested that OXSR1 might promote hippocampal neurons injury to facilitate epilepsy progression.
Downregulated OXSR1 relieved SE-stimulated hippocampal neurons injury. Hippocampal neurons from SE mice were transfected with si-NC or si-OXSR1. Hippocampal neurons from sham mice were used as control. (A) The OXSR1 protein expression was measured by WB analysis. (B) The concentrations of IL-6 and TNF-α were measured using ELISA assay. MTT assay (C) and EdU staining (D) were performed to detect cell viability. (E) Cell apoptosis rate was analyzed by flow cytometry. (F) The Bax and Bcl-2 protein levels were evaluated using WB analysis. (G-H) The productions of SOD and MDA were measured by corresponding Assay Kits. **P < 0.01, ***P < 0.001, ****P < 0.0001
MiR-15a-5p targeted OXSR1 to regulate SE-induced hippocampal neurons injury
To further confirm that miR-15a-5p indeed targeted OXSR1 to regulate epilepsy progression, we performed the rescue experiments. The detection of OXSR1 protein expression showed that pcDNA OXSR1 overexpression vector could significantly enhance OXSR1 expression (Fig. 7A). Then, SE-induced hippocampal neurons were transfected with miR-15a-5p mimic and pcDNA OXSR1 overexpression vector. The decreasing effect of miR-15a-5p mimic on OXSR1 protein expression could be promoted by the addition of pcDNA OXSR1 overexpression vector (Fig. 7B). By assessing the inflammation and viability of SE-induced hippocampal neurons, we found that miR-15a-5p overexpression reduced the concentrations of IL-6 and TNF-α, accelerated cell viability, and increased the EdU positive cells, while these effects could be reversed by overexpressing OXSR1 (Fig. 7C-E). Furthermore, miR-15a-5p also suppressed the apoptosis rate, decreased Bax protein expression and increased Bcl-2 protein expression in SE-induced hippocampal neurons, while OXSR1 overexpression also overturned these effects (Fig. 7F-H). In addition, overexpressed OXSR1 abolished the increasing effect of miR-15a-5p on SOD production and the decreasing effect on MDA production in SE-induced hippocampal neurons (Fig. 7I-J). Hence, our data illuminated that miR-15a-5p targeted OXSR1 to inhibit epilepsy progression.
MiR-15a-5p targeted OXSR1 to regulate SE-induced hippocampal neurons injury. (A) The transfection efficiency of pcDNA OXSR1 overexpression vector was assessed by detecting OXSR1 protein expression using WB analysis. Hippocampal neurons from SE mice were transfected with miR-NC, miR-15a-5p, miR-15a-5p + pcDNA or miR-15a-5p + OXSR1. Hippocampal neurons from sham mice were used as control. (B) WB analysis was used to determine the OXSR1 protein expression. (C) ELISA assay was utilized to detect the concentrations of IL-6 and TNF-α. Cell viability were analyzed by MTT assay (D) and EdU staining (E). (F-G) Cell apoptosis rate was evaluated using flow cytometry. (H) WB analysis was performed to measure the Bax and Bcl-2 protein levels. (I-J) The productions of SOD and MDA were examined by corresponding Assay Kits. **P < 0.01, ***P < 0.001, ****P < 0.0001
ZFAS1 regulated OXSR1 expression by sponging miR-15a-5p
The above results showed that ZFAS1 could sponge miR-15a-5p, and miR-15a-5p could target OXSR1. To reveal the regulation of ZFAS1 on OXSR1, we measured OXSR1 expression in SE-induced hippocampal neurons transfected with si-ZFAS1 and anti-miR-15a-5p. The results suggested that ZFAS1 knockdown had an inhibition on OXSR1 mRNA and protein expression, while these effects could be reversed by anti-miR-15a-5p (Fig. 8A-B). All data indicated that ZFAS1 sponged miR-15a-5p to positive regulate OXSR1.
ZFAS1 regulated OXSR1 expression by sponging miR-15a-5p. Hippocampal neurons from SE mice were transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-miR-NC or si-ZFAS1 + anti-miR-15a-5p. Hippocampal neurons from sham mice were used as control. The mRNA and protein expression of OXSR1 was measured by qRT-PCR (A) and WB analysis (B). ***P < 0.001, ****P < 0.0001
ZFAS1/miR-15a-5p/OXSR1 axis regulated the activity of NF-κB pathway
NF-κB pathway is a key signaling pathway that mediates cellular inflammatory response, and is closely related to the occurrence of cell injury (Chen et al. 2018; Wan et al. 2020). In this, we assessed the activity of NF-κB pathway. After SE induction, we found that the protein expression levels of p-P65 and p-IκBα were markedly enhanced in hippocampal neurons (Fig. 9A), confirming that the NF-κB pathway was activated in the hippocampal neurons from SE mice. Additionally, ZFAS1 knockdown reduced the protein expression levels of p-P65 and p-IκBα in SE-induced hippocampal neurons, while these effect could be reversed by miR-15a-5p inhibitor or OXSR1 overexpression (Fig. 9B). Therefore, we confirmed that the ZFAS1/miR-15a-5p/OXSR1 axis mediated the activity of NF-κB pathway. Above all, our results showed that ZFAS1 inhibited hippocampal neurons viability, while promoted apoptosis, inflammation and oxidative stress to facilitate the progression of epilepsy through regulating the miR-15a-5p/OXSR1/NF-κB pathway (Fig. 10).
ZFAS1/miR-15a-5p/OXSR1 axis regulated the activity of NF-κB pathway. (A) The protein expression levels of p-P65 and p-IκBα were detected by WB analysis in hippocampal neurons from SE mice or sham mice. (B) Under different transfection conditions, the p-P65 and p-IκBα protein expression in SE-induced hippocampal neurons was determined by WB analysis. ****P < 0.0001
Discussion
As a part of the limbic system, hippocampus plays a vital role in information coding, short-term memory, long-term memory, and spatial navigation (Courellis et al. 2019; Sakaguchi and Sakurai 2020). Studies have shown that the impairment of hippocampus function is one of the important causes for the development of many neurological diseases, including epilepsy (Qian et al. 2019a; Ying et al. 2020). Therefore, elucidating the molecular mechanisms affecting hippocampal injury may provide effective molecular targets for the treatment of epilepsy. He et al. showed that ZFAS1 was upregulated in temporal lobe epilepsy patients, and it could inhibit hippocampal neurons viability, while enhanced apoptosis and inflammation (He et al. 2021). Moreover, Hu et al. reported that ZFAS1 silencing had an inhibition on the apoptosis and autophagy of SE-induced hippocampal neurons (Hu et al. 2020). Consistent with these results, our data suggested that ZFAS1 was highly expressed in SE mice and SE-induced hippocampal neurons, and its knockdown could alleviate SE-induced hippocampal neurons injury, which was characterized by a significant increase in cell viability, and a significant decrease in inflammation, apoptosis and oxidative stress. These results confirmed that ZFAS1 might be a potential target for treating epilepsy.
In terms of mechanism, we discovered that ZFAS1 acted as miR-15a-5p sponge. MiR-15a-5p has been shown to regulate the malignant progression of many cancers, including endometrial cancer (Wang et al. 2017), prostate cancer (Wu et al. 2020), and cervical cancer (Zhao et al. 2019). Also, many studies had shown that miR-15a-5p could alleviate LPS-induced chondrocytes injury (Zhang et al. 2020). It was reported that miR-15a-5p was under-expressed in temporal lobe epilepsy children, which could improve hippocampal neurons viability and suppress apoptosis (Li et al. 2020a). Additionally, propofol was found to suppress the apoptosis of SE-induced hippocampal neurons by increasing miR-15a-5p expression (Liu et al. 2020). In this, our data verified that miR-15a-5p indeed had a suppressive role on inflammation, apoptosis and oxidative stress in SE-induced hippocampal neurons. The reversal effect of anti-miR-15a-5p on the function of si-ZFAS1 in SE-induced hippocampal neurons injury confirmed that ZFAS1 sponged miR-15a-5p to promote epilepsy progression.
OXSR1 is a marker for oxidative stress and is widely expressed in cancer and a variety of human diseases (Chen et al. 2020; Li et al. 2021). Li et al. proposed that downregulated OXSR1 could repress LPS-induced kidney cell injury (Li et al. 2021). Past research had shown that miR-25-3p inhibited OXSR1 expression to alleviate epileptiform discharges by reducing the oxidative stress and apoptosis of hippocampal neurons (Li et al. 2020b). In our research, we found that OXSR1 could be targeted by miR-15a-5p and was positively regulated by lncRNA ZFAS1. Furthermore, OXSR1 knockdown enhanced the viability and hindered the inflammation, apoptosis and oxidative stress in SE-induced hippocampal neurons, suggesting that OXSR1 played an active role in epilepsy progression. In addition, OXSR1 overexpression also reversed the negative regulation of miR-15a-5p on SE-induced hippocampal neurons injury. The above data confirmed the existent of ZFAS1/miR-15a-5p/OXSR1 axis in epilepsy.
NF-κB is a key signaling pathway in the regulation of neuro-inflammatory processes, and is associated with the progression of a variety of neurological diseases (Shabab et al. 2017; Singh et al. 2020). In many research, the activation of NF-κB pathway was found to promote the inflammatory process of hippocampal neurons or astrocytes, and thus accelerating the occurrence and development of epilepsy (Qi et al. 2020; Yan et al. 2019; Yu et al. 2020). Here, we discovered that the activity of NF-κB pathway was significantly enhanced in SE-induced hippocampal neurons. Importantly, further experiments also revealed that the ZFAS1/miR-15a-5p/OXSR1 axis could positively regulate the activity of NF-κB pathway.
In conclusion, our study demonstrated that lncRNA ZFAS1 contributed to SE-induced hippocampal neurons injury via regulating the miR-15a-5p/OXSR1/NF-κB pathway. These findings helped us to deeply understand the molecular mechanism of epilepsy and provided new evidence for ZFAS1 as a potential therapeutic target for epilepsy.
Data availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
References
Cavanna AE, Ali F (2011) Epilepsy: the quintessential pathology of consciousness. Behav Neurol 24(1):3–10
Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation 15(1):116
Chen J, Zhou J, Fu H, Ni X, Shan Y (2020) Upregulation of oxidative stress-responsive 1(OXSR1) predicts poor prognosis and promotes hepatocellular carcinoma progression. Bioengineered 11(1):958–971
Courellis HS, Nummela SU, Metke M, Diehl GW, Bussell R, Cauwenberghs G, Miller CT (2019) Spatial encoding in primate hippocampus during free navigation. PLoS Biol 17(12):e3000546
Ghafouri-Fard S, Esmaeili M, Taheri M (2020) H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother 123:109774
Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, Hu W, Zhang JG, Li L, Meng FG (2018) Long non-coding RNA H19 contributes to apoptosis of hippocampal neurons by inhibiting let-7b in a rat model of temporal lobe epilepsy. Cell Death Dis 9(6):617
He C, Su C, Zhang W, Zhou Q, Shen X, Yang J, Shi N (2021) Modulatory potential of LncRNA Zfas1 for inflammation and neuronal apoptosis in temporal lobe epilepsy. Yonsei Med J 62(3):215–223
Hu F, Shao L, Zhang J, Zhang H, Wen A, Zhang P (2020) Knockdown of ZFAS1 inhibits hippocampal neurons apoptosis and autophagy by activating the PI3K/AKt pathway via up-regulating miR-421 in epilepsy. Neurochem Res 45(10):2433–2441
Huang Y (2018) The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 22(12):5768–5775
Jafarpour S, Hodgeman RM, De Marchi Capeletto C, de Lima MTA, Kapur K, Tasker RC, Loddenkemper T (2018) New-onset status epilepticus in pediatric patients: Causes, characteristics, and outcomes. Pediatr Neurol 80:61–9
Klein P, Friedman A, Hameed MQ, Kaminski RM, Bar-Klein G, Klitgaard H, Koepp M, Jozwiak S, Prince DA, Rotenberg A, Twyman R, Vezzani A, Wong M, Loscher W (2020) Repurposed molecules for antiepileptogenesis: Missing an opportunity to prevent epilepsy? Epilepsia 61(3):359–86
Li X, Giri V, Cui Y, Yin M, Xian Z, Li J (2019) LncRNA FTX inhibits hippocampal neuron apoptosis by regulating miR-21-5p/SOX7 axis in a rat model of temporal lobe epilepsy. Biochem Biophys Res Commun 512(1):79–86
Li N, Pan J, Liu W, Li Y, Li F, Liu M (2020a) MicroRNA-15a-5p serves as a potential biomarker and regulates the viability and apoptosis of hippocampus neuron in children with temporal lobe epilepsy. Diagn Pathol 15(1):46
Li R, Wen Y, Wu B, He M, Zhang P, Zhang Q, Chen Y (2020b) MicroRNA-25-3p suppresses epileptiform discharges through inhibiting oxidative stress and apoptosis via targeting OXSR1 in neurons. Biochem Biophys Res Commun 523(4):859–866
Li H, Zhang X, Wang P, Zhou X, Liang H, Li C (2021) Knockdown of circ-FANCA alleviates LPS-induced HK2 cell injury via targeting miR-93-5p/OXSR1 axis in septic acute kidney injury. Diabetol Metab Syndr 13(1):7
Liu X, Geng J, Guo H, Zhao H, Ai Y (2020) Propofol inhibited apoptosis of hippocampal neurons in status epilepticus through miR-15a-5p/NR2B/ERK1/2 pathway. Cell Cycle 19(9):1000–1011
Nelson SE, Varelas PN (2018) Status epilepticus, refractory status epilepticus, and super-refractory status epilepticus. Continuum (Minneap Minn) 24(6):1683–1707
Pondal-Sordo M, Diosy D, Tellez-Zenteno JF, Girvin JP, Wiebe S (2006) Epilepsy surgery involving the sensory-motor cortex. Brain 129(Pt 12):3307–3314
Qi Y, Qian R, Jia L, Fei X, Zhang D, Zhang Y, Jiang S, Fu X (2020) Overexpressed microRNA-494 represses RIPK1 to attenuate hippocampal neuron injury in epilepsy rats by inactivating the NF-kappaB signaling pathway. Cell Cycle 19(11):1298–1313
Qian X, Wang ZR, Zheng JJ, Ding JQ, Zhong JG, Zhang TY, Li W, Zhang M (2019a) Baicalein improves cognitive deficits and hippocampus impairments in temporal lobe epilepsy rats. Brain Res 1714:111–118
Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK (2019b) Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci 44(1):33–52
Rao VR, Lowenstein DH (2015) Epilepsy. Curr Biol 25(17):R742–R746
Sakaguchi Y, Sakurai Y (2020) Left-right functional difference of the rat dorsal hippocampus for short-term memory and long-term memory. Behav Brain Res 382:112478
Schmidt D, Schachter SC (2014) Drug treatment of epilepsy in adults. BMJ 348:g254
Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127(7):624–633
Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP (2020) NF-kappaB-mediated neuroinflammation in parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox Res 37(3):491–507
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701
Wan G, An Y, Tao J, Wang Y, Zhou Q, Yang R, Liang Q (2020) MicroRNA-129-5p alleviates spinal cord injury in mice via suppressing the apoptosis and inflammatory response through HMGB1/TLR4/NF-kappaB pathway. Biosci Rep 40(3):BSR20193315
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY, Wang DM (2017) miR-15a-5p suppresses endometrial cancer cell growth via Wnt/beta-catenin signaling pathway by inhibiting WNT3A. Eur Rev Med Pharmacol Sci 21(21):4810–4818
Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu J, Yang H, Chen Q, Chen M, Ye L, Wu H, Zhang Q (2020) Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother 121:109627
Wen X, Han XR, Wang YJ, Wang S, Shen M, Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, Hu B, Sun CH, Wu DM, Lu J, Zheng YL (2018) MicroRNA-421 suppresses the apoptosis and autophagy of hippocampal neurons in epilepsy mice model by inhibition of the TLR/MYD88 pathway. J Cell Physiol 233(9):7022–7034
Wu Z, Wu P, Zuo X, Yu N, Qin Y, Xu Q, He S, Cen B, Liao W, Ji A (2017) LncRNA-N1LR Enhances Neuroprotection Against Ischemic Stroke Probably by Inhibiting p53 Phosphorylation. Mol Neurobiol 54(10):7670–7685
Wu H, Tian X, Zhu C (2020) Knockdown of lncRNA PVT1 inhibits prostate cancer progression in vitro and in vivo by the suppression of KIF23 through stimulating miR-15a-5p. Cancer Cell Int 20:283
Xiaoying G, Guo M, Jie L, Yanmei Z, Ying C, Shengjie S, Haiyan G, Feixiang S, Sihua Q, Jiahang S (2020) CircHivep2 contributes to microglia activation and inflammation via miR-181a-5p/SOCS2 signalling in mice with kainic acid-induced epileptic seizures. J Cell Mol Med 24(22):12980–12993
Yan Y, Xia H, Hu J, Zhang B (2019) MicroRNA-542-3p regulates P-glycoprotein expression in rat epilepsy via the toll-like receptor 4/Nuclear Factor-kappaB signaling pathway. Curr Neurovasc Res 16(5):433–440
Ying C, Ying L, Yanxia L, Le W, Lili C (2020) High mobility group box 1 antibody represses autophagy and alleviates hippocampus damage in pilocarpine-induced mouse epilepsy model. Acta Histochem 122(2):151485
Yu Q, Zhao MW, Yang P (2020) LncRNA UCA1 suppresses the inflammation via modulating miR-203-mediated regulation of MEF2C/NF-kappaB signaling pathway in epilepsy. Neurochem Res 45(4):783–795
Zhang G, Zhang Q, Zhu J, Tang J, Nie M (2020) LncRNA ARFRP1 knockdown inhibits LPS-induced the injury of chondrocytes by regulation of NF-kappaB pathway through modulating miR-15a-5p/TLR4 axis. Life Sci 261:118429
Zhao XQ, Tang H, Yang J, Gu XY, Wang SM, Ding Y (2019) MicroRNA-15a-5p down-regulation inhibits cervical cancer by targeting TP53INP1 in vitro. Eur Rev Med Pharmacol Sci 23(19):8219–8229
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: Zhimin Na and Ying Cui; Formal analysis and Data curation: Ying Cui, Chunjie Wei and Shuqiu Wang; Validation and Investigation: Zengmian Wang and Zhimin Na; Writing - original draft preparation and Writing - review and editing: Zengmian Wang, Zhimin Na, and Ying Cui; Approval of final manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the ethical review committee of School of Basic Medicine, Jiamusi University. Written informed consent was obtained from all enrolled patients.
Consent for publication
Patients agree to participate in this work.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Knockdown of ZFAS1 alleviates SE-induced hippocampal neurons injury.
2. ZFAS1 acts as a sponge for miR-15a-5p.
3. MiR-15a-5p targets OXSR1.
4. The ZFAS1/miR-15a-5p/OXSR1 regulates the activity of NF-κB pathway.
Supplementary Information

Supplementary Fig. 1
Anti-miR-miR-15a-5p accelerated SE-stimulated hippocampal neurons injury. Hippocampal neurons from SE mice were transfected with anti-miR-NC or anti-miR-miR-15a-5p. Hippocampal neurons from sham mice were used as control. (A) MiR-15a-5p expression was measured by qRT-PCR. (B) The concentrations of IL-6 and TNF-α were examined by ELISA assay. MTT assay (C) and EdU staining (D) were used to analyze cell viability. (E) Cell apoptosis rate was tested using flow cytometry. (F-G) The productions of SOD and MDA were determined using corresponding Assay Kits. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (PNG 202 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Na, Z., Cui, Y. et al. LncRNA ZFAS1 regulates the hippocampal neurons injury in epilepsy through the miR-15a-5p/OXSR1/NF-κB pathway. Metab Brain Dis 37, 2277–2290 (2022). https://doi.org/10.1007/s11011-022-01013-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01013-5