Abstract
Alchemilla kiwuensis Engl. (Rosaceae) (A. kiwuensis) is an herbaceous plant traditionally used by Cameroonians to treat epilepsy and other central nervous system disorders. The present study evaluated the antiepileptogenic and antiepileptic effects of A. kiwuensis (40 mg/kg, 80 mg/kg) following Pentylenetetrazole (PTZ)-induced kindling as well as its sub-chronic toxicity. Following an initial i.p administration of a challenge dose (70 mg/kg), Wistar rats of both sexes received sub convulsive doses (35 mg/kg) of PTZ every other day, one hour after the oral gavage of animals with treatments, until two consecutive stage 4, in all animals of negative control. Seizure progression, latency, duration, and repetition were noted. Twenty-four hours later, animals were dissected to extract their hippocampi. The resulting homogenates were used to evaluate Malondialdehyde, reduced glutathione, catalase activity, GABA, GABA-Transaminase, glutamate, glutamate transporter 2, IL-1β and TGF-1 β. Sub-chronic toxicity was conducted according to OECD 407 guidelines. The lyophilisate of A. kiwuensis significantly increased the latency of seizure appearance, delayed seizure progression and decreased seizure repetition and duration. Biochemical analysis revealed that the lyophilisate significantly increased the catalase activity, reduced glutathione, GABA, glutamate transporter 2 and TGF-1B levels. The lyophilisate equally caused a significant decreased in the GABA-Transaminase activity, malondialdehyde, and IL-1 β levels. There was no noticeable sign of toxicity. A. kiwuensis possesses antiepileptic and antiepiletogenic effects by enhancing GABAergic neurotransmission and antioxidant properties, coupled to modulation of glutamatergic and neuroinflammatory pathways and is innocuous in a sub-chronic model. These justifies its local use for the treatment of epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common neurological disorders with a high global disability and mortality burden [1]. It is a chronic neurological disorder characterized by recurrent and unprovoked seizures [2, 3]. The clinical hallmark of epilepsy is a seizure, which represents the brain’s uncontrolled and abnormal electrical activity that may cause motor alterations and changes in the level of consciousness and behaviour [4, 5]. An unavoidable aspect of the pathophysiology of epilepsy is the homeostatic imbalance observed between inhibitory and excitatory currents in favour of excitatory currents. Glutamate is the major excitatory neurotransmitter in the CNS and accounts for about 50% of all the CNS’ synaptic transmission [6, 7]. It is, however, subject to several homeostatic mechanisms, such as the activity of its transporters, mainly glutamate transporter 2 (or excitatory amino acid transporter 2: EAAT-2), which is responsible for its synaptic clearance following its release [8]. The role of GABAergic-neurotransmission in the pathology of epilepsy has been well documented [9,10,11]. GABA represents the major inhibitory neurotransmitter whose main role is to dampen excitatory tone. Its transmission is also contingent on homeostatic mechanisms such as the catabolic activity of GABA transaminase, which is responsible for its synaptic clearance [12,13,14]. In case of homeostatic failure of the inhibitory/excitatory balance, an apparent latent period known as epileptogenesis characterised by plastic changes that modify a normal brain into an epileptic brain, will take over [15,16,17]. The subsequent enhanced glutamatergic transmission triggers several cascades of which, neuroinflammation and oxidative stress appear to be the most important and the most targeted by researchers [18, 19].
Kindling is a phenomenon which progressively alters brain structures rendering it susceptible to unprovoked seizures. Kindling models originally described by Goddaard et al. [20] have been extensively used over the past 30 decades to screen potential anti-epileptic drugs [21, 22]; with PTZ-induced kindling taking the lead over the other methods [23,24,25]. PTZ kindling produces robust and reproducible sequences of molecular and cellular alterations in neuronal circuits, which enables the study of the mechanisms of epileptogenesis [25, 26]. This model can produce distortions in both GABA-transmission and glutamate-transmission [27, 28], inducing the production of pro-inflammatory and pro-oxidative cascades [22, 29,30,31].
Modern medicine provides several antiepileptic drugs such as sodium valproate, phenobarbital, phenytoin, benzodiazepines, etc., which control seizures, delay epileptogenesis and hence protect against epilepsy [32,33,34]. However, these medications are efficient at controlling only 70% of seizure cases [35,36,37]. In addition, the toxicity of these drugs is a safety concern to patients due to their wide range of side effects ranging from weight gain to initiation and worsening of neuropsychiatric symptoms [38]. Moreover, they generally fail to prevent or control the progression of the pathology but rather target its symptoms [5]. Despite several efforts made by researchers and pharmaceutical companies, there is still no safe and potent drug to treat epilepsy [39]. This health crisis highlights the urgency to find better alternatives for the management of epilepsy. With over 80% of the world’s population relying on plant-based medicine, many researchers are redirecting their focus towards finding phyto-therapeutic alternatives to highly toxic medications [40, 41].
Alchemilla kiwuensis Engl. is a medical plant used both by European, Asian and African populations for various pathologies [42,43,44]. The medicinal plant is mainly used by these populations to treat bacterial infections, fungal infections, anaemia, haemorrhages and diarrhoea [45, 46]. Characterised by a high phenolic contents, the aerial part of species in the genus Alchemilla are traditionally used as sedative, anti-inflammatory agents, antioxidant agents, neuroprotective agents and hence against neurodegenerative disorders [47,48,49]. Early scientific studies with Alchemilla kiwuensis Engl. have shown its anti-inflammatory [43] and the immunomodulatory properties [45]. Our previous studies on A. kiwuensis, have demonstrated its anticonvulsant properties following PTZ and picrotoxine-induced acute seizures, as well as its innocuousness following acute toxicity [23].
The present study was undertaken to continue the exploration of the therapeutic potentials of A. kiwuensis. This work sought at investigating the antiepileptogenic and antiepileptic properties of Alchemilla kiwuensis following the PTZ-induced kindling model as well as further illustrating its safety following a sub-chronic toxicity study.
Materials and Methods
Animals
A total of thirty-five albinos Wistar rats (Ratus norvegicus) of both sexes aged between 2 and 3 months were used for the antiepileptic and antiepileptogenic tests and their weight range at the beginning of the test was between 150 and 200 g, and each group consisted of 3 females and 4 males. For the sub-chronic toxicity test, a total of thirty animals were used, five males and five females per group. The animals were raised in the research unit of animal physiology and phytopharmacology of the department of animal biology of the University of Dschang in Cameroon. Since antiepileptic drugs are both used by men and women, the decision to use both gender in this study was taken. Indeed, studies have shown that sex bias in research is responsible for the failure of designed treatment that work for one gender and not the other, and for some side effects of drugs that are equally applicable for one gender only [50]. Animals were kept according to sex, at room temperature and subjected to the natural dark/light cycle. The sex’s distribution in groups was done according to Miller et al. [51]. In addition, they received food and water ad libitum. Animals were treated following the guidelines of Cameroon’s Bioethics committee (reg N. FWA IRB00001954) and the NIH-Care and Use of Laboratory Animals manual. As such, efforts were made to minimise animal suffering and reduce the number of animals used in the experiment.
Plant
The plant material consisted of the entire Alchemilla kiwuensis plant harvested in the west region of Cameroon, precisely in Bamboutos division at the locality of Balatchi during the dry season and registered at Cameroon’s National Herbarium in Yaoundé. The plant was compared to specimens’ number 35,613/HNC (Y.A.) and 35,614/HNC (Y.A.) for identity. The harvested plant was shade-dried for several days and pulverised into powder. The resulting powder was used for extract preparation.
Extract Preparation and Administration
Fifty grammes of the powder were introduced in 1 L of distilled water, and the mixture was boiled for 10 min. The resulting extract was allowed to cool, then filtered with a filter tissue, cotton, and filter paper n° 4 successively. The filtrate obtained was dried by lyophilisation at 0°c at the Institute for Medicinal research and study of Medicinal Plants (IMPM) in Yaoundé, Cameroon. The extraction yield following lyophilisation was 13.33%. The lyophilisate was administered p.o at a volume of 10 ml/kg body weight.
Drugs and Chemicals
PTZ procured by Sigma-Aldrich (St. Louis, USA) was used as an inducing agent and was prepared by dissolving 70 and 35 mg in 10 ml of distilled water to obtain the respective concentrations of 7 and 3.5 mg/ml. These solutions were administered by i.p injection at a volume of administration of 10 ml/kg, yielding administration doses of 70 mg/kg and 35 mg/kg, respectively [22]. Sodium valproate was purchased from Sanofi-Aventis (Paris, France).
Experimental Protocol: PTZ Kindling Induction
A total of thirty-five Wistar rats of both sexes were distributed into five groups of seven animals each and received treatments as indicated below:
-
Group 1: received only distilled water (10 mL/kg: vehicle);
-
Group 2: received distilled water (10 mL/kg) (negative control) + inducing agent (PTZ);
-
Group 3: received the plant lyophilisate at the dose of 40 mg/kg + inducing agent (PTZ);
-
Group 4: received the plant lyophilisate at the dose of 80 mg/kg + inducing agent (PTZ);
-
Group 5: received sodium valproate at 300 mg/kg dose + inducing agent (PTZ).
PTZ-induced kindling is an established and generally used model to screen antiepileptogenic and antiepileptic potentials of drugs. This is a simplification of reality and it is a model used both in the development of ideas on epileptogenesis (understanding of the pathogenesis) and in the identification of drugs effective against epilepsy [52]. Moreover, neuronal and histological changes observed in the brain tissues of epileptic patients also occur in the brain regions of PTZ-kindled animals [26, 53].
Kindling was induced following the method described by Ngoupaye et al. [22]. Briefly, animals received an initial convulsive dose of PTZ (70 mg/kg). Forty-eight hours later, animals received sub-convulsive doses of PTZ (35 mg/kg) and were observed for a duration of 30 min. An hour before administrations of sub-convulsive doses of PTZ, animals received various treatments (distilled water, the lyophilisate of A. kiwuensis at various doses, valproate 300 mg/kg). Administration of sub-convulsive doses was repeated every other day to all the treatment groups apart from the vehicle group, until the appearance of stage 4, two consecutive times (the kindled state) as described by Racine scale (1972) in all animals of the negative control group. Animals of the vehicle group were treated only with distilled water. Animals were observed for seizure progression (to assess the antiepileptogenic effect), the latency of appearance of the kindled state, seizure repetitions (the number of times the animal repeated stages 4 or 5 on the kindled day), and seizure durations (the cumulative duration of seizures following the kindled state), to assess the antiepileptic effect.
Biochemical Analysis
Twenty-four hours (24 h) after the end of the kindling induction period, the animals were sacrificed by decapitation and immediately dissected for their hippocampi. These hippocampi were weighed and frozen at -20 °C to assay some elements of the GABAergic signalling pathway, such as GABA level and GABA transaminase (GABA-T) activity as well as some parameters of the glutamatergic signalisation such as glutamate level and its transporter EAAT-2. Inflammatory cytokines IL-1β and TGF-1β, oxidative stress markers, malondialdehyde (MDA), reduced glutathione (GSH) and Catalase activity were also assayed.
Homogenates Preparation
Hippocampi were ground to homogenates (10% w/v) in a porcelain mortar. Each homogenate was prepared using a 0.1 M phosphate buffer containing 1% Triton-100X (pH 7.4). These homogenates were centrifuged for 15 min (3000 rpm) at room temperature, and the supernatants were collected for various assays [54].
Determination of Malondialdehyde Level
A volume of 250 µL of the supernatant was introduced in test tubes. 250 µl of 20% trichloroacetic acid (TCA), 500 µl of 0.67% thiobarbituric acid (TBA), and 10 µl of 0.1% BHT (Butylated hydroxytoluene) were added to each tube. The blank solution consisted of all the above-stated elements except the homogenate. The tubes were sealed and incubated for 10 min at 90 °C and then cooled with tap water. They were then centrifuged at 3000 rpm for 10 min at room temperature. The supernatant was pipetted, and the absorbance read at 532 nm on a BIORAD spectrophotometer, SMART SPEC 3000 (USA), against the blank, and results were expressed in nmol/mg of wet tissue [55].
Determination Reduced Glutathione Level
A volume of 1500 µL of Ellman reagent DTNB (0.1 mM 5,5’-dithiol bis-2-nitrobenzoic acid in 0.3 M phosphate buffer with 1% of sodium citrate solution) was introduced into test tubes containing 100 µL of supernatant. Next, 100 µL of phosphate buffer (PBS) was added and then the mixtures were incubated for 1 h at room temperature. The absorbance was read using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 412 nm against the blank, and results were expressed in nmol/mg of wet tissue [55].
Determination of Catalase Activity
A 375 µL phosphate buffer (PBS) was introduced into the test tube containing 25 µL of tissue supernatant. Subsequently, 100 µL of 50 mM hydrogen peroxide (H2O2) was introduced into the tube, and the reaction proceeded for 1 min, after which 1000 µL of the solution of 5% potassium dichromate + acetic acid was introduced into the reaction medium to stop the reaction. The tubes were then brought to boil for 10 min in a boiling water bath. After cooling, the reading was done using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 570 nm against the blank. The activity was established from an H2O2 calibration curve, and results were expressed in µmol of H2O2 decomposed/ min/ mg of proteins [55].
Determination of GABA Transaminase Activity
The activity of GABA transaminase (GABA-T) was evaluated according to the method described by Moto et al. [56] and is based on the staining formed by succinic semialdehyde acid resulting from the degradation of GABA by GABA-T. The reaction medium consisted of 15 µmol of α-oxoglutarate, 15 µmol of GABA, and 10 µg of pyridoxal phosphate. For this reaction, either 100 µl of supernatant (test tubes) or 100 µl of 5% methanol (blank tube) was added and the volume of each tube was filled up to 3 ml using tris-HCl buffer (50 mM, pH 7.4). The tubes were then incubated at 37 ° C for 30 min. After incubation, 500 µl of 20% TCA was introduced into each tube, and 30 s later, 1000 µl of 12% FeCl3 was introduced into each tube. The absorbance was immediately read with a spectrophotometer at 610 nm against the blank, and then a second reading was taken 60 s after the first reading. The absorbance was proportional to the activity of GABA-T in the sample. GABA-T activity was expressed as nmol of GABA decomposed/min/mg protein.
Determination of GABA Level
GABA was evaluated according to the method described by Moto et al. [56] and is based on the coloration formed by the reaction of GABA and ninhydrin in an alkaline medium in the presence of glutamate. In the reaction medium consisting of 200 µl of 0.14 M ninhydrin [prepared in carbonate-bicarbonate buffer (0.5 M, pH 9.9)] and 100 µl of glacial trichloroacetic acid (TCA) at 10%, 100 µl of homogenate was introduced, and the entire solution was incubated at 60 °C for 30 min. After cooling, each tube’s contents were introduced into tubes containing 5000 µl copper tartrate. The tubes were then incubated at 25 °C for 10 min. The absorbance was then read on a spectrophotometer at 451 nm against the blank and was proportional to the concentration of GABA in the sample. The concentration of GABA in each sample was expressed in µg/mg of tissue.
Determination of Glutamate Level
Glutamate level was evaluated by the colorimetric method according to the manufacturer’s instructions (BioVision glutamate colorimetric assay kit), and it was based on the ability of the Glutamate mix enzyme to recognize glutamate as a specific substrate leading to a proportional colour formation. The results were expressed as pmol/µL.
Determination of EAAT-2
Excitatory amino acid transporter 2 (EAAT-2) level was evaluated with a commercially available CLOUD-CLONE enzyme-linked immunosorbent assay kit. The assay was conducted according to the manufacturer’s protocol.
Determination of IL-1β and TGF-1β
The tissue levels of the pro-inflammatory cytokine IL-1β and TGF-1β were evaluated with ELABSCIENCE enzyme-linked immunosorbent assay kits commercially available. The assay was conducted according to the manufacturer’s protocol.
Quantitative Phytochemical Characterization
Determination of Tannins Content
Tannins were determined by the method described by Brainbridge et al. [57]. A volume of 0.25 mL of each extract was added to each test tube coated with aluminium foil to avoid light, followed by the addition of 0.75 mL of a freshly prepared solution of 4% vanillin in ethanol (w/v). After agitation, 0.25 mL of concentrated hydrochloric acid (HCl) was added to each tube and then incubated at room temperature for 15 min. The absorbance of the mixture was read at 500 nm against a blank. The experiment was performed in triplicate. The amount of tannins was calculated using a standard calibration curve of tannic acid (0.1-1 mg/mL). The results were expressed in milligrams of tannic acid equivalent per gram of dry extract (mg TAE/g extract).
Determination of Total Flavonoid Content
The flavonoid content was determined following the method described by Chang et al. [58] with some modifications using quercetin as the standard. 500 µL of each extract (2 mg/ml) was mixed with 150 µL of 5% sodium nitrate (NaNO2) and incubated for 5 min at room temperature. A volume of 150 µL of 10% aluminium trichloride (AlCl3), followed 6 min later by the addition of 1 ml of 1 M sodium hydroxide (NaOH) was then added to the mixture which was later incubated for 15 min at room temperature, and the absorbance was read with a spectrophotometer at 510 nm against a blank. The total flavonoid content was calculated using the calibration curve of Quercetin (0.1-1 mg/ml). The total flavonoid content was expressed as mg quercetin equivalent per gram of extracts (mg QE/g extract).
Determination of the Total Alkaloids
Quantification of the total alkaloids in the different extracts was performed according to the method described by Singh et al. [59] with slight modifications. 100 µL each extract (2 mg/ml) was introduced into a test tube, followed by the addition of 1 mL of acidified ferric chloride solution (FeCl3 0.025 M; 0.5 M HCl) and 1 mL of an ethanolic solution of 1,10-phenanthroline (0.05 M). The mixture was homogenized and incubated for 30 min in a water bath at 100 °C. The absorbance of the reddish complex formed was read at 510 nm against the blank. Quinine at a concentration of 10–100 µg/ml was used as standard, and the alkaloid content was expressed as milligrams of quinine equivalent per gram of dry extract (µg QuE/g extract).
Sub-chronic Toxicity
The oral subchronic toxicity test was carried out according to OECD guidelines 407 [60]. A total of 30 animals aged between 10 and 12 weeks were divided into three groups of 10 animals each and treated with distilled waters (group 1) and the plant extract at treatment doses of 40 and 80 mg/kg (group 2 and 3), for 28 days. Animals were kept in groups of 2 or 3 animals of the same sex. Their daily masses, food, and water consumption were recorded. On the 28th day of treatment, animals were starved for 8 h. On the 29th day, they received an i.p injection of diazepam (0.2 ml/ 100 g of body weight) and ketamine (0.1 ml/ 100 g of body weight). Following this sedation, some blood was collected in EDTA tubes for a blood count. Some blood was also collected in EDTA-free tubes and centrifuged at 3000 turns/minute for 15 min. The resulting serum was collected and stored in Eppendorf tubes at -20 °C for biochemical analysis (total and direct bilirubin, creatinine, ALAT, and ASAT). Urine was also collected for the assessment of creatinine. After blood collection, some vital organs were collected for macroscopic observation, rinsed with 0.9% saline solution and weighed to determine their relative masses. Part of the liver and a kidney were stored in formaldehyde 10% for histological analysis.
Determination of ALAT Level
Serum level of ALAT was determined using a commercial kit with prepared reagents (Dutch Diagnostics, Netherlands). The absorbance was read using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 340 nm against the blank, and results were expressed in U/L.
Determination of ASAT Level
Serum level of ASAT was determined using a commercial kit with prepared reagents (Dutch Diagnostics, Netherlands). The absorbance was read using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 340 nm against the blank, and results were expressed in U/L.
Determination of Direct and Total Bilirubin
Serum level of direct and total bilirubin was determined using a commercial kit with prepared reagents (Dutch Diagnostics, Netherlands). The absorbance was read using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 530 nm against the blank, and results were expressed in mg/dL.
Determination of Creatinine Level
The creatinine level in urine was determined using a commercial kit with prepared reagents (Dutch Diagnostics, Netherlands). The absorbance was read using a BIORAD spectrophotometer SMART SPEC 3000 (USA) at 480 nm against the blank, and results were expressed in mg/dL.
Histological Analysis
The histological analysis was performed according to the procedure described by Suvarna et al. [61]. The procedure consisted of the following steps: fixation, trimming, dehydration, inclusion, cutting, staining, mounting and observation.
Fixation
This enabled the conservation of the cells in a state nearest to the living cells. Organs were fixed in buffered formaldehyde 4%.
Trimming
Following the fixation, a fine cut of each organ was collected and placed in labelled plastic cassettes.
Dehydration
The plastic cassettes were initially placed in 6 tanks containing ethanol of increasing concentrations: tank 1, 70° (1 h); tank 2, 90° (1 h and 1h30 minutes); tank 3, 100° (1 h, 1h30 minutes and 2 h) (dehydration proper). Following dehydration at 100°, the cassettes were kept in two baths of xylene for 1 h and 1h30, respectively, then transferred into a series of 3 tanks of molten paraffin for 1 h, 1h30 minutes, and 2 h.
Inclusion
This step provides solid external support to tissues for fine cuttings. Organs were carefully placed in stainless steel molds; then, the molds were covered by the base of cassettes and filled with molten paraffin. The setup was allowed to harden on a cooling plate.
Cutting
With a microtome Reichet Jung 2030, paraffin blocs containing the organs were cut into 5 μm. The obtained ribbon sections were allowed to unfold in a water bath (at 40 °C) containing frozen water (1%). They were then collected on labelled slides. The slides were kept in an oven at 45 °C for 24 h.
Staining
Gives room to obtain staining differentiation of cellular and tissue elements. Herein paraffin was removed (3 baths of xylene, 5 min per bath), then the cuts were rehydrated (3 baths of alcohol of decreasing concentration) before coloration. The slide was then dipped in Mayer’s hematoxylin (essential substances which give blue stain to nuclear elements) for 10 min, then rinsed with water. They were then dipped in ethanol 70° and 90° for 5 min each. They were finally kept in alcoholic eosin 0.5% (an acidic substance that gives a pink-red stain to the cytoplasm.
Mounting and Observation
Following staining, the slides were dipped in three baths of 100% alcohol and three baths of xylene (5 min each). Few drops of resin were then placed on the slide and then covered for microscopic observation. The microscope (Scientico STM-50) used had a digital camera (Pierron 15,601) connected to a H.P. computer core i7 for photographic shots.
Statistical Analysis
The results obtained were analyzed using Graph Pad Prism software version 5.03. They were presented as mean ± Standard Error on Mean (SEM). Normal distribution of data was assessed using the Shapiro-Wilk normality test. The one-way ANOVA followed by Newman-keuls post hoc test was used for multiple comparisons of groups in one-variable tests, and two-way ANOVA followed by the Bonferonni test compared the averages for two-variable tests. The tests were significant when p < 0.05.
Results
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Seizure Progression and the Onset time of Kindled State
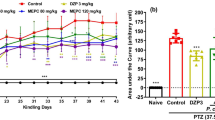
Figure 1 depicts the effect of the aqueous lyophilisate of Alchemilla kiwuensis against the process of epileptogenesis marked by the seizure progression and the onset time of kindled state.
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on seizure progression. (A) and the onset time of kindled state (B). Data expressed as mean ± SEM; n = 7; *p < 0.05, **p < 0.01, ***p < 0.001, versus negative control; ###p < 0.001, versus vehicle, ANOVA two ways followed by Bonferroni post hoc test (Fig. 1a), ANOVA one way followed by Newman-keuls post hoc test (Fig. 1b); VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
Figure 1a represents the progression of the seizures to reach the kindled state (repeated stage 4). Significant difference in the appearance of stage three between animals of the negative control group and those of the vehicle group was noted [F (1, 12) = 64.00; p < 0.0001]. There was a treatment effect as all the treatments delayed the appearance of stage three [F (3, 24) = 11.94; p = 0.012 p < 0.00015]. The treatment with the aqueous lyophilisate of Alchemilla kiwuensis at all doses delayed the appearance of stage three [F (2, 18) = 5.652; p = 0.0125]. This delay was marked at the dose 80 mg/kg [F (1, 12) = 12.52; p = 0.0041]. There was a kindling effect as animals from the negative control reached the kindled state (repeated stage 4) [F (1, 12) = 60.50; p < 0.0001]. This state was delayed by treatment with the aqueous lyophilisate of Alchemilla kiwuensis [F (2, 18) = 17.17; p < 0.0001], with significant reductions [F (1, 12) = 13, 50; p = 0.0032]; [(1, 12) = 60.50; p < 0.0001] observed at doses 40 mg/kg and 80 mg/kg respectively. The group of animals treated with sodium valproate delayed the kindled state to in a significant manner [F (1, 12) = 5.654; p = 0.0349].
Figure 1b depicts the variation in the onset time of appearance of a fully kindled state on the kindled day between various treatment groups. There was a kindled effect as animals treated with distilled water showed an average onset time of 11.88 ± 2.33 min to reach the kindled state [F (2, 22) = 69.95; p < 0.0001]. This latency time was increased after different treatments [F (4, 39) = 69.95; p < 0.0001]. This latency which was 11.88 ± 2.33 min on the negative control was delayed to 29.19 ± 0.81 min at the dose of 40 mg/kg [F (2, 20) = 44.25; p < 0.0001] and 30 ± 0.00 min at the dose 80 mg/kg [F (2, 20) = 60.23; p < 0.0001]. Similarly a significant increase was observed in animals treated with the reference drug showing an average of 23.91 ± 3.95 min [F (2, 20) = 3.960; p = 0.0.0376].
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Seizure Repetition and Seizure Duration on the Kindled Day
The variation in the number of times animals repeated stage four on the kindled day between various treatment groups and the duration of seizures are illustrated in Fig. 2. Figure 2a shows that the vehicle group had a value of 0 repetitions. The average number of repetitions displayed by the animals treated with distilled water was significantly increased to 80.00 ± 27.67 repetitions compared to the vehicle [F (2, 20) = 8.359; p = 0.0027]. This average value was significantly reduced with all treatment doses of the aqueous lyophilisate of Alchemilla kiwuensis [F (2, 20) = 8.359; p = 0.0027], showing values of 2.00 ± 1.29 repetitions and 0.00 ± 0.00 repetitions at respective doses of 40 mg/kg and 80 mg/kg. Sodium valproate also reduced the seizure compared to the negative control to 3.50 ± 1.87 repetitions [F (2, 23) = 7.912; p = 0.0034].
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on seizure repetition (A) and seizure duration (B) on the kindled day. Data expressed as mean ± SEM; n = 7; **p < 0.01; ***p < 0.001, versus negative control; ##p < 0.01; ###p < 0.001, versus vehicle, ANOVA one-way followed by Newman-keuls post hoc test; VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
Figure 2b depicts the average duration of seizures on the kindled day between various treatments. Animals from the negative control significantly increased the seizure duration on the kindled day, which moved from 0 s in the vehicle group to 22.30 ± 4.30 s [F (2, 20) = 26.91; p < 0.0001] in the negative control group. This average seizure duration was significantly decreased with all the treatment doses of the aqueous lyophilisate of Alchemilla kiwuensis [F (2, 20) = 26.72; p < 0.0001]. Sodium Valproate significantly decreased the seizure duration of kiwuensis [F (2, 20) = 26.37; p < 0.0001].
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Some Parameters of the GABAergic Signalling Pathway
Figure 3 shows the variation of GABA levels and the activity of the enzyme GABA-T between treatment groups following PTZ kindling. GABA level shows a tendency to reduce in animals from the negative control compared to the vehicle [F (2, 11) = 4.118; p = 0.0537], with an average of 0.00023 ± 0.00006 µg/mg when compared to the vehicle group (0.00043 ± 0.00005 µg/mg). The extract of A. kiwuensis significantly increased the level of GABA concentration [F (3, 15) = 18.06; p = 0.0114]. This level moved from 0.00023 ± 0.00006 µg/mg in the negative control to 0.00051 ± 0.00002 [F (2, 11) = 8.048; p = 0.0099] and 0.00083 ± 0.00019 µg/mg F (2, 11) = 9.041; p = 0.0070], at the respective doses of 40 mg/kg and 80 mg/kg. Sodium valproate also showed a significant increase in the GABA level in the tissue with an average value of 0.00066 ± 0.00004 µg/mg [F (2, 11) = 18.99; p = 0.0006] (Fig. 3a).
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on GABA. (A) level and the activity of the enzyme GABA-T (B). Data expressed as mean ± SEM; n = 4; *p < 0.05, **p < 0.01; ***p < 0.001, versus negative control; #p < 0.05, versus vehicle, ANOVA one way followed by Newman-keuls post hoc test; VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
Figure 3b shows the variation in the activity of the enzyme GABA-T between various groups following the PTZ induction procedure. The graph reveals that kindling increased the activity of GABA-T as the animals of the negative control group showed an average activity of 0.44 ± 0.11 µM of decomposed GABA/min/mg of protein, compared to the vehicle group, which displayed an average of 0.19 ± 0.07 µM of decomposed GABA/min/mg of protein [F (2, 11) = 4.615; p = 0.0417]. The activity of GABA-T was significantly reduced at respective doses of 40 mg/kg [F (2, 11) = 4.847; p = 0.0375] and 80 mg/kg [F (2, 11) = 10.02; p = 0.0051], with average activities of 0.17 ± 0.03 and 0.02 ± 0.05 µM of decomposed GABA/min/mg of protein respectively. Animals treated with sodium valproate showed an average of 0.12 ± 0.04 µM of decomposed GABA/min/mg of protein, corresponding to a significant reduction of this activity[F (2, 11) = 6.537; p = 0.0176] (Fig. 3b).
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Some Parameters of the Excitatory Signaling Pathway
Figure 4 illustrates the changes observed in glutamate and glutamate transporter levels following kindling induction. Figure 4a shows that the level of glutamate was significantly increased in the negative control group when compared to the vehicle group, with respective averages of 17.93 ± 0.81 pmol/µL and 13.81 ± 0.29 pmol/µL [F (2, 11) = 20.23; p = 0.0005]. Neither the plant extract nor the reference drug did correct this increase in a significant manner. Nevertheless, a decreasing tendency was observed with both treatments [F (3, 15) = 1.445; p = 0.2786].
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on the level of glutamate (A) and the expression of glutamate transporter EAAT-2 (B). Data expressed as mean ± SEM; n = 4; *p < 0.05, versus negative control, ###p < 0.001, versus vehicle; ANOVA one way followed by Newman-keuls post hoc test; VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
The variations in the expression of the transporter for glutamate EAAT-2 concerning the different treatments are shown in Fig. 4b. The figure reveals that the level of the transporter was reduced in animals treated with distilled water showing an average of 192.11 ± 19.94 pg/mL when compared with the vehicle group, which showed an average of 227.05 ± 14.43 pg/mL [F (2, 11) = 1.500; p = 0.2741]. The decrease in the negative control was corrected by various treatments, and a significant increase was observed at the dose 80 mg/kg with an average expression of 243.23 ± 1.91 pg/mL [F (2, 11) = 6.455; p = 0.0142].
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on some Parameters of Oxidative Stress
The effect of Alchemilla kiiwuensis extract on lipid peroxidation, reduced glutathione, and catalase activities are shown in Fig. 5.
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on lipid peroxidation (A), level of reduced glutathione (B), and catalase activity (C). Data expressed as mean ± SEM; n = 4; **p < 0.01; ***p < 0.001, versus negative control; ##p < 0.01; ###p < 0.001, versus vehicle, ANOVA one way followed by Newman-keuls post hoc test; VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
Figure 5a depicts that the kindling procedure has increased lipid peroxidation as shown by an increase, although not significant, in the amount of MDA in the group of animals which received distilled water as treatment (5676.73 ± 1435.15 nmol/mg) when compared with the vehicle group (3164.16 ± 750.70 nmol/mg) [F (2, 11) = 1.981; p = 0.1937]. The aqueous lyophilisate of Alchemilla kiiwuensis at 80 mg/kg dose significantly decreased lipid peroxidation, which moved from 5676.73 ± 1435.15 nmol/mg in the negative control to 1250.75 ± 617.93 nmol/mg [F (2, 11) = 6.938; p = 0.0150].
The variation in the strength of the antioxidant mechanism, as revealed by the level of reduced glutathione, is shown in Fig. 5b. The group of animals from negative control significantly diminished the level of reduced glutathione (38477.67 ± 7664.17 nmol/mg) compared with the vehicle group (92846.23 ± 10537.33 nmol/mg) [F (2, 11) = 10.53; p = 0.0044]. The treatment with the lyophilisate of Alchemilla kiwuensis has significantly increased the level of reduced glutathione at the respective doses of 40 and 80 mg/kg, with the respective averages of 80677.84 ± 2671.35 [F (2, 11) = 24.39 p = 0.0002] and 105,771. ± 10252.89 nmol/mg [F (2, 11) = 20.34; p = 0.0005]. Valproate equally significantly increased the level of reduced glutathione with an average of 102753.51 ± 11168.16 nmol/mg [F (2, 11) = 17.06; p = 0.0009].
Figure 5c depicts the variation in catalase activity between various groups. Animals from the negative control which received distilled water exhibited a significant reduction in the activity of this enzyme, from an average value of 0.0015 ± 0.0002 µM/min/mg in the vehicle group to a value of 0.0002 ± 0.0003 µM/min/mg[F (2, 11) = 9.053; p = 0.0070]. The experimental treatment, together with the reference drug, significantly increased the activity of this enzyme [F (3, 15) = 12.91; p = 0.0005] as they exhibit an average activities of 0.0014 ± 0.0003 [F (2, 11) = 5.642; p = 0.0258], 0.0024 ± 0.0003 µM/min/mg [F (2, 11) = 20.02; p = 0.0005] for plant extract at the respective doses of 40 and 80 mg/kg, and 0.0021 ± 0.0002 µM/min/mg[F (2, 11) = 16.90; p = 0.0009] for sodium valproate.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on some Parameters of Neuroinflammation
The variations in the effects of the experimental treatments on TGF-1β (Fig. 6a) and IL-1β (Fig. 6b) are shown below. Figure 6a shows no noticeable change in the levels of TGF-1β between the vehicle and the negative control group. However, sodium valproate and A. kiwuensis at the dose of 80 mg/kg have shown a noticeable increase in tissue levels of this anti-inflammatory cytokine; this increase was significant [F (2, 11) = 6.207; p = 0.0202] with the lyophilisate showing an average value of 1012.42 ± 136.67 pg/mL when compared to the negative control which displayed an average value of 433.51 ± 128.99 pg/mL.
Effect of the aqueous lyophilisate of Alchemilla kiwuensis on the levels of TGF-1B (A) and IL-1B (B). Data expressed as mean ± SEM; n = 4; *p < 0.05, versus negative control, ANOVA one way followed by Newman-keuls post hoc test VEH = vehicle; H2O = distilled water; PTZ = Pentylenetetrazole 35 mg/kg; VAL = Sodium Valproate 300 mg/kg
As far as the level of IL-1β is concerned, though not significant, kindled animals displayed an increase in tissue levels of IL-1β (1059.72 ± 84.28 pg/mL) compared to the vehicle (998.95 ± 12.95 pg/mL). A. kiwuensis at the dose of 80 mg/kg showed a significant decrease in tissue levels of this pro-inflammatory cytokine, with an average value of 916.61 ± 16.75 pg/ml when compared to the negative control, which displayed an average value of 1064.77 ± 84.28 Pg/ml [F (2, 11) = 4.719; p = 0.0397] (Fig. 6b).
Quantitative Phytochemical Characterization of the Aqueous Lyophilisate of Alchemilla Kiwuensis
Based on our previous qualitative findings, quantification of flavonoids, alkaloids, and tannins was realized, and the values are shown in Table 1 below.
Sub-chronic Toxicity Study
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Body Weight
The variation in the body weight of the animals following 28 days of oral gavages is shown in Fig. 7 (A and B) below. No significant change was observed between treatment groups. However, there was a general increase in body weight in males (Fig. 7a) and females (Fig. 7b) in all the groups.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Food and Water Consumption
Table 2 below depicts the variation in the amount of food and the quantity of water consumed by the animals during the test period. There was a significant treatment effect on the food consumed in males [F (2, 48) = 7.604; p = 0.0074]. A significant decrease was observed with the animals receiving the plant extracts at the dose 80 mg/kg in the fourth week of treatment, showing an average consumption of 39.70 ± 7.82 mg when compared to the vehicle group, which had an average of 41.90 ± 3.56 mg of food [F (2, 14) = 3.696; p = 0.0358]. Similarly, a general decreasing tendency in the amount of water consumed by males was observed. However, no significant change between treated groups was observed [F (8, 48) = 0.3824; p = 0.6902]. Similarly, the amount of food consumed by females was significantly reduced [F (8, 48) = 8.056; p = 0.0061] and water not [F (8, 48) = 1.430; p = 0.2772] throughout the weeks. At the beginning of the test, significant variations in the amount of food consumed by these females were observed at the doses 80 mg/kg with average consumption values of 65.00 ± 5.75 mg, when compared with the vehicle group having an average of 47.60 ± 4.47 mg[F (2, 14) = 10.67; p = 0.0022]. Moreover, at the end of the first week of treatment, the animals treated with the plant extract at the dose 40 mg/kg exhibited a significant reduction in the amount of food consumed, as they had an average of 23.8 ± 3.55 mg compared to the vehicle with an average of 40.00 ± 4.81 mg [F (2, 14) = 25.422; p = 0.0210]. There was a significant reduction in water consumption among the females treated with the lyophilisate at the dose 40 mg/kg at the end of the first week of treatment, showing an average of 17.00 ± 1.86 ml of water consumed, compared to an average of 31.40 ± 3.91 ml consumed by the vehicle group [F (2, 14) = 7.023; p = 0.0096].
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on the Relative Masses of Organs
Table 3 below shows the differences in the relative masses of some vital body organs following 28 days of treatments. Overall no significant difference was observed neither between the males nor the females.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on some Biochemical Parameters
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on ALAT, ASAT, and Creatinine Levels
The differences in the ALAT, ASAT, and creatinine levels are shown in Fig. 8 (A to F) below. Although certain variations appear for some parameters, overall, there was no significant change in the average value of each parameter when compared to the corresponding vehicle.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Total and Direct Bilirubin
Figure 9 (A to D) expresses the differences in the average levels of direct and total bilirubin in males (A and B) and females (C and D). No significant difference was observed in both parameters, both males and females.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on some Hematological Parameters
Table 4 below illustrates the differences in hematological parameters following 28 days of treatment in males and females. From the table, the group of animals treated with the plant extract at the dose 40 mg/kg had a significant increased the number of granulocytes [F (2, 14) = 14.60; p = 0.0006] and white blood cells [F (2, 14) = 7.944; p = 0.0063] respectively when compared to the vehicle group, with average values of 7.24 ± 0.92 and 0.70 ± 0.09 × 109/L respectively, when compared to the vehicle with average values of 5.01 ± 0.27 and 0.33 ± 0.07 × 109/L respectively. No significant change was observed for the other parameters, both males and females.
Effect of the Aqueous Lyophilisate of Alchemilla Kiwuensis on Renal and Hepatic Tissue
In the course of evaluating the safety of the plant extract, its effects on the tissue morphology of some vital organs like the kidney and the liver were assessed (Fig. 10). Histological analysis following the hematoxylin stain revealed no toxicity for the liver or kidneys.
Photomicrographs of males’ and females’ liver and kidneys following sub-chronic toxicity study. A: Vehicle; B:A. kiwuensis, 40 mg/kg; C:A. kiwuensis, 80 mg/kg; Liver, V.P.: hepatic portal vein; He: hepatocytes; Cs: sinusoid capillary; Cb: Bile canal; kidney, Gl: glomerulus; Eu: Urinary space; Tcd: distal convoluted tubule; Tcp: proximal convoluted tubule; hematoxylin stain
Discussion
The present study was undertaken to evaluate the antiepileptic and the antiepileptogenic properties of the aqueous lyophilisate of Alchemilla kiwuensis and the involvement of some essential and relevant signalling pathways such as the GABAergic, glutamatergic, inflammatory and antioxidative signalling pathways, following PTZ-induced kindling. Kindling is a process by which repeatedly induced seizures lead to an increased duration of seizures and enhanced behavioural consequences of those induced seizures until a plateau is attained [21, 25]. PTZ-induced kindling is an experimental model which has been widely used for decades to screen potential antiepileptic drugs [62]. As such, repetitive administration of a sub-convulsive dose of PTZ replicates the appearance and the progressive increase of convulsions, which culminates in generalised seizures [63]. This progressive nature of seizure was evidenced in this study by the behaviour of the negative control group whose stages gradually increased from 0 to the kindled stage (successive repetition of stage 4), which appeared around the fifth day of treatment on average. The lyophilisate of Alchemillia kiwensis (A. kiwuensis) was able to delay the process of epileptogenesis, as evidenced by the significant delay in the occurrence of stages 3 and 4 by the two treatment doses. This same effect was also observed with the reference drug sodium valproate. Indeed, the behavioural progression in the kindling process suggests that it starts with a limited number of neuronal circuits and subsequently recruits additional circuits as the behavioural component of the seizure evolves to convulsion [21], an effect which accounts for the progressive nature of epilepsy. Present day antiepileptic drugs fail to fully control the pathology because they fail to inhibit the progression of the pathology and focus on the symptomatic expression of the seizures only [5]. The ability to influence the progression of the pathology will be a step forward in the quest for better treatments. In addition to the influence on the progression of the pathology, control of the severity of seizures has been stated to be a determining factor in the potency of antiepileptic drug [22, 25]. To evaluate this effect, the latency of the appearance of seizures, repetition of seizures and duration of seizures were recorded on the kindled day. Just like sodium valproate, the lyophilisate of A. kiwuensis successfully increased seizures onset time and decreased the duration and repetition of seizures. The increase in seizure duration suggests that the existing resistance of the circuits to seizure activity is gradually weakened [21, 63]. As such, by reducing seizure duration, the extracts of A. kiwuensis and sodium valproate could strengthen the circuits’ resistance to seizures, thus reducing seizure susceptibility. This pharmacological property is a necessary aspect to inhibit the progression of epilepsy. Pharmacological substances capable of delaying and/or inhibiting epileptogenesis (seizure progression) are equally good as antiepileptic substances [25]. This shows that A. kiwuensis is both an antiepileptogenic and an antiepileptic agent.
Alterations caused by progressive excitability and a marked imbalance in the brain’s excitatory-inhibitory neurotransmissions characterise kindling [27]. PTZ is a blocker of the GABAA receptor Cl− channel [64], thus it produces seizures by inhibiting the GABA pathway in the CNS [65]. The action of GABA on the GABAA receptor prevents seizure initiation and propagation. This implies that drugs or treatments that activate the GABAA receptor or elevate synaptic GABA levels by blocking its breakdown or reuptake show anticonvulsants and antiepileptic properties [32, 66]. Evaluation of tissue levels of GABA demonstrated a reduced GABA content in kindled animals, an observation which confirms the implication of GABA in controlling seizure initiation and it subsequent propagation. This condition was prevented with the administration of A. kiwensis at doses 40 and 80 mg/kg as well as with the reference drug, all of which significantly increased the tissue GABA content. The activity of GABA-T, an enzyme responsible for the synaptic breakdown of GABA [14, 67, 68], was also evaluated. This activity was elevated in kindled animals compared to the vehicle. This disturbance was corrected with the aqueous lyophilisate of A. kiwuensis and sodium valproate which significantly reduced the activity of this enzyme. These results on GABA and GABA-T suggest that the antiepileptogenic and hence the antiepileptic effect of A. kiwuensis and valproate previously observed are due to their interaction with the GABAergic-neurotransmission. These results are in accordance with our previous study, which demonstrated the anticonvulsant effects of the plant following PTZ- and picrotoxin-induced seizure, which are both antagonists of the GABAA receptor [23], showing the affinity the plant extract has with the GABA signalling pathway.
Altered GABA-glutamate neurotransmissions are commonly observed in brain tissues of kindle animals [69]. Given that GABA maintains the inhibitory tone that counterbalances neuronal excitation [12], a reduction in GABA levels is generally accompanied by a simultaneous increase in excitatory signalisation, evidenced by the high release of glutamate in postsynaptic terminals [28]. For this reason, glutamate levels and the levels of its major transporter (EAAT-2) were evaluated. Results revealed that glutamate levels were significantly increased in the hippocampus of kindled animals compared to the vehicle. However, a decreasing tendency in glutamate was observed in the hippocampus of animals treated with either the plant extract or sodium valproate. Similarly, the glutamate transporter was reduced in kindled animals, and only the aqueous lyophilisate of A. kiwuensis at the single dose of 80 mg/kg was able to cause a significant increase of this transporter, thus facilitating the synaptic clearance of the excitatory neurotransmitter. Glutamate is highly implicated in nearly all aspects of normal brain function, including learning, memory, movements, cognition, and development [6]. Therefore, uncontrolled, or careless modulation of this transmission may be accompanied by a host of consequences. As such, modulation of this signalling pathway should be handled carefully [70]. These reasons explain the plant extract’s light and smooth effects on this signalization pathway. However, the lyophilisate has shown its ability to increase the expression of glutamate’s major transporter. An increase of glutamate clearance from the extracellular space would probably normalize the default observed in the kindled animals compared to the vehicle, hence accounting for the modulated behavioural outcome.
PTZ-induced kindling and the subsequent excitotoxicity are most often the cause of an overproduction of free radicals enough to set an imbalance between the pro-oxidant and the antioxidant systems of the brain [71], thus generating a condition which can be characterised as oxidative stress. The resulting free radicals attack unsaturated sites of lipids and other biological molecules in the cells [28, 72], these contributes to seizure propagation and hence the genesis of spontaneous seizures [73, 74]. These assertions were confirmed in the present study as kindled animals depicted marked lipid peroxidation evidenced by an increase in the tissue levels of MDA. Coupled to the increase in lipid peroxidation, a reduction in the antioxidant potentials was observed in the hippocampi of these animals, as evidenced by a decrease in GSH levels and catalase activity. The lyophilisate of A. kiwuensis and sodium valproate inhibited all these kindling phenomena. Only the lyophilisate at the single dose of 80 mg/kg produced a significant reduction of the level of MDA, marking its strong influence on lipid peroxidation compared to other treatments in this study. However, the increase in the levels and activities of antioxidants was significant in all treatments. The harmful effects of ROS in the brain appear when they are in excessive concentrations, and therefore when the ability of the neuronal antioxidant mechanisms to counterbalance the damaging reaction is dampened. Given the high oxygen-consuming nature of the brain, it is subjected to particular damages in such situations. Its hippocampus is one of the most vulnerable structures to oxidative stress and to epilepsy in general [55, 72, 73]. Therefore, treatments with antioxidant properties could be endowed with antiepileptic potentials. In line with these findings, recent and similar studies have shown that the antioxidative potentials of some plant treatments contribute to their protective effects against epilepsy [28, 64, 74]. The latent phase of epileptogenesis offers a unique opportunity for therapeutic trials [75]. Given the conspicuous involvement of oxidative stress in this stage of the pathology, it will be of high therapeutic value to exert a homeostatic correction of the imbalance between the pro and antioxidant species at this stage of the disease. These suggest that the observed antiepileptic and antiepileptogenic properties of A. kiwuensis are at least partly due to its antioxidant properties.
Together with oxidative stress, inflammation is one of the hallmarks of the latent phase of epileptogenesis [75]. It has been shown above that kindling-induced oxidative stress increases circulating free radicals. At high quantities, these free radicals may be considered and recognized as damage-associated molecular patterns by the brain’s immune system and thus activate immune responses mainly through the activation of NADPH oxidase [76, 77]. This activation leads to the production of pro-inflammatory cytokines such as IL-1β and the reduction of anti-inflammatory cytokines such as TGF-1β [78, 79, 80]. Indeed, kindled animals in the present study expressed increased levels of IL-1β, compared to unkindled and naïve animals. The lyophilisate of A. kiwuensis and sodium valproate inhibit the increase in IL-1β levels, and boost the production of TGF-1β. TGF-1β is an anti-inflammatory cytokine known to protect neurons from injury and down-regulate the reactivity of glial cells [81]. These suggest the modulatory properties of the treatments on neuroinflammation as a contributing factor to their antiepileptogenic and antiepileptic properties. Indeed, we can recall that A. kiwuensis at all doses showed its effect on the GABAergic and oxidative stress. However, the dose 80 mg/kg which did not reach the kindled state decreased glutamate as well as the IL1β and increased glutamate transporter and the TGF-1β suggesting that its antiepileptic activities could be done through the modulation of the glutamatergic pathway and neuroinflammation.
Quantitative phytochemical analysis revealed that flavonoids represented the most significant proportion of bioactive molecules in Alchemilla kiwuensis. Flavonoids are compounds of the polyphenol subclass whose relatively side effect-free therapeutic potential as antiepileptic treatment has been demonstrated [82]. This bioactive compound has been shown to act both on the GABAA receptor subtype [83] and on the ionotropic GABA receptor subtype [84] to produce their antiepileptic effects. In addition, quercetin, one of the most widely occurring flavonoids, has been shown to modulate the GABAergic [85, 86], inflammatory [87, 88], and antioxidant [89] pathways. Taken together, the flavonoid content of the lyophilisate of Alchemilla kiwuensis would have been responsible for its therapeutic effects in the present study.
A subchronic toxicity test was conducted to evaluate the safety of the lyophilisate and in continuum with previous studies. Overall, the extract of A. kiwuensis did not show signs of toxic effects following this investigation. However, a significant reduction in the food consumed was observed in males at the end of the 28 days of treatments. This reduction may not be considered a toxic effect, because this reduction in food consumption was not accompanied by a decrease in body weight which was increased overall; instead of food consumption, body weight is a sign of animal health status [90, 91]. Analysis of some biochemical parameters such as ALAT, ASAT, creatinine, total and direct bilirubin revealed no significant changes with the lyophilisate, indicating no sign of toxicity in some vital organs such as the liver and kidney. These organs are implicated in the metabolism of xenobiotic, detoxification, and fluid purification respectively [92, 93]. These results are backed by histological analysis which showed regular tissue organisation. A significant elevation in granulocyte levels and WBC was noticed in males at the dose of 40 mg/kg. Granulocytes constitute the first line of defence against infection and are predominantly activated during parasitic, viral, and bacterial infections [94], and are most often associated to acute phase infections [95]. All these suggest that the increase in granulocyte count may not be associated to the chronic treatment with the plant extract but rather with a possible acute infection restricted to animals of this group. Granulocytes and neutrophils represent the most significant proportion of all WBC (60–70%) [96], therefore an elevation in granulocyte blood count will undoubtedly lead to a similar elevation in WBC count, justifying the increase of WBC observed herein. Moreover, the absence of significant increase in the spleen’s relative mass suggests an acute increase in blood count. The observed effects following toxicity study can be categorised as “no observed adverse effect” (NOAEL) [97].
Conclusion
The results obtained from this study reveal that the aqueous lyophilisate of Alchemilla kiwuensis is endowed with antiepileptogenic and antiepileptic properties and is relatively safe and non- toxic. The antiepileptic and antiepileptogenic results are obtained due to their ability to restore the homeostatic imbalance between inhibitory and excitatory signalization. This justifies its local use in the treatment of epilepsy.
Data Availability
The datasets generated during and/or analysed during the current study are adequately contained within the manuscript. Further information will be made available from the corresponding author on request from qualified investigators.
Change history
18 July 2023
The original article were updated due to removal of co-authors email addresses.
References
Linehan C, Benson A, Gunko A, Christensen J, Sun Y, Tomson T, Marson A, Forsgren L, Trinka E, Iliescu C, Althoehn J, Sonderup, Dreier JW, Sandu C, Leanca M, Rainer L, Kobulashvili T, Granbichler AC, Delanty N, Doherty C, Staines A, Shahwan A (2021) Exploring the prevalence and profile of epilepsy across Europe using a standard retrospective chart review: challenges and opportunities. Epilepsia 62:2651–2666. https://doi.org/10.1111/epi.17057
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Perez ER, Scheffer I, Zuberi SM (2017) Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classifcation and Terminology. Epilepsia 58:522–530. https://doi.org/10.1111/epi.13670
WHO (2019) Executive board 46th session provisional agenda item. 11 11:6
Jessica F-W, Scheffer IE, Robert S, Fisher (2018) The new definition and classification of seizures and epilepsy. Epilepsy Res 139:73–79. https://doi.org/10.1016/j.eplepsyres.2017.11.015
Mishra CB, Kumari S, Siraj F, Yadav R, Kumari S, Tiwari AK, Tiwari M (2018) The anti-epileptogenic and cognition enhancing effect of the novel 1-[4-(4-benzo [1,3] dioxol-5-ylmethyl-piperazin-1-yl)-phenyl]-3phenyl-urea (BPPU) in pentylenetetrazole induced chronic rat model of epilepsy. Biomed Pharmacother 105:470–480. https://doi.org/10.1016/j.biopha.2018.05.140
Rousseaux CG (2008) A review of glutamate receptor I: current understanding of their biology. J Toxicol Pathol 21:25–51. https://doi.org/10.1293/TOX.21.25
Sazhina TA, Sitovskaya DA, Zabrodskaya Y, Bazhanova ED (2020) Functional imbalance of glutamate-and GABAergic neuronal systems in the pathogenesis of focal drug-resistant epilepsy in humans. Bull Exp Biol Med 168:519–522. https://doi.org/10.1007/s10517-020-04747-3
Cho CH (2013) New mechanisms for glutamate hypothesis in epilepsy. Font Cell Neurosci 127:2. https://doi.org/10.3389/fncel.2013.00127
Nielsen EB, Suzdak PD, Andersen KE, Knutsen LJ, Sonnewald U, Braestrup C (1991) Characterisation of tiagabine (NO-328), a new potent and selective GABA uptake inhibitor. Eur J Pharmacol 196:257–266. https://doi.org/10.1016/0014-2999(91)90438-V
Greenfield JLJ (2013) Molecular mechanisms of antiseizure drug activity at GABAA receptor. Seizure 2013: 12p. doi: https://doi.org/10.1016/j.seizure.2013.04.015
Bjornsen LPBW (2016) Glutamate and GABA transporters in mesial temporal lobe epilepsy. Thesis for the degree of philosophiae Doctor. University of Oslo, Norway 2016: 42p. http://urn.nb.no/URN:NBN:no-53026
Treiman DM (2001) GABAergic mechanisms in Epilepsy. Epilepsia 42:8–12. https://doi.org/10.1046/j.1528-1157.2001.042suppl.3008.x
Fueta Y, Vasilets LA, Takeda K, Kawamura M, Schwarz W (2003) Down-regulation of GABA-transporter function by hippocampal translation products: its possible role in epilepsy. Neuroscience 118:371–378. https://doi.org/10.1016/s0306-4522(02)00924-7
Squire LR, Bloom FE, Spitzer NC, Du Lac S, Ghosh A, Berg D (2008) Fundamental neuroscience 3th Edition. Elsevier Academic press, Burlington
Pitkänen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10:173–186. https://doi.org/10.1016/S1474-4422(10)70310-0
Reddy SD (2014) Clinical pharmacology of current antiepileptic drugs. IJPSN 1:2305–2319. https://doi.org/10.37285/ijpsn.2014.7.1.1
Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN (2020) Revisiting the role of neurotransmitters in epilepsy: an updated review. J Life Sci 265:60. https://doi.org/10.1016/j.lfs.2020.118826
Basulto JJJ, Martinez YJ, Cervantes MCR, Guerrero MEU, Velasco AIF, Zarate CB (2018) Interactions between epilepsy and plasticity. Pharmaceuticals 11:18. https://doi.org/10.3390/ph11010017
Reutt KKB, Czuczwar SJ (2020) Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep 72:1218–1226. https://doi.org/10.1007/s43440-020-00143-w
Goddard VG, McIntyre DC, Leech CK (1969) A permanent change in brain functioning resulting from daily electrical stimulation. Exp Neurol 25:295–330. https://doi.org/10.1016/0014-4886(69)90128-9
Bertram E (2007) The relevance of kindling for human epilepsy. Epilepsia 48:67–74. https://doi.org/10.1111/j.1528-1167.2007.01068.x
Ngoupaye GT, Adassi MB, Foutsop FA, Yassi BF, Ngo bum E (2022) Pentylenetetrazole kindling-induced epilepsy rat models: insight on the severity state, a comparative study. IBRO neurosci Rep 13:164–176. https://doi.org/10.1016/j.ibneur.2022.08.003
Ngoupaye GT, Adassi MB, Foutsop FA, Noungoua MC, Yaya J, Kom DT, Ngo bum E (2021) Anticonvulsant effects and acute toxicity study of the aqueous lyophilised extract of four medicinal plants of Cameroon: Malvaviscus arboreus, Alchemilla kiwuensis and a mixture of Drymaria cordata and Markhamia lutea. Adv Tradit Med 22:177–191. https://doi.org/10.1007/s13596-020-00525-8
Adassi MA, Ngoupaye GT, Yassi FB, Foutsop AF, Kom TD, Ngo Bum E (2022) Revealing the most effective anticonvulsant part of Malvaviscus arboreus dill. Ex Cav. And its acute and sub-acute toxicity. J ethnopharmacol 303:21. https://doi.org/10.1016/j.jep.2022.115995
Singh T, Mishra A, Goel RK (2021) PTZ kindling model for epileptogeneis, refractory epilepsy, and associated comorbidities: relevance and reliability. Metab Brain Dis 36:1573–1590. https://doi.org/10.1007/s11011-021-00823-3
Erkeç ÖE, Arihan O (2015) Pentylenetetrazole Kindling epilepsy model. Epilepsia 2:6–12. https://doi.org/10.5505/epilepsi.2015.08108
Zhu X, Dong J, Shen K, Bai Y, Chao J, Yao H (2016) Neuronal nitric oxide synthase contributes to pentylenetetrazole-kindling induced hippocampal neurogenesis. Brain Res Bull 121:138–147. https://doi.org/10.1016/j.brainresbull.2016.01.010
De Souza A, Filho AJMC, Oliveira JVS, De Souza DAA, Lopes IS, De Carvalho MAJ, De Lima KV, Sousa FCF, Vasconcelos SMM, Macedo D, Fonteles MMD (2019) Prevention of Pentylenetetrazole-induced kindling in behavioural comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: involvement of brain antioxidant and BDNF upregulating properties. Biomed Pharmacother 109:429–439. https://doi.org/10.1016/j.biopha.2018.10.066
Vilela LR, Lima IV, Kunsch EB, Pinto HPP, De Miranda AS, Vieira ELM, De Oliveira ACP, Moraes MFD, Teixeira AL, Moreira FA (2017) Anticonvulsant effects of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav 75:29–35. https://doi.org/10.1016/j.yebeh.2017.07.014
Anissian D, Ghasemi-Kasman M, Khalili-Fomeshi M, Akbari A, Hashemian M, Kazemi S, Moghadamnia AA (2018) Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling models of epilepsy. Int J Bio Macromol 107:973–983. https://doi.org/10.1016/j.ijbiomac.2017.09.073
Rohani R, Aliaghaei A, Abdollahifar MA, Sadeghi Y, Zare L, Dehghan S, Heidari MH (2021) Long-term effects of hippocampal low-frequency stimulations on pro-inflammatory factors and astrocytes activity in kindled rays. Cell J 23:85–92. https://doi.org/10.22074/cellj.2021.7139
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi O, Costa C (2019) Valproic acid and epilepsy: from molecular mechanism to clinical evidences. Curr Neuropharmacol 17:926–946. https://doi.org/10.2174/1570159X17666181227165722
Stienen M, Haghikia A, Dambach H, Thöne J, Wiemann M, Gold R, Chan A, Dermietzel R, Faustmann MP, Hinkerohe D, Prochnow N (2011) Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGF1β regulation. Br J Pharmacol 162:491–507. https://doi.org/10.1111/j.1476-5381.2010.01038.x
Mahdavi A, Naeini AA, Najafi M, Maracy M, Ghazvini (2020) Effect of levetiracetam drug on antioxidant and liver enzymes in the epileptic patients: case-control study. Afr Health Sci 20:984–990. https://doi.org/10.4314/ahs.v20i2.55
Hauser WA (1997) Incidence and prevalence. In: Engel Jr J, Pedly TA, editors. Epilepsy, a comprehensive textbook. Philadelphia: Lippincott-Raven Publishers 47–57. doi:https://doi.org/10.1001/jama.300.4.443-a
Kwan P, Brodie MJ (2003) Early identification of refractory epilepsy. N Engl J Med 342:314–319. https://doi.org/10.1056/NEJM200002033420503
Wahab A (2010) Difficulties in treatment and management of epilepsy and challenges in new drugs development. Pharmaceuticals 3:2090–2110. https://doi.org/10.3390/ph3072090
Beghi M, Cornaggia CM, Frigeni, Beghi E (2006) Learning disorders in epilepsy. Epilepsia 47:14–18. https://doi.org/10.1111/j.1528-1167.2006.00681.x
Viswanatha GL, Venkataranganna MV, Prasad NBL (2017) Ameliorative potential of Colebrookea oppositifolia methanolic root extract against experimental models of epilepsy: possible role of GABA mediated mechanism. Biomed Pharmacother 90:455–465. https://doi.org/10.1016/j.biopha.2017.03.078
Muazu J, Kaita A (2008) A review of traditional plants used in the treatment of epilepsy among the Hausa/Fulani tribes of the northern Nigeria. Afr J Tradt Complement Altern Med 5:387–390. https://doi.org/10.4314/ajtcam.v5i4.31294
Ekor M (2013) The growing use of herbal medicine: issues realting to adverse reactions and challenges in monitoring safety. Front Pharmacol 177:10. https://doi.org/10.3389/fphar.2013.00177
Akbulut S, Bayramoglu MM (2013) The trade and use of some medicinal and aromatic herbs in turkey. Ethno Med 7:67–77. https://doi.org/10.1080/09735070.2013.11886446
Kamtchueng MO, Baylan R, Mouokeu RS, Tume C, Banerjee C, Chawla SA, Oumar M, Kuiate JR (2017) Anti-inflammatory activity of methanol extracts and fraction from Alchemilla kiwuensis eng on LPS activated macrophages. IJPPR 9:473–481. https://doi.org/10.25258/phyto.v9i2.8117
Kurtul E, Eryilmaz M, Sarialtin S, Tekin M, Acikara ÖB, Coban T (2022) Bioactivities of Alchemilla mollis, Alchemilla persica and their active constituents. Braz J Pharm Sci 58:10. https://doi.org/10.1590/s2175-97902022e18373
Kamtchueng MO, Mouokeu RS, Tume C, Djafoua YM, Kuiate JR (2013) Evaluation of immunomodulatory activity of the methanol extracts of Alchemilla kiwuensis Eng. IJPPR:349–355
Karhagomba IB, Mirindi TA, Mushagalusa TB, Nabino VB, Koh K, Kim HS (2013) The cultivation of wild food and medicinal plants for improving community livelihood: the case of the Buhozi site, DR Congo. Nutr Res Pract 7:510–518. https://doi.org/10.4162/nrp.2013.7.6.510
Tadic V, Krgovic N, Zugic A (2020) Lady’s mantle (Alchemilla vulgaris L., Rosaceae): a review of traditional uses, phytochemical profile, and biological properties. tr. Nat Med Mater 40:66–74. https://doi.org/10.5937/leksir2040066T
Vlaisavljevic S, Jelaca S, Zengin G, Mimica-Dukic N, Berezni S, Miljic M, Stevanovic Z (2019) Alchemilla vulgaris agg (Lady’s mantle) from central Balkan: antioxidant, anticancer and enzyme inhibition properties. RSC adv 9:37474–37483. https://doi.org/10.1039/c9ra08231j
Plevkova J, Brozmanova M, Harsanyiova J, Sterusky M, Honetschlager J, Buday T (2020) Various aspects of sex and gender bias in biomedical research. Physiol Res 69:367–378. https://doi.org/10.33549/physiolres.934593
Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S (2017) Considering sex as a biological variable in preclinical research. FASEB J 31:29–34. https://doi.org/10.1096/fj.201600781R
Jefferys JGR (2003) Models and mechanisms of experimental epilepsies. Epilepsia 44:44–50
Kebriaeezadeh A, Emami S, Ebrahimi M, Sharifzadeh M, Khorosani R, Shafiee A (2008) Anticonvulsant and antiepileptogenic effects of azolylchromanones on lithium-pilocarpine induced seizures and pentylenetetrazole-kindling model of epilepsy. Biomed Pharmacother 62:208–211
Ngoupaye GT, Pahaye DB, Ngondi J, Moto FCO, Ngo Bum E (2017) Gladiolus dalenii lyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed Pharmacother 91:350–357. https://doi.org/10.1016/j.biopha.2017.04.061
Ngoupaye GT, Kom TD, Adassi MB, Yaya J, Noungoua CM, Foutsop AF, Ngo Bum E (2020) Antiamnesic effect of aqueous lyophilisate of Drymaria cordata on scopolamine-induced amnesia and oxidative stress in mice. Invest Med Chem Pharmacol 3:45. https://doi.org/10.1016/j.biopha.2020.110603
Dimo T, Tsala DE, Dzeufiet DPD, Penlap BV, Njifutie N (2006) Effects of Alafia multiflora Stapf on lipid peroxidation antioxydant enzyme statuts in carbon tetrachloride-treated rats. Pharmacologyonline 2:76–89
Moto FCO, Arsa’a A, Ngoupaye TG, Taiwe GS, Njapdounke JSK, Kandeda AK, Nkantchoua GCN, Omam OJP, Pale S, Kouemou NE, Ayissi MER, Pahaye DB, Ojong L, Mairara V, Ngo Bum E (2018) Anxiolytic and antiepileptic properties of the aqueous extract of Cissus quadrangularis (Vitaceae) in mice pilocarpine model of epilepsy. Front Pharmacol 9:10. https://doi.org/10.3389/fphar.2018.00751
Bainbridge Z, Tomlins K, Wellings K, Westb A (1996) Methods for assessing Quality characteristics of Non-Grains Starch Staples (Part 3. Laboratory methods). NRI, Chatham, UK
Chang C, Yang M, Wan H (2020) Estimation of the total flavonoid content in Propolis by two complimentary calorimetric methods. J Food Drug Anal 10:178–182. https://doi.org/10.38212/2224-6614.2748
Singh DK, Srivastava B, Sahu A (2004) Spectrophotometric determination of rauwolfia alkaloids: estimation of Reserpine in Pharmaceuticals. Anal Sci 20:571–573. https://doi.org/10.2116/analsci.20.571
OECD (2008) OECD guidelines for the testing of chemicals N°407: repeated dose 28. -day oral toxicity study in rodent
Suvarna K, Layton C, Bancroft (2019) Bancroft’s theory and practise of histological techniques 9th edition. Elsevier: 557p
Akyüz E (2020) A new view of the racing scoring system in the pentylenetetrazol epilepsy model. J Harran Univ Med Fac 17:306–310. https://doi.org/10.35440/hutfd.763232
Deeba F, Sanz-Leon P, Robinson PA (2020) Effects of physiological parameter evolution on the dynamics of tonic-clonic seizures. PLoS ONE 15:25. https://doi.org/10.1371/journal.pcbi.1006387
Bhardwaj M, Kumar A (2016) Neuroprotective effects of Lycopene against PTZ-induced kindling seizures in mice: possible behavioral, biochemical and mitochondrial dysfunction. Phytother Res 30:306–313. https://doi.org/10.1002/ptr.5533
Kumar A, Lalitha S, Mishra J (2013) Possible nitric oxide mechanism in the protective effect of hesperidin against petylenetetrazol (PTZ)-induced kindling and associated cognitive dysfunction in mice. Epilepsy Behav 29:103–111. https://doi.org/10.1016/j.yebeh.2013.06.007
Taiwe GS, Dabole B, Tchoya TB, Menanga JR, Dzeufiet PDD, De Waard M (2016) Anticonvulsant effects of iridoid glycosides fraction purified from Feretia apodanthera Del. (Rubiaceae) in experimental mice models of generalized tonic-clonic seizures. BMC Complement Altern Med 16:285–307. https://doi.org/10.1186/s12906-016-1269-8
Tran V, Hatalski CG, Yan X, Baram TZ (2011) Effects of blocking GABA degradation on corticotropin releasing hormone gene expression in selected brain region. Epilepsia 40:1190–1197. https://doi.org/10.1111/j.1528-1157.1999.tb00847.x
Kennedy AD, Pappan KL, Donti T, Delgado M, Shinawi M, Pearson TS, Lalani SR, Craigen WJ, Sutton VR, Evans AM, Sun Q, Emrick LT, Elsea SH (2019) 2-Pyrrolidinone and succinimide as clinical screening biomarkers for GABA-transaminase deficiency: ant seizure medications impact accurate diagnosis. Front Neurosci 13:12. https://doi.org/10.3389/fnins.2019.00394
Kaur N, Singh T, Kumar S, Goel RK (2017) Neurochemical evidence based suggested therapy for safe management of epileptogenesis. Epilepsy behave 72:8–16. https://doi.org/10.1016/j.yebeh.2017.4.004
Zhou Y, Danbolt NC (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121:799–817. https://doi.org/10.1007/s00702-014-1180-8
Mazhar F, Malhi SM, Simjee SU (2017) Comparative study in the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J Basic Clin Physiol Pharmacol 28:31–42. https://doi.org/10.1515/jbcpp-2016-0034
Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM (2013) ROS and brain diseases: the good, the bad and the ugly. Oxid. Med. Cell. Longev 2013: 14p. doi: https://doi.org/10.1155/2013/963520
Roganovic M, Pantovic S, Dizdarevic (2019) Role of the oxidative stress in the pathogenesis of epilepsy. Neurol Sci Neurophysiol 36:1–8. https://doi.org/10.5152/NSN.2019.11632
Fokoua AR, Ajayi AM, Ben-Azu B, Chouna R, Folarin O, Olopade J, Nkeng-Efouet AP, Aderibigbe AO, Umukoro S, Nguelefack TB (2021) The antioxidant and neuroprotective effects of the Psychotria camptopus Verd. Hook. (Rubiaceae) stem bark methanol extract contributes to its antiepileptogenic activity against pentylenetetrazol kindling in male Wistar rats. Metab Brain Dis 36:2015–2027. https://doi.org/10.1007/s11011-021-00825-1
Borowicz-Reutt KK, Czuczwar SJ (2020) Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep 72:1218–1226. https://doi.org/10.1007/s43440-020-00143-w
Simpson DSA, Oliver PL (2020) ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9:27. https://doi.org/10.3390/antiox9080743
Ganguly U, Kaur U, Chakrabarti SS, Sharma P, Agrawal BK, Saso L, Chakrabarti S (2021) Oxidative stress, neuroinflammation, and NADPH oxidase: implication in the pathogenesis and treatment of Alzheimer’s disease. Oxid. Med. Cell. Longev 2021: 19p. https://doi.org/10.1155/2021/7086512
Thompson KK, Tsirka SE (2017) The diverse role of microglia in neurodegenerative aspect of central nervous system (CNS) autoimmunity. Int J Mol Sci 18:15. https://doi.org/10.3390/ijms18030504
Hiragi T, Ikegaya Y, Koyama (2018) Microglia after seizure and in epilepsy. Cell 7:12. https://doi.org/10.3390/cells7040026
Wolinski P, Ksiazek-Winiarek D, Glabinski A (2022) Cytokines and neurodegeneration in epileptogenesis. Brain Sci 12:17. https://doi.org/10.3390/brainsci12030380
Vitkovic L, Maeda S, Sternberg E (2001) Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation 1:295–312. https://doi.org/10.1159/000059387
Kwon JY, Jeon M, Jung UJ, Kim DW, Moon GJ, Kim SR (2019) Perspective: therapeutic potentials of flavonoids as alternative medicines in epilepsy. ASN 10:778–790. https://doi.org/10.1080/01926230252824725
Wasowski C, Marder M (2012) Flavonoids as GABAA receptor ligands: the whole story? J Exp Pharmaco 4:9–24. https://doi.org/10.2147/JEP.S23105
Hanrahan JR, Chebib M, Johnston GA (2015) Interactions of flavonoids with ionotropic GABA receptors. Adv Pharmaco 72:189–200. https://doi.org/10.1016/bs.apha.2014.10.007
Sefil F, Kahraman I, Dokuyucu R, Gokce H, Ozturk A, Tutuk O, Aydin M, Ozkan U, Pinar N (2014) Ameliorating effects of quercentin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp 7:2471–2477 PMCID: PMC4211749
Moghbelinejad S, Alizadeh S, Mohammadi G, Khodabandehloo F, Rashvand Z, Najafipour R, Nassiri-asl M (2017) The effects of quercentin on the gene expression of the GABAA receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J Physiol Sci 67:339–343. https://doi.org/10.1007/s12576-016-0497-5
Impellizzeri D, Cordaro M, Campolo M, Gugliandolo E, Esposito E, Benedetto F, Cuzzocrea S, Navarra M (2016) Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (bje) in a mouse model of intestinal ischemia/perfusion injury. Front Pharmacol 7:7. https://doi.org/10.3389/fphar.2016.00203
Leyva-lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DI, Heredia JB (2016) Flavonoids as cytokine modulators: a possible therapy for inflammation-related disease. Int J Mol Sci 17:15. https://doi.org/10.3390/ijms17060921
Nassiri-asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi MR, Lotfizadeh M, Bazahang P (2013à Effects of quercentin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav 28: 151–155. doi: https://doi.org/10.1016/j.yebeh.2013.04.019
Cho H, Jeon S, Lee M, Kang K, Kang H, Park E, Kim M, Hong S, Seo S (2020) Analysis of the factors influencing body weight variation in Hanwoo Steers using an automated weighing system. Animals 10:8. https://doi.org/10.3390/ani10081270
Ghasemi A, Jeddi S, Kashfi K (2021) The laboratory rat: age and body weight matter. J Exp Clin Med 20:1431–1445. https://doi.org/10.17179/excli2021-4072
Uhuo EN, Ogugua VN, Emeka G (2014) A comparative study of the effects of ethanol bark and methanol leaf extracts of Kigelia africana on some biochemical parameters in alloxan induced diabetic rats. Glob J biotechnol Biochem 9:30–34. https://doi.org/10.5829/idosi.gjbb.2014.9.8491
Alsaadi BH, Shoaa HA, Sabrin RM, Amal AE, Dina SE, Gamal AM (2018) Hepatoprotective activity of costus species (Koen. Ex. Retz.) Against paracetamol-induced liver inhury in mice. Afr J Tradit Complement Altern Med 15:35–34. https://doi.org/10.21010/ajtcamv15i2.5
Jones R, Manickam C, Ram DR, Kroll K, Hueber B, Woolley G, Shah SV, Smith S, Varner V, Reeves K (2021) Systemic and mucosal mobilisation of granulocytes subsets during lentiviral infection. Immunology 164:348–357. https://doi.org/10.1111/imm.13376
Margraft A, Lowell CA, Zarbock A (2022) Neutrophils in acute inflammation: current concepts and translational implications. Blood 139:2130–2144. https://doi.org/10.1182/blood.2021012295
Sieminska I, Poljanska E, Baran J (2021) Granulocytes and cells of granulocytes origin-the relevant players in colorectal cancer. Int J Mol Sci 22:14. https://doi.org/10.3390/ijms22073801
Lewis RW, Bilington R, Debryune E, Gamer A, Lang B, Carpanini F (2002) Recognition of adverse and nonadverse effects in toxicity studies. Toxicol pathol 30:66–74. https://doi.org/10.1080/01926230252824725
Acknowledgements
The authors acknowledge the Research Unit of Animal Physiology and Phytopharmacology (URPAP) who hosted the present work.
Funding
Partial financial support was received by the International brain research organisation [IBRO Early Career grant] and the international society for neurochemistry [ISN CAEN − 1B] grants.
Author information
Authors and Affiliations
Contributions
A.F.F. co-designed the work, and conducted the laboratory trials, data analysis, and manuscript writing as part of its PhD thesis. G.T. N. designed the work and supervised the experiments, data analysis, and manuscript writing. Material preparation and data collection were performed by A.F.F. assisted by M.B.A., F. B.Y., T. D. K., C.M.N., and A.P., and supervised by G.T.N. and G.A. The first draft of the manuscript was written by A.F.F. and G.T.N. and G. A. commented the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval and consent to participate
Treatment of animals was done following the guidelines of the Cameroon bioethics committee (reg N. FWA IRB00001954) and in accordance with NIH-Care and use of laboratory animals manual, all of which are in line with Directives 2010/63/EU for animal experiments. Briefly, efforts were made to minimise animal suffering and to reduce the number of animal used in the experiment.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article were updated due to removal of co-authors email addresses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foutsop, A.F., Ateufack, G., Adassi, B.M. et al. The Aqueous Lyophilisate of Alchemilla Kiwuensis Engl. (Rosaceae) Displays Antiepileptogenic and Antiepileptic Effects on PTZ-induced Kindling in rats: Evidence of Modulation of Glutamatergic and GABAergic Pathways Coupled to Antioxidant Properties. Neurochem Res 48, 3228–3248 (2023). https://doi.org/10.1007/s11064-023-03982-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03982-0