Abstract
A substantial number of epileptic patients are resistant to the current medication thus necessitating the search for alternative therapies for intractable forms of the disease. Previous studies demonstrated the acute anticonvulsant properties of the methanol extract of the stem bark of Psychotria camptopus (MEPC) in rats. This study investigated the effects of MEPC on pentylenetetrazole-kindled Wistar rats. Kindling was induced by intraperitoneal injection of pentylenetetrazole (37.5 mg/kg) on every alternate day, 1 h after each daily oral pretreatment of rats (8 ≤ n ≤ 10) with MEPC (40, 80 and 120 mg/kg), vehicle or diazepam (3 mg/kg) for 43 days. The kindling development was monitored based on seizure episodes and severity. Rats’ brains were collected on day 43 for the determination of oxidative stress parameters. The histomorphological features and neuronal cell viability of the prefrontal cortex (PFC) and hippocampus were also assessed using H&E and Cresyl violet stains. Chronic administration of pentylenetetrazole time-dependently decreased the latency to myoclonic and generalized seizures, and increased seizure scores and the number of kindled rats. MEPC and diazepam significantly increased the latencies to myoclonic jerks and generalized tonic-clonic seizures. These substances also reduced seizure score and the number of rats with PTZ-kindling. MEPC improved glutathione status and decreased lipid peroxidation in the brains of kindled rats. MEPC also exhibited neuroprotection against pentylenetetrazole-induced hippocampal and PFC neuronal damages. These results suggest that P. camptopus has antiepileptogenic activity, which might be related to the augmentation of antioxidant and neuroprotective defense mechanisms, and further confirm its usefulness in the management of epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a neurological disorder characterized by recurrent seizures, which has been ascribed to abnormal excessive and synchronous neuronal activity in the brain (Fisher et al. 2017). It has been described clinically as occurrence of at least two consecutive unprovoked seizures at interval of 24 h or one unprovoked seizure with the possibility of reoccurrence after ten years (Scheffer et al. 2017). The etiological insult that converts normal brain into an epileptic brain entails a series of epileptogenic events known as epileptogenesis. The latent period between the initial insult and the development of spontaneous recurrent seizure has been reported to range from several weeks in animals to years in humans (Pitkänen et al. 2015; Devinsky et al. 2018). This period is usually characterized by brain remodeling and other neurobiochemical changes in some brain regions that prime the organism for seizure development.
Cumulative evidences from rodent models of epilepsy showed that epileptogenic events and seizure activity are sustained by neuroinflammatory and oxidative stress mechanisms (Vezzani et al. 2012; Taiwe et al. 2016; Ravizza et al. 2017; Rana and Musto 2018; Singh et al. 2018, 2019). In a healthy brain, the levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), are known to be very low and well-regulated to prevent their harmful effects on biological molecules and cell structures (Singh et al. 2018). However, in an epileptic condition, activation of pro-inflammatory pathways and the production of exponential amount of ROS and RNS, and loss of antioxidant mechanisms occurs (Nigar et al. 2016; Pearson-Smith and Patel 2017). This leads to accumulation of ROS and RNS in the brain, which in turns promote membrane lipid peroxidation, mitochondrial DNA damage, depletion of antioxidant defense system and apoptosis (Nigar et al. 2016; Pearson-Smith and Patel 2017; Roganovic et al. 2019). These cellular and molecular changes result in increased seizure activity, neurodegeneration and abnormal synaptic rewiring, which might contribute to disease development and neurological complications associated with epilepsy (Devinsky et al. 2018; Roganovic et al. 2019).
PTZ-kindling is a well-established model to elucidate the pathophysiology of epilepsy and for development of new antiepileptic drugs. One of the advantages of the PTZ-kindling is that it models epileptogenic events and chronic intractable epilepsy (Dhir 2012; Erkec 2015), making it an ideal paradigm for novel antiepileptic drugs discovery. It is known that PTZ kindling induces seizures that are relevant to the human temporal lobe epilepsy (TLE) and generalized tonic-clonic convulsions (Kumar and Kumar 2017). Studies revealed that PTZ-kindling induces neuronal hyperexcitability, neuroinflammation, oxidative stress and cell loss in specific brain regions including the hippocampus and prefrontal cortex (Erkec 2015; Bascuñana et al. 2016; Kola et al. 2017; Zhu et al. 2017; Samokhina and Samokhin 2018).
Over last decade, epilepsy researches have emphasized on the elucidation of molecular pathways and cellular mechanisms that can be targeted in the development of novel therapies. Even with the availability of a stream of new antiepileptic drugs, of which some act on specific molecular targets, many patients still do not achieve adequate seizure control. Indeed, more than 30% of epileptic patients fail to relieve under current medication (Wahab 2010). These drugs are generally anti-symptomatic than disease modifying molecules, and could be classified as anti-seizures instead of antiepileptic drugs (Kubova 2016a; Saletti et al. 2019). The inability of these drugs to modify the core pathological abnormality in epileptic brains, coupled with numerous adverse effects, depressive and cognitive comorbidities indicate the need to search for new medicines for this disease. It is worthy to note that current research trends are directed towards the development of molecules with antioxidant, anti-inflammatory and neuroprotective properties for patients with epilepsy (Tang et al. 2017; Saletti et al. 2019). Indeed, previous preclinical studies have shown that medicinal plants and plant-derived compounds with antioxidant and anti-inflammatory properties exhibited promising antiepileptic effect (Taiwe et al. 2015, 2016; Singh and Goel 2016; Moto et al. 2018).
Our previous studies have demonstrated that the stem bark extracts of P. camptopus has acute anticonvulsant properties against PTZ, strychnine, picrotoxin and thiosemicarbazide-induced seizure in Wistar rats (Fokoua et al. 2021). We proposed in this study to investigate the effects of the methanol extract of Psychotria camptopus on PTZ-kindling, a type of progressive epileptogenic process that closely replicate the human intractable temporal lobe epilepsy, in rats.
Materials and methods
Drug and chemicals
Sodium Hydrogen Phosphate (Cat# BDH9298-500G), Sodium Carbonate (Cat# BDH92284-500G), Formaldehyde (Cat# 10790–708), Potassium Carbonate (Cat# BDH9256-500G) and Sodium Chloride (Cat# BDH9286-500G) were obtained from BDH Poole (England). Trichloroacetic acid (Cat# T6399-100G), Thiobarbituric acid (Cat# T5500-100G), 5,5’-Dithiobis-2-nitrobenzoic acid (Cat# D8130-10G), Sulfanilamide (Cat# S9251-100G), N-(1-Naphthyl)ethylenediamine dihydrochloride (Cat# 222488-25G), Sodium Nitrite (Cat# 237213-5G) and Pentylene tetrazole (Cat# P6500-100G) were purchased from Sigma Aldrich, St Louis, USA.
Plant extraction
The stem barks used in this study were harvested in Wabane highlands forest, South West region, Cameroon with the aid of an ethnobotanist, Dr. Tacham N.W. (Department of Biological Sciences, University of Bamenda). A voucher of the plant was authenticated at the Yaoundé National Herbarium as previously identified by Focho et al. (2009) under the identification number No: 56353/HNC (Focho et al. 2009). The methanol extract was obtained as previously described (Fokoua et al. 2021).
Experimental animals
Male Wistar rats weighting between 140 and 160 g (8–10 weeks old) were used in the study. They were obtained from the Central Animal House, College of Medicine, University of Ibadan, Nigeria and housed for two weeks in the Department of Pharmacology and Therapeutics, College of Medicine, University of Ibadan, Nigeria, prior to the beginning of the study. They were housed in 80 × 50 × 50 cm plastic cages embedded with sawdust. The animals were maintained under natural dark/light cycle and room temperature, with free access to water and standard rodent pellet (Oladekude Food Ltd., Nigeria) except during the treatment and video-recording periods. The animals were handled in accordance with the Guide for the Care and Use of Laboratory Animal published by the United States National Institutes of Health (NIH publication No: 85–23, revised in 1996) (Clark et al. 1997). In addition, the experimental protocols were approved by the Animal Care, Use and Research Ethics Committee of the University of Ibadan (UI-ACUREC/19/00054). All the experiments were conducted between 7:00 and 11:30 AM every day to avoid bias from the circadian rhythm.
Study design
Fifty-six male Wistar rats were randomly divided into six groups. The naive group (n = 8) received vehicle (DMSO 2%, 10 mL/kg). The control group (n = 10) received vehicle (DMSO 2%, 10 mL/kg/day) and intraperitoneal injection of a sub-convulsive dose of PTZ (37.5 mg/kg) on every alternate day till the end of the kindling process (43 days). The diazepam group (n = 8) and the MEPC groups (n = 10 each) were treated orally everyday with Diazepam 3 mg/kg and P. camptopus methanol extract at the doses of 40, 80 or 120 mg/kg respectively. The sample size was determined based on the statistics conveniences, the study design and the ethical guidelines (UI-ACUREC/19/00054), with the prediction that the kindling induction could lead to animal death. The doses used in the present work are from our previous studies (Fokoua et al. 2021). They were selected based on a screening and following the instructions given by the traditional healer.
On PTZ injection days, diazepam and MEPC were administered 1 h prior to PTZ. After the injection of PTZ, rats were individually placed in plexiglas boxes (50 × 40 × 25 cm) and video-monitored during 20 min for seizure behaviours.
Kindling procedure
The standard PTZ kindling protocol previously described by Davoudi et al (Davoudi et al. 2013) was used in this study. Briefly, PTZ-induced kindling model of seizure was induced in adult male Wistar rats by intraperitoneal injection of PTZ (37.5 mg/kg) on alternate days for 43 days. The behaviours of the animals were video monitored for a period of 20 min after each PTZ injection. Seizure behaviour scoring was operated using a revised Racine’s (0–6) scale. The seizure stages were scored and classified as follows: no response (0); ear and facial twitching with sudden arrest behaviour (1); hyperactivity, rearing with convulsive waves through the body (2); rearing and myoclonic jerks (3); clonic-tonic convulsions, turn over into side position (4); generalized clonic-tonic seizures, loss of postural control and wild jumping (5); generalized tonic-clonic seizure followed by death (6) (Corda et al. 1991). Rats were considered fully kindled after at least three consecutive manifestations of stage 4 and/or 5 seizures following injection of PTZ. Animals that did not show any sign of seizure activity within the period (20 min) of observation, received the highest latency corresponding to the duration of the observation.
Preparation for brain tissues for biochemical and histomorphological studies
After the behavioural testing on day 43, 5 animals per group were sacrificed by cervical dislocation and their brains were removed, rinsed and transferred into ice cold sodium phosphate buffer (PBS; 0.1 M, pH = 7.4). The hippocampus and the prefrontal cortex were dissected out and homogenized in PBS. After centrifugation (10,000 rpm, 4 °C, 10 min) (Olugbemide et al. 2021), the supernatant was collected into eppendorf tubes and stored at -20 °C for analysis of oxidative stress biomarkers (MDA, GSH, SOD, CAT and nitrite). The remaining animals (n = 3 or 2) were trans-cardiacly perfused with normal saline followed by 10% neutral buffer formalin (NBF) and the brains were dissected out and stored in 10% NBF fixative for histological analysis.
Evaluation of the anti-oxidant effects of P. camptopus methanol extract in PTZ kindled rats
The concentrations of reduced GSH were determined in the supernatants of hippocampus and prefrontal cortex tissues as described by Jollow et al (Jollow et al. 1974). Glutathione levels were extrapolated from a standard curve of glutathione (0–200 μM) and expressed as μmol/mg protein.
Catalase activity in the samples was estimated by a colorimetric assay based on the stable yellow complex formed with ammonium molybdate and hydrogen peroxide (Góth 1991). Catalase activity in the samples was expressed U/mg protein.
The malondialdehyde (MDA) level used as a marker of lipid peroxidation, was measured in the hippocampus and prefrontal cortex homogenates using the thiobarbituric reacting substances (TBARs) assay as previously described (Tseuguem et al. 2019). The MDA contents were determined using the molar extinction coefficient 1.56 × 105 M/cm and expressed as nmol/mg protein.
The brain level of SOD activity was estimated by a modified method previously described (Tseuguem et al. 2019) based on the superoxide dependent adrenaline auto-oxidation inhibition in a basic medium. The SOD activity expressed as Unit/mg protein, representing the amount of SOD necessary to cause 50% inhibition of the oxidation of adrenaline.
The concentrations of nitrite in the hippocampus and prefrontal cortex samples were used as a mean to evaluate the production of nitric oxide in the brain using the Greiss reagent colorimetric assay protocol (Green et al. 2000). The amount of nitrite in the homogenates were estimated from sodium nitrite standard curve and expressed as μmol/mg protein.
Histomorphological studies
The neutral buffer formalin fixed brains were processed and paraffin embedded. The blocks were then sagittaly sectioned into 4 μm thick, mounted on gelatine-coated charged slide and dried for 2 h at 60 °C in an oven. After dewaxing and rehydration, sections were stained with Haematoxylin & Eosin (H&E) or Cresyl violet stains, rehydrated and finally cover-slipped and observed at 100 and 400X magnification using a Leica DM500 microscope equipped with a Leica ICC50 E camera for image acquisition. For each rat, we performed 3 different microphotographies per region of interest in each brain hemisphere. That means for each animal per region of interest, we had 3 × 2 = 6 different values. An average was made per region of interest and per animal and thereafter, the mean of the group was determined and plotted as bar graph. The Nissl staining sections were used for neuronal cell counting, using the automated cell counting plugin of the FIJI ImageJ software. Neuronal damages and alterations were examined on the H&E sections. The histomorphological alterations in the prefrontal cortex, CA1 and CA3 hippocampus regions were identified by observing and quantifying one or more of the following damages: the cytoplasmic vacuolation and cytoplasmic eosinophilia, nuclear chromatin clumping and fragmentation, condensed cytoplasm and fragmentation of the neuronal cells (Singh et al. 2018).
Statistical analysis
The normality test was performed with the QQ plot for data with two variables. For data with one variable, normality was tested using Shapiro-Wilk and Kolmogorov-Smirnov tests (Results are reported in the supplementary file). Parametric data are expressed as means ± standard deviation (SD) and analyzed using one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparisons test. Non-parametric data are reported as median and interquartile range and analyzed using Dunn’s test. All data analysis were performed with Graph Pad Prism Software, version 8.4. Values were considered statistically significant at p ˂ 0.05.
Results
P. camptopus methanol extract reduces seizure scores in PTZ kindled rats
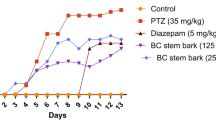
This study was carried out to evaluate the antiepileptogenic effect of the plant extract. The seizure stages or scores were different in all the groups during the PTZ-kindling process as shown in Fig. 1. Repeated injections of PTZ (37.5 mg/kg, i.p) on alternate days for 43 days produced a gradual increase in seizure scores, cumulative seizure episodes in rats (Fig. 1a-b) and up to 30% animal death (Supplementary Table S1). However, the administration of MEPC at all the tested doses and diazepam significantly [two way ANOVA main effects time (F (11, 432) = 55.36, p < 0.0001), treatment (F (5, 432) = 308.3, p < 0.0001) and interaction (F (55, 432) = 3.132, p < 0.0001)] reduced the seizure scores in PTZ-kindled rats, with MEPC at 120 mg/kg being the most active (Fig. 1a). Figure 1b represents the area under the curve plotted from the curve of the seizure scores and it clearly shows the dose-dependent anti-kindling effect of MEPC. Diazepam also significantly [Dunnett’s multiple comparisons test (F (5, 36) = 46.66] (p < 0.0001) reduced the seizure severity and prevented kindling as evidenced by the reduced area under the curve.
Effects of Psychotria camptopus methanol extract on seizure stage during the PTZ-kindling in Wistar rats. (a) Seizure scores evolution during kindling. (b) Area under the curve of the seizure score graph, expressing the cumulative seizure scores for each group. Each point in panel A represents the mean ± SD of 7 animals/group. *p < 0.05, **p < 0.01 and ***p < 0.001 relative to PTZ control (DMSO 2%). Panel (a) was analysed with two-way ANOVA followed by Dunnett’s multiple comparisons test while panel (b) was analysed using one-way ANOVA followed by Dunnett’s multiple comparisons test
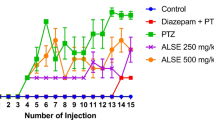
P. camptopus methanol extract increases the latency to convulsions and reduces the number of rats with PTZ-kindling
To determine the effect of repeated administration MEPC on the disease severity and the global response to treatment, we evaluated the latency to convulsion and the percentage of animal fully kindled. As shown in Fig. 2A, repeated intraperitoneal administration of PTZ (37.5 mg/kg) induced myoclonic jerks and tonic-clonic convulsions respectively at the stage 3 and 4 of the PTZ-kindling in rats. Daily pre-treatment of the rats with the plant extract or diazepam significantly [two way ANOVA main effects time (F (5, 216) = 26.78, p < 0.0001), treatment (F (5, 216) = 33.08, p < 0.0001) and interaction (F (25, 216) = 1.760, p = 0.0175)] delayed the latencies to myoclonic jerks and tonic-clonic convulsions when compared with the PTZ control group (Fig. 2A). With the time point analysis, only MEPC (120 mg/kg) and diazepam could significantly [Dunnett’s multiple comaprisons test] (p = 0.0036) reduce the pathology occurrence.
Effects of Psychotria camptopus extract on stage 3 or 4 latency (a) and the percentage of fully kindled rats (b) during the PTZ-kindling process. Each point or bar represents the mean ± SD of 7 animals/group. *p < 0.05, **p < 0.01 and ***p < 0.001 relative to PTZ control (DMSO2%). Panel A was analysed with two-way ANOVA followed by Dunnett’s multiple comparisons test while panel B was analysed using Chi square test
As presented in Fig. 2B, 85.7% of rats treated with intraperitoneal injection of PTZ developed kindling behaviour. However, the plant extract and diazepam significantly [[chi]2 (df, N = 30) = 1953] (p < 0.001) reduced the percentage of PTZ- kindled rats.
P. camptopus methanol extract reduces MDA and nitrite contents in the brains of PTZ-kindled rats
The extension of the oxidative/nitrosative stress was evaluated by assessing the MDA and nitrite contents in the regions of interest of the brain. PTZ-kindling rats had increased MDA and nitrite contents in the PFC and the hippocampus when compared with vehicle (Fig. 3). Repeated oral administration of rats with MEPC significantly reduced the MDA (Fig. 3A-B) level in both pre-frontal cortex and hippocampus [Dunnett’s multiple comparisons test] (p = 0.001). The nitrite content (Fig. 3C-D) was also significantly [Dunnett’s multiple comparisons test] (p = 0.0044) reduced by the MEPC treatment. Diazepam was only able to significantly [Dunnett’s multiple comparisons test] (p = 0.0156) reduce the MDA content in the pre-frontal cortex (Fig. 3A).
Effects of the methanol extract of P. camptopus on MDA (a-b) and nitrite (c-d) levels in the prefrontal cortex (PFC) and hippocampus of PTZ kindled rats. Each bar represents the mean ± SD of 5 animals/group. *p < 0.05, **p < 0.01 and ***p < 0.001 relative to PTZ control. Data were analysed using one-way ANOVA followed by Dunnett’s multiple comparisons test
P. camptopus methanol extract boosts antioxidant profiles in the brains of PTZ-kindled rats
To examine the effect of MEPC on the endogenous antioxidant system, we assessed the catalase and superoxide dismutase activities as well as the GSH content. As presented in Figs. 4, repeated injection of sub-convulsive dose of PTZ significantly decreased the antioxidant enzymes (CAT and SOD) and GSH contents in the PFC and the hippocampus [Dunnett’s multiple comparisons test] (p from 0.0444 to <0.001) when compared with the naive control. Administration of the extract (120 mg/kg) significantly augmented CAT activity in the hippocampus [Dunnett’s multiple comparisons test] (p = 0.0039) but not in the PFC (Fig. 4A-B). MEPC did not exert any significant effect on the SOD activity (Fig. 4C-D) and GSH levels (Fig. 4E-F) in the PFC and hippocampus of rats when compared with PTZ kindling group.
Effects of the methanol extracts of P. camptopus on catalase activity (a-b), super oxide dismutase activity (c-d) and reduced-Glutathione (e-f) in the prefrontal cortex (PFC) and hippocampus of PTZ-kindled rats. Each bar represents the mean ± SD of 5 animals/group. *p < 0.05, **p < 0.01 and ***p < 0.001 relative to PTZ control. Data were analysed using one-way ANOVA followed by Dunnett’s multiple comparisons test
P. camptopus exhibits neuroprotective effect against PTZ-kindling in rats
The H&E and Nissl staining were used to evaluate the neuroprotective effects of MEPC on brain cells and neurons, respectively. H&E staining of the PFC and the hippocampus (CA3) regions showed normal morphological features of the PFC and hippocampus in naive rats. In contrast, PTZ-kindling (control) resulted in marked neuropathological alterations in the PFC with increased number of nuclear pyknosis and cytoplasmic vacuolation. There were also distortions of the hippocampus CA3 and CA1 cells in PTZ-kindled rats, with cellular disorganization (Fig. 5A). These damages were reduced by the treatment with the plant extract at the dose of 120 mg/kg. Diazepam (3 mg/kg) failed to restore the cellular architecture of the hippocampal neurons both in the CA1 and CA3 (Fig. 5). Quantitative and qualitative analysis of the H&E sections revealed that PTZ kindling reduced cell density in the PFC and hippocampus CA3 of rats as compare to naive group. Also, this cell lost was correlated to an increase of cell damages as the percentage of altered cells increased in the PTZ-control group as compare to the naive animals. As observed on Fig. 5B, MEPC and DZP pretreatments prevented cell lost in the PFC and Hippocampus hCA1 and hCA3 regions as compare to the control group.
Effect of extract of P. camptopus on histomorphological changes of H&E stained sections of the prefrontal cortex and hippocampus of PTZ-kindled rats. (a)-Slices from naive group revealed normal histological open chromatin pattern and cytoarchitecture (white arrows) of viable neurons in all the brain regions observed. Control slices showed abnormal histomorphological features, with some pyknotic cells (black arrows), increased cytoplasmic vacuolations (arrow heads) and cell disorganization (red arrow) in the hCA3. Slices from DZP 3 mg/kg and MEPC 120 mg/kg treated rats presented ameliorated histomorphological distortions with reduced cellular damages. Original magnification X400, Calibration bar = 50 μm for all microphotographs. (b)- Number of cells and damaged cells in the PFC and hippocampus CA1 and CA3 regions. Each bar represents the mean of microphotographs per group (n = 3 or 2, for each animal 3 different microphotographs per region of interest × 2 hemispheres)
The Nissl staining was performed to focus on neuronal cells. This staining revealed normal cell morphology and distribution in the prefrontal cortex and the hippocampus from naive rats. Altered neuronal cells morphology and organization was observed in PTZ-kindling rat and these changes were attenuated by the methanol extract of P. Camptopus (120 mg/kg). Quantitative analysis of these brain sections showed that PTZ kindling significantly reduced the number of viable neuronal cells in the PFC and hippocampus CA3 when compared with Naive (Fig. 6A and B). However, treatment with the extract of P. Camptopus (120 mg/kg) significantly attenuated neuronal cell death in these brain regions. Diazepam significantly prevented cell death in the PFC and the hCA1. This substance instead tends to worsen the condition in the CA3 region (Fig. 6A and B).
Effect of P. camptopus methanol extract on histomorphological changes of Nissl stained section of the prefrontal cortex and the hippocampus of PTZ-kindled rats. (a)-Microphotograhs of PFC and hippocampus sections. Slices from naive rats revealed normal histological open chromatin pattern and cytoarchitecture of viable neurons (white arrow) and a normal cellular organization of the hCA3. Control slices showed abnormal histomorphological features, with some pyknotic cells (black arrows) and loss of cellular organization in the hCA3 (red arrow). Diazepam (3 mg/kg) and MEPC 120 mg/kg revealed ameliorative features of the alterations induced by kindling. Original magnification X40, Calibration bar = 50 μm for all figures. (b)- Number of Nissl stained neurons in the PFC and hippocampus CA1 and CA3 regions. Each bar represents the mean of microphotographs per group (n = 2 or 3, for each animal 3 different microphotographs per region of interest × 2 hemispheres
Discussion
The present study was undertaken to evaluate the antiepileptogenic and neuroprotective effects of the methanol extract from the stem bark of P. camptopus (MEPC) in PTZ-induced kindled rats. Chronic administration of pentylenetetrazole time-dependently decreased the latency to myoclonic and generalized seizures. An increase in seizure scores and kindled rats number was also observed. MEPC and diazepam significantly increased the latencies to myoclonic jerks and generalized tonic-clonic seizures. These substances also reduced seizure score and the number of rats with PTZ-kindling. MEPC improved glutathione status and decreased lipid peroxidation in the brains of kindled rats. MEPC also exhibited neuroprotective activity against pentylenetetrazole-induced hippocampal and PFC neuronal damages.
Pentylenetetrazole-induced kindling is a type of progressive epileptogenic process and a well-established model in rodents that closely replicates the pathogenesis of refractory epilepsy in humans. It is also used to elucidate neurological complications associated with epilepsy, including oxidative stress (Taiwe et al. 2016) and neurodegeneration (Erkec 2015; Singh et al. 2018). These characteristics justify the use of pentylenetetrazole-induced kindling model in the present study. The inability of most antiepileptic drugs to modify the changes in oxidative stress in epileptic brain may perhaps contribute to their ineffectiveness in certain patients, who still experienced seizures despite treatments (Kubova 2016b; Tang et al. 2017). Thus, phytochemicals with antioxidant and neuroprotective properties are increasingly being proposed as promising strategy for treatment of epilepsy particularly the intractable forms (Ashrafi et al. 2007; Dariani et al. 2013; Annamaria Vezzani 2014; Tang et al. 2017). The results of this study showed that the methanol extract of P. camptopus exhibited antiepileptogenic effect against PTZ-induced kindling as evidenced by increased latency to myoclonic jerks and tonic-clonic convulsions, reduced seizure scores, and number of rats with seizure episodes. The extract also reduced MDA and nitrite levels in the PFC and hippocampus of PTZ-kindled rats. The altered histomorphological features of the PFC and the hippocampus of rats with PTZ-kindling were ameliorated by the plant extract.
The kindling process results in gradual increase in seizure susceptibility and amplification of seizure activity that often culminate in generalized tonic-clonic seizures (Davoudi et al. 2013; Shimada and Yamagata 2018). In this model, antiepileptogenic activity is proven based on the ability of the test compound to prolong the latency to myoclonic and generalized seizures, to reduce seizure scores and the number of kindled rats. Our data showed that the extract of P. camptopus increased the latencies to myoclonic jerks and generalized tonic-clonic seizures, decreased seizure scores and reduced the number of rats with PTZ-kindling. Thus, suggesting that the extract of P. camptopus possesses antiepileptogenic activity and further support its use as a remedy for generalized tonic-clonic seizures in ethnomedicine. These findings are in accordance with our previous study that demonstrated the anticonvulsant effects of the same extract on acute models of convulsion (Fokoua et al. 2021).
It has been reported that PTZ-kindling results in an increase in brain levels of glutamate, increased NO-mediated activation of NMDA receptors and decreased brain GABA levels (De Luca et al. 2005; Abdel-Zaher et al. 2017) that play a vital role in epileptogenesis and seizure activity (Kumar and Kumar 2017). The increase in the excitability of neurons due to antagonism of GABA and activation of NMDA receptors results in copious production of free radicals and oxidative stress-mediated neuronal cell loss (Roganovic et al. 2019). Indeed, there are increasing evidences implicating oxidative stress in the development and progression of epilepsy (Waldbaum and Patel 2010; Geronzi et al. 2018). In fact, the hyper-excitability of neurons in the process or during persistent seizure distorts the antioxidant-oxidant equilibrium. This imbalance causes neuronal cells injuries through membrane peroxidative activity of ROS and RNS (Ilhan et al. 2005; Roganovic et al. 2019). Brain tissues are extremely susceptible to oxidative stress due to high metabolism, increased blood perfusion, enriched membrane lipids and reduced antioxidant defense mechanisms (Ilhan et al. 2005; Nigar et al. 2016; Pearson-Smith and Patel 2017). The oxidative stress status will lead to membrane lipid peroxidation with increased MDA content, biological molecule oxidation, DNA alteration and other cell damages, which ultimately culminate into neuronal cell death (Waldbaum and Patel 2010; Snehunsu et al. 2015; Pearson-Smith and Patel 2017). The findings that increased free radicals activity and lowered endogenous antioxidant molecules correlated with decreased seizure threshold and neuronal cell loss further support the role of oxidative stress in the pathophysiology of epilepsy (Nigar et al. 2016; Abdel-Zaher et al. 2017; Pearson-Smith and Patel 2017; Roganovic et al. 2019). The cortex and limbic structures including the prefrontal cortex, amygdala and the hippocampus have been reported to be the most vulnerable brain regions during epileptogenesis (Roganovic et al. 2019). These prompted us to evaluate whether the antiepileptogenic effects of MEPC could be related to a probable antioxidant effect in target structures, namely the prefrontal cortex and the hippocampus.
In agreement with the literature, repeated administration of sub-convulsive dose of PTZ resulted in oxidative stress, as depicted by elevated MDA and nitrite levels accompanied by decreased antioxidant (GSH, CAT and SOD) contents in the PFC and hippocampus of PTZ-kindled rats. MEPC significantly reduced MDA in these brain regions. Besides, MEPC significantly increased the catalase activity in the hippocampus as compared to the PTZ-kindling rats. These results suggest in vivo antioxidant property of MEPC, which might contribute to its antiepileptogenic effect.
It is worth noting that MEPC did not have any significant effect on the GSH content, neither on the SOD activity, suggesting that the plant extract might be unable to boost these endogenous antioxidant parameters. The potentiation of the catalase activity alone might be insufficient to justify the potent effect of the plant extract against lipid peroxidation. It could be hypothesized that MEPC possess intrinsic antioxidant activity, serving as primary antioxidant substance.
Studies have revealed increased cell damages in the frontal cortex and hippocampus of animals after PTZ kindling (Samokhina and Samokhin 2018). Specifically, PTZ kindling has been shown to cause cell damages and reduced neuronal cell density in PFC, CA1 and CA3 brain regions (Snehunsu et al. 2015; Vasil’ev et al. 2015; Aldawsari et al. 2017; Erkec et al. 2018; Muke et al. 2018; Samokhina and Samokhin 2018; Singh et al. 2018, 2019). Concordantly, results from the present study showed a close correlation between seizure severity, oxidative stress and neuronal cell damages in the PFC, CA1 and CA3 of rats with PTZ kindling. The fact that MEPC ameliorates the histomorphological distortions and reduced the loss of PFC and hippocampal neuronal cells suggest it has neuroprotective activity. Pentylenetetrazol initially blocks GABAA receptors and increases glutamate production by reducing the inhibitory GABAergic influxes (Lin et al. 2019). This results in excitotoxicity-induced cell damages. It is reported that establishment of functional GABAergic system and restoration of the inhibitory/excitatory balance attenuates seizure sensitivity and prevents neurons damages during epileptogenesis (Moto et al. 2018; Lin et al. 2019; Righes Marafiga et al. 2020; Sünnetçi et al. 2021). It is worthy to note that previous studies have also established the presence in MEPC of bioactive compounds with neuroprotective and antioxidant properties. Furthermore, MEPC was able to inhibit thiosemicarbazide- and picrotoxin-induced seizures in wistar rats (Fokoua et al. 2021). In this study, we found that MEPC protects nervous cells from PTZ-induced cell damages, suggesting a neuroprotective effect that may be mediated trough GABAergic modulation and antioxidant effects. However, the relevance of these bioactive compounds in the antiepileptogenic and neuroprotective activities of P. camptopus against PTZ-induced kindling requires further investigations.
Strengths of the present study are the clear demonstration of the anti-epileptogenic and neuroprotective effects of the methanol extract of P. camptopus at the doses that are in the pharmacological range. The study also demonstrated the antioxidant effect of the plant extract and linked it to the anti-epileptogenic and neuroprotective activities observed.
Its limitations are the lack of precise mechanism underlying the antiepileptogenic and neuroprotective effects of the plant extract. In addition, the effects of the plant extract on neuropsychiatric and cognitive alterations were not assessed. However, these limitations are currently under investigation in our laboratory.
Conclusion
The results of this study showed that the methanol extract of Psychotria camptopus stem bark exhibited antiepileptogenic and neuroprotective activities against PTZ-induced kindling in rats. The antioxidant effects of this plant extract may contribute to its antiepileptogenic and neuroprotective activities. These findings support the potential of Psychotria camptopus in the management of epilepsy and especially the intractable forms.
Abbreviations
- MEPC:
-
Methanol extract of the stem bark of Psychotria camptopus
- PFC:
-
prefrontal cortex
- ROS:
-
reactive oxygen species
- RNS:
-
reactive nitrogen species
- PTZ:
-
pentylenetetrazole
- TLE:
-
temporal lobe epilepsy
- NBF:
-
neutral buffer formalin
- MDA:
-
malondialdehyde
- GSH:
-
glutathione
- SOD:
-
superoxide dismutase
- CAT:
-
catalase
References
Abdel-Zaher AO, Farghaly HSM, Farrag MMY et al (2017) A potential mechanism for the ameliorative effect of thymoquinone on pentylenetetrazole-induced kindling and cognitive impairments in mice. Biomed Pharmacother 88:553–561. https://doi.org/10.1016/j.biopha.2017.01.009
Aldawsari HM, Eid BG, Neamatallah T et al (2017) Anticonvulsant and Neuroprotective Activities of Phragmanthera austroarabica Extract in Pentylenetetrazole-Kindled Mice Evidence-based. Complement Altern Med:2017. https://doi.org/10.1155/2017/5148219
Ashrafi MR, Shams S, Nouri M et al (2007) A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia 48:1750–1755. https://doi.org/10.1111/j.1528-1167.2007.01143.x
Bascuñana P, Javela J, Delgado M et al (2016) [18F]FDG PET neuroimaging predicts Pentylenetetrazole (PTZ) kindling outcome in rats. Mol Imaging Biol 18:733–740. https://doi.org/10.1007/s11307-016-0950-0
Clark JD, Gebhart GF, Gonder JC et al (1997) The 1996 guide for the care and use of laboratory animals. ILAR J 38:41–48. https://doi.org/10.1093/ilar.38.1.41
Corda MG, Orlandi M, Lecca D et al (1991) Pentylenetetrazol-induced kindling in rats: effect of GABA function inhibitors. Pharmacol Biochem Behav 40:329–333. https://doi.org/10.1016/0091-3057(91)90562-G
Dariani S, Baluchnejadmojarad T, Roghani M (2013) Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J Mol Neurosci 51:679–686. https://doi.org/10.1007/s12031-013-0043-3
Davoudi M, Shojaei A, Palizvan MR et al (2013) Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res 106:54–63. https://doi.org/10.1016/j.eplepsyres.2013.03.016
De Luca G, Di Giorgio RM, Macaione S et al (2005) Amino acid levels in some lethargic mouse brain areas before and after pentylenetetrazole kindling. Pharmacol Biochem Behav 81:47–53. https://doi.org/10.1016/j.pbb.2005.02.012
Devinsky O, Vezzani A, O’Brien TJ et al (2018) Epilepsy. Nat Rev Dis Prim 3:1–24. https://doi.org/10.1038/nrdp.2018.24
Dhir A (2012) Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci 1:1–12. https://doi.org/10.1002/0471142301.ns0937s58
Erkec EO (2015) Pentylenetetrazol Kindling Epilepsy Model. J Turkish Epilepsi Soc 21:6–12. https://doi.org/10.5505/epilepsi.2015.08108
Erkec OE, Arihan O, Kara M et al (2018) Effects of Leontice leontopetalum and Bongardia chrysogonum on oxidative stress and neuroprotection in PTZ kindling epilepsy in rats. Cell Mol Biol 64:71–77. https://doi.org/10.1079/9780851990804.0013
Fisher RS, Cross JH, French JA et al (2017) Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:522–530. https://doi.org/10.1111/epi.13670
Focho DA, Ndam WT, Fonge BA (2009) Medicinal plants of Aguambu - Bamumbu in the Lebialem highlands, southwest province of Cameroon. African J Pharm Pharmacol 3:001–013
Fokoua AR, Ndjenda MK, Kaptué Wuyt A et al (2021) Anticonvulsant effects of the aqueous and methanol extracts from the stem bark of Psychotria camptopus Verdc. (Rubiacaea) in rats. J Ethnopharmacol 272:113955. https://doi.org/10.1016/j.jep.2021.113955
Geronzi U, Lotti F, Grosso S (2018) Oxidative stress in epilepsy. Expert Rev Neurother 18:427–434. https://doi.org/10.1080/14737175.2018.1465410
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151. https://doi.org/10.1016/0009-8981(91)90067-M
Green MF, Kern RS, Braff DL, Mintz J (2000) Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26:119–136
Ilhan A, Gurel A, Armutcu F et al (2005) Antiepileptogenic and antioxidant effects of Nigella sativa oil against pentylenetetrazol-induced kindling in mice. Neuropharmacology 49:456–464. https://doi.org/10.1016/j.neuropharm.2005.04.004
Jollow D, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Kola PK, Akula A, NissankaraRao LS, Danduga RCSR (2017) Protective effect of naringin on pentylenetetrazole (PTZ)-induced kindling; possible mechanisms of antikindling, memory improvement, and neuroprotection. Epilepsy Behav 75:114–126. https://doi.org/10.1016/j.yebeh.2017.07.011
Kubova H (2016a) Side effects of antiepileptic drugs. In: Talevi a, Rocha L (eds) antiepileptic drug discovery., methods in. Humana press, New York, New York, NY, pp 329–250
Kubova H (2016b) Chapter 17 side effects of antiepileptic drugs. doi: https://doi.org/10.1007/978-1-4939-6355-3
Kumar M, Kumar P (2017) Protective effect of spermine against pentylenetetrazole kindling epilepsy induced comorbidities in mice. Neurosci Res 120:8–17. https://doi.org/10.1016/j.neures.2017.02.003
Lin X, Cui Y, Wang L, Chen W (2019) Chronic exercise buffers the cognitive dysfunction and decreases the susceptibility to seizures in PTZ-treated rats. Epilepsy Behav 98:173–187. https://doi.org/10.1016/j.yebeh.2019.07.032
Moto FCO, Arsa’a A, Ngoupaye GT et al (2018) Anxiolytic and antiepileptic properties of the aqueous extract of Cissus quadrangularis (Vitaceae) in mice pilocarpine model of epilepsy. Front Pharmacol 9:1–10. https://doi.org/10.3389/fphar.2018.00751
Muke S, Kaikini A, Peshattiwar V et al (2018) Neuroprotective effect of coumarin nasal formulation: kindling model assessment of epilepsy. Front Pharmacol 9:1–16. https://doi.org/10.3389/fphar.2018.00992
Nigar S, Pottoo F, Tabassum N et al (2016) Molecular insights into the role of inflammation and oxidative stress in epilepsy. J Adv Med Pharm Sci 10:1–9. https://doi.org/10.9734/jamps/2016/24441
Olugbemide AS, Ben-Azu B, Bakre AG et al (2021) Naringenin improves depressive- and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res Bull 169:214–227. https://doi.org/10.1016/j.brainresbull.2020.12.003
Pearson-Smith JN, Patel M (2017) Metabolic dysfunction and oxidative stress in epilepsy. Int J Mol Sci 18:1–13. https://doi.org/10.3390/ijms18112365
Pitkänen A, Lukasiuk K, Dudek FE, Staley KJ (2015) Epileptogenesis. Cold Spring Harb Perspect Med 5:1–18. https://doi.org/10.1101/cshperspect.a022822
Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflammation 15:1–12
Ravizza T, Balosso S, Marchi N (2017) Experimental models of inflammation in epilepsy research. In: Models of seizures and epilepsy, Second edn. Elsevier Inc., Second Edi, pp 961–974
Righes Marafiga J, Vendramin Pasquetti M, Calcagnotto ME (2020) GABAergic interneurons in epilepsy: more than a simple change in inhibition. Epilepsy Behav
Roganovic M, Pantovic S, Dizdarevic S (2019) Role of the oxidative stress in the pathogenesis of epilepsy. Neurol Sci Neurophysiol 36:1–8. https://doi.org/10.5152/NSN.2019.11632
Saletti PG, Ali I, Casillas-Espinosa PM et al (2019) In search of antiepileptogenic treatments for post-traumatic epilepsy. Neurobiol Dis 123:86–99
Samokhina E, Samokhin A (2018) Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int J Neurosci 128:1086–1096. https://doi.org/10.1080/00207454.2018.1481064
Scheffer IE, Berkovic S, Capovilla G et al (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521. https://doi.org/10.1111/epi.13709
Shimada T, Yamagata K (2018) Pentylenetetrazole-induced kindling mouse model. J Vis Exp 2018:e56573. https://doi.org/10.3791/56573
Singh D, Goel RK (2016) Anticonvulsant mechanism of saponins fraction from adventitious roots of Ficus religiosa: possible modulation of GABAergic, calcium and sodium channel functions. Rev Bras Farmacogn 26:579–585. https://doi.org/10.1016/j.bjp.2015.10.007
Singh N, Saha L, Kumari P et al (2019) Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res Bull 144:233–245. https://doi.org/10.1016/j.brainresbull.2018.11.013
Singh N, Vijayanti S, Saha L et al (2018) Neuroprotective effect of Nrf2 activator dimethyl fumarate, on the hippocampal neurons in chemical kindling model in rat. Epilepsy Res 143:98–104. https://doi.org/10.1016/j.eplepsyres.2018.02.011
Snehunsu A, Ghosal C, Kandwal M et al (2015) 1-Triacontanol cerotate; isolated from Marsilea quadrifolia Linn. Ameliorates reactive oxidative damage in the frontal cortex and hippocampus of chronic epileptic rats. J Ethnopharmacol 172:80–84. https://doi.org/10.1016/j.jep.2015.06.020
Sünnetçi E, Solmaz V, Erbaş O (2021) Chronic oxytocin treatment has long lasting therapeutic potential in a rat model of neonatal hypercapnic-hypoxia injury, through enhanced GABAergic signaling and by reducing hippocampal gliosis with its anti-inflammatory feature. Peptides 135. https://doi.org/10.1016/j.peptides.2020.170398
Taiwe GS, Moto FCO, Ayissi ERM et al (2015) Effects of a lyophilized aqueous extract of Feretia apodanthera Del. (Rubiaceae) on pentylenetetrazole-induced kindling, oxidative stress, and cognitive impairment in mice. Epilepsy Behav 43:100–108. https://doi.org/10.1016/j.yebeh.2014.11.022
Taiwe GS, Moto FCO, Pale S et al (2016) Extracts of Feretia apodanthera Del. Demonstrated anticonvulsant activities against seizures induced by chemicals and maximal electroshock. Epilepsy Res 127:30–39. https://doi.org/10.1016/j.eplepsyres.2016.08.009
Tang F, Hartz AMS, Bauer B (2017) Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol 8:1–19. https://doi.org/10.3389/fneur.2017.00301
Tseuguem PP, Nguelefack TB, Piégang BN, Mbankou Ngassam S (2019, 2019) Aqueous and methanol extracts of Paullinia pinnata (Sapindaceae) improve monosodium Urate-induced gouty arthritis in rat: analgesic, anti-inflammatory, and antioxidant effects. Evidence-based Complement Altern Med. https://doi.org/10.1155/2019/5946291
Vasil’ev DS, Tumanova NL, Lavrent’eva VV et al (2015) The ability of NMDA-type glutamate receptor blockers to prevent the development of Pentylenetetrazole kindling and morphological changes to pyramidal neurons in the mouse Hippocampus. Neurosci Behav Physiol 45:528–535. https://doi.org/10.1007/s11055-015-0106-8
Vezzani A (2014) Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr 14:3–7
Vezzani A, Balosso S, Ravizza T (2012) Inflammation and epilepsy. In: handbook of clinical neurology, 1st edn. Elsevier B.V., pp 163–175
Wahab A (2010) Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals 3:2090–2110. https://doi.org/10.3390/ph3072090
Waldbaum S, Patel M (2010) Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res 88:23–45. https://doi.org/10.1016/j.eplepsyres.2009.09.020
Zhu X, Dong J, Han B et al (2017) Neuronal nitric oxide synthase contributes to PTZ kindling-induced cognitive impairment and depressive-like behavior. Front Behav Neurosci 11:1–12. https://doi.org/10.3389/fnbeh.2017.00203
Acknowledgements
The authors are grateful to the West African Research Association (WARA) for the financial support through the WARA travel grant 2019. We thank the University of Ibadan, Oyo state, Nigeria for the facilities provided.
Data availability
All the data and material from this study are available on demand.
Funding
This work benefits from the support of the West African Research Association (WARA) through the WARA Travel Grant 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The experimental protocols were approved by the Animal Care, Use and Research Ethics Committee of the University of Ibadan (UI-ACUREC/19/00054).
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 2806 kb)
Rights and permissions
About this article
Cite this article
Fokoua, A.R., Ajayi, A.M., Ben-Azu, B. et al. The antioxidant and neuroprotective effects of the Psychotria camptopus Verd. Hook. (Rubiaceae) stem bark methanol extract contributes to its antiepileptogenic activity against pentylenetetrazol kindling in male Wistar rats. Metab Brain Dis 36, 2015–2027 (2021). https://doi.org/10.1007/s11011-021-00825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00825-1