Abstract
Neuropathy is considered a critical complication of diabetes mellitus (DM). Scientific studies are needed to relieve these painful complications. The current study aims to estimate the ameliorative role of Physalis juice (PJ) against neurological impairment in streptozotocin (STZ)-induced diabetic rats. Type 1 DM was induced after one week of injecting rats with 55 mg STZ/kg body weight. PJ-treated rats were orally administered 5 ml PJ/kg body weight per day for 28 days after induction of diabetes. A small piece of the cerebral cortex of rats was fixed and used for histopathological investigations. The remaining portion of the cerebral cortex was homogenized for biochemical and molecular analyses. As compared to the controls, STZ-injected rats showed significant elevations in the levels of blood glucose, tumor necrosis factor alfa, interleukin-1β, malondialdehyde, nitric oxide, and expression levels of caspase-3 and B-cell lymphoma-2 associated X-protein. Additionally, remarkable declines in the levels of brain-derived neurotrophic factor, monoamines, B-cell lymphoma-2, glutathione, as well as the activities and gene expression levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in STZ-treated rats were reported. Moreover, some histopathological alterations were observed in the brain cortex of the STZ-treated rats. On the other hand, the administration of PJ substantially reduced the blood glucose and alleviated the above-mentioned alterations in all the studied parameters of the cerebral cortex. In conclusion, an oral administration of 5 ml PJ/kg revealed a neuroprotective action against neurodegenerative diabetes-induced complications in rats, which might be due to the reported antioxidative and anti-inflammatory actions of PJ. Thus, further therapeutic studies are recommended to apply PJ in the treatment regimen of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is considered as one of the most common leading causes of death (Kang et al. 2016). Globally, the International Diabetes Federation expected that the number of diabetic patients to be about 693 million by 2045 (Cho et al. 2018). In Egypt, among all the adults, the prevalence of diabetes is around 15.56 % (Hegazi et al. 2015). The chief role of insulin is to decrease blood glucose levels by facilitating cellular glucose uptake. In diabetic patients, the body cannot regulate the amount of blood glucose leading to hyperglycemia due to increased insulin resistance or an insufficient insulin secretion (Rorsman and Ashcroft 2018). Type 1 of DM (T1DM) or insulin-dependent originates caused by insufficient secretion of insulin due to autoimmune destruction of β-cell in the pancreas (Zhang et al. 2019). However, type 2 of DM (T2DM), or insulin-independent, results from insulin resistance that is accompanied by a remarkable decline in the secretion of insulin by pancreatic β-cell (Kang et al. 2016).
Complications of diabetes also are considered a crisis that increases the rate of morbidity and mortality. Complications of DM are of two types; microvascular and macrovascular. Microvascular complications include neuropathy, nephropathy, and retinopathy (Huang et al. 2017). However, macrovascular complications involve coronary artery disease (CAD), peripheral arterial disease (PAD), and cerebrovascular disease (Vadivelu and Vijayvergiya 2018). Generally, DM complications can be due to the excessive production of free radical species following glucose oxidation (Sivaraman et al. 2013).
Several anti-diabetic chemical drugs are now on the market, but most of them neither prevent the complications of DM nor eliminate the disease. Furthermore, these chemical drugs mostly have undesirable side effects. Nowadays, scientific research aims to study the potential therapeutic and protective roles of natural products against various diseases and their complications. Physalis, locally known as Harankash, is a tropical fruit that belongs to the Solanaceae family (Dong et al. 2019). Recently, the antioxidative, nutraceutical, and medicinal characteristics of Physalis fruits are documented (El-Beltagi et al. 2019). To our knowledge, no scientific research was designed to investigate the protective role of physalis against the neurological complications of DM. Accordingly, the present work aims to evaluate the potential protective role of Physalis pubescens L. plant extract against the neuronal damage in male streptozotocin (STZ)-induced diabetic rats.
Materials and methods
Experimental model
Adult male Wistar rats, weighing 180–200 g, of 3 months old, were purchased from the Holding Company for Biological Products and Vaccines (VACSERA, Egypt). Rats were acclimatized for one week in the animal house of the Zoology Department, Faculty of Science, Cairo University. During acclimatization, rats were kept at a temperature of 22-25oC and were exposed to a 12/12 h light-dark cycle. Rats had access to ad libitum rodent chow and clean water.
Induction of diabetes
T1DM was induced in rats, after one week following a single intraperitoneal injection with 55 mg STZ/kg body weight (Al-Quraishy et al. 2015). STZ was diluted in a 0.05 M citrate buffer (pH 4.5) and was freshly prepared immediately before injection. Few blood drops were drained from the tail of STZ-injected rats every three days, following STZ injection to test the blood glucose levels using an Accu-check blood glucose meter (Roche Diagnostics, Basel, Switzerland).
Physalis juice preparation and stability
The fresh fruits of Physalis pubescens L. (10 kg) were separated from the outer calyxes and homogenized. The pulp was filtered off. The clear yellow filtrate was immediately diluted with distilled water in ratio 1:5 (V/V) to produce Physalis juice (PJ). The resultant PJ was stored at a temperature of 4 °C.
Experimental design
Thirty rats were equally and randomly divided into five groups (n = 6 rats per group), as shown in Table 1. The applied doses of PJ and Met were according to Dewi and Sulchan (2018) and Waisundara et al. (2008), respectively.
Sampling
Rats were suddenly decapitated at 24 h after the last treatment, and the brain was removed. The obtained brains were used for further histological, molecular, and biochemical studies. Brains were rapidly excised from skulls, blotted, and chilled. The brain tissue was rapidly wiped dry with filter paper. Dissection was performed on an ice-cooled glass plate, and then the cerebral cortex was extracted as described by Onaolapo et al. (2019).
Preparation of brain homogenates
The extracted cerebral cortex was divided into two parts. The first part was homogenized in ten volumes of the ice-cold medium of 50 mM Tris–HCl (pH 7.4). The homogenate was centrifuged at 1000 rpm for 10 min at 4ºC. The supernatant was then separated and was stored at – 80ºC till the beginning of biochemical measurements. In the tissue homogenates, the protein level analysis was according to the method described by Simeonova et al. (2019). For the determination of neurotransmitters, part of the cerebral cortex was homogenized in 75 % aqueous HPLC grade methanol (10 % w/v). The produced homogenate was spun at 4000 rpm for 10 min.
Biochemical parameters
Estimation of blood glucose levels
On the 28th day, few blood drops were collected by piercing the tail to detect the glucose levels. The blood glucose levels were analysed using an Accu-check blood glucose meter (Roche Diagnostics, Basel, Switzerland).
Determination of tumour necrosis factor- alpha and interleukin-1 beta levels
Quantitative estimation of interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) levels in the brain cortex were executed using enzyme-linked immunosorbent assay (ELISA) kits specified for rats supplied by Novus Biologicals (Centennial, CO, USA) based on the manufacturer’s protocols (Wang et al. 2019a, c).
Determination of nitric oxide level
Quantitative determination of nitric oxide (NO) concentrations in the brain cortex was achieved by determination of total nitrate (NO3) and nitrite (NO2) concentrations. Through acid reduction method in an acidic medium with the presence of nitrite. The formed nitrous acid diazotize sulphanilamide and the product was coupled with N-(1-naphthyl) ethylenediamine and the resultant was bright reddish-purple azo dye which was measured at 540 nm (R&D Systems, Minneapolis, USA) adopted by Green et al. (1982).
Quantification of brain‐derived neurotrophic factor
Quantitative estimation of brain-derived neurotrophic factor (BDNF) was performed using ELISA kits (BDNF, Cat. no. EM2IL1B, ThermoFisher Scientific) as described by Dalise et al. (2017).
Determination of monoamines neurotransmitters concentrations
The levels of monoamines (Norepinephrine, dopamine, and serotonin “5-HT”) in the cerebral cortex were detected using HPLC in the 1st half of the supernatant according to the method of Pagel et al. (2000).
Determination of apoptotic protein levels
Quantitative estimation of B-cell lymphoma 2 (Bcl-2) and Bcl-2 associated X (Bax) levels were estimated through using ELISA kits supplied by Life Span Bio Sciences, Inc., (Seattle, WA, USA). The Bax and Bcl-2 protein levels were estimated according to the methods described by Baty et al. (2020) and Albasher et al. (2020), respectively.
Determination of lipid peroxidation
In an acidic medium, LPO was determined in terms of malondialdehyde (MDA) formation in the brain cortex in all experimental groups according to the method described by Ohkawa et al. (1979). The resulted red pigment was extracted with the n-butanol-pyridine mixture and estimated by the absorbance at 532nm.
Determination of antioxidants
The antioxidant levels in the cerebral cortex homogenate were analyzed using Bio-diagnostics kits (Dokki, Giza, Egypt). The glutathione (GSH) levels and the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were estimated according to the techniques described by Sayed et al. (2018). However, glutathione reductase (GR) activity was analyzed using the method described by Abdel-Ghaffar et al. (2018).
Quantitative real time polymerase chain reaction
Total RNA was extracted from the brain cortex by using triazole, and then RNA was converted to complementary DNA (cDNA) using cDNA Synthesis Kit (Bio-Rad, Belgium). For gene expression analysis using quantitative real-time polymerase chain reaction (RT-PCR), cDNA of the oxidative stress enzyme, and apoptotic markers (GPX1, GR, SOD2, CAT, Bcl-2, Bax, and Cas 3) were utilized as a template. QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) and the corresponding forward and reverse primers were used. Primers were obtained from (Jena Bioscience GmbH, Jena, Germany). All experiments were carried out in triplicate using Applied Biosystems 7500 Instrument (Thermo Fisher Scientific, CA, USA).
Histological investigations
The cerebral cortex was fixed in 10 % formalin for 24 h. Then, alcohol was applied for dehydration. The fixed tissues were cleared using xylene and then mounted in molten paraplast. Tissues were cut by microtome into thin sections (4–5 μm). Then, sections were stained by hematoxylin and eosin. Nikon light microscope (Eclipse E200-LED, Tokyo, Japan) was utilized to investigate the stained specimens.
Statistical analysis
Data statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 23. According to Kolmogorov-Smirnova and Shapiro-Wilk tests, data showed normal distribution within each of the experimental groups. Thus, one-way analysis of variance test was recommended to study the effect of different treatments on the studied parameters. Post-hoc multi-comparison Duncan’s test was applied to study homogeneity in the studied variables among the experimental groups. The changes between the means were considered statistically significant when the value of p < 0.05. The data were expressed as mean ± standard errors of the mean.
Results
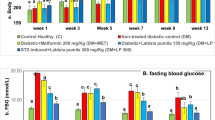
Effect on the levels of blood glucose
The concentrations of blood glucose in all the experimental groups were estimated in Table 2. In the rats of all STZ-induced diabetic groups either without any treatment or after treatment with PJ or Met, the blood glucose levels were significantly higher than those of the controls. However, STZ + PJ-treated rats exhibited a significant decline in the blood glucose levels, as compared to those of the STZ-treated and STZ + Met-treated groups, towards the control levels.
Effect on the levels of tumour necrosis factor- alpha and interleukin-1 beta
In Table 2, the TNF-α and IL-1β levels in the brain cortex of all rats were recorded. The TNF-α and IL-1β levels of the STZ + PJ-treated group were remarkably higher than the control but substantially lower than STZ-treated group. As compared to STZ + Met-treated group, the IL-1β level of STZ + PJ rats was reduced markedly.
Effect on the levels of nitric oxide
The data of NO levels in the brain of all groups were estimated (Table 2). In the rats of STZ + PJ-treated and STZ + Met-treated groups, the NO levels were remarkably higher than the controls, whereas substantially lower than those of STZ-treated group.
Effect on the levels of brain‐derived neurotrophic factor
The BDNF levels in the cerebral cortex homogenate were measured (Table 3). As compared to the control group, the BDNF levels in the cerebral cortex of all the experimental rats did not exhibit any significant changes except for a marked decline in the STZ-treated group.
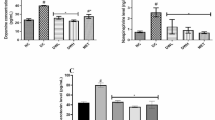
Effect on the levels of monoamine neurotransmitters
The levels of monoamine neurotransmitters (norepinephrine, serotonin, and dopamine) in the brain cortex of all the experimental rats, were displayed in Table 3. As compared to the controls, all the studied monoamine levels in the brain cortex were markedly elevated in the PJ-treated group, whereas were significantly reduced in the rats administered STZ alone. As compared to the STZ-treated group, the levels of all the studied monoamines in the cerebral cortex of STZ + PJ-treated and STZ + Met-treated groups were markedly elevated towards the control values.
Effect on Bcl-2 family of proteins and caspases
Gene expression levels of Bcl-2, Bax, and Cas-3, as well as the concentrations of Bcl-2 and Bax in the brain cortex of all rats, were reported in Table 4. In the rats of the PJ-treated group, the mRNA expression level of Bcl-2 was significantly upregulated as compared to the control group. In the STZ-treated and STZ + Met-treated groups, mRNA expression level and protein level of Bcl-2 were remarkably lower than the corresponding controls. As compared to STZ-treated group, the mRNA expression level and protein level of Bcl-2 in the cerebral cortex of STZ + PJ-treated group were markedly elevated towards the normal values of controls. On the other hand, the mRNA expression levels, and the concentrations of Bax in the brain cortex of STZ + PJ-treated and STZ + Met-treated groups, were remarkably higher than the control, but substantially lower than those of STZ-treated group. In the brain cortex of STZ + PJ-treated and STZ + Met-treated groups, the mRNA expression levels of Cas-3 were markedly higher than the control but were meaningfully lower than those administered STZ alone.
Effect on the levels of lipid peroxidation and endogenous antioxidants
The levels of MDA, GSH, and mRNA expression levels and activities of SOD, CAT, GPx, and GR in the brain cortex of rats were estimated (Table 5).
The MDA levels in the STZ + PJ and STZ + Met groups were significantly lower than those of STZ-treated group but were remarkably higher than the control rats. As compared to the STZ-treated group, the GSH content and SOD, CAT, GPx, and GR activities of STZ + PJ-treated group were significantly elevated towards the control values.
In comparison to the controls, gene expression levels of SOD, CAT, GPx, and GR in the cerebral cortex of STZ-treated group were markedly downregulated. On the other hand, the mRNA levels of all the studied enzymatic antioxidants in the PJ-treated group were significantly higher than the controls. As compared to STZ-treated group, the gene expression levels of SOD, CAT, GPx, and GR of STZ + PJ-treated group were remarkably increased towards the control levels.
Effect on histological investigation
Histopathological examination of the cerebral cortex with light microscopy of all the experimental rats was displayed in Fig. 1. In rats of the control and PJ-treated group, the cerebral cortex showed regular histological architecture. In contrast, the brain tissue of STZ-treated group showed degenerated nerve cells with deeply stained nuclei, several apoptotic neurons, inflammatory cell infiltration in the cerebral cortex. Attractively, daily oral administration of PJ was able to abolish most of the histopathological alterations in the brain cortex of the STZ-induced diabetic rats.
Light microscope photography of cross sections in cerebral cortex stained with hematoxylin & eosin from the control rats (a) and those orally administered physalis-juice (PJ) alone (b), as well as those of STZ-induced diabetic rats without treatment (c) or after treatment with PJ (d) or metformin (e). White arrow indicates degenerated neurons. Blue arrow indicates apoptotic neurons. Red arrow indicates inflammatory cell infiltration
Discussion
Before starting the experiments, hyperglycemia in all the rats injected with STZ was manifested (blood glucose levels > 200 mg/dl). After 28 days, rats of the STZ-induced diabetic group with no treatment showed a significant elevation in the blood glucose levels above 350 mg/dL. It is documented that intraperitoneal injection of rats with a large dose of STZ (50–60 mg) can lead to the destruction of β-cells in the pancreas causing a reduced secretion of insulin and subsequently induce T1DM (Zhang et al. 2019). Structurally, STZ contains a glucose moiety that enables its facilitated diffusion via glucose transporter in the β-cell membranes (Bouafir et al. 2017). Similarly, Kim et al. (2017) reported that the blood glucose levels of rats injected with 50 mg of STZ significantly exceeded 300 mg/dL. They considered that as a sign of diabetes induction.
On the other hand, the blood glucose levels of rats administered PJ after the induction of diabetes with STZ were markedly reduced to less than 200 mg/dL, in the present data. It is noteworthy that administering PJ caused a significant reduction in the blood glucose levels even, greater than the administration of Met. Physalis-induced hypoglycemia may be due to the inhibition of α-glucosidase, which is involved in the intestinal digestion of complex carbohydrates (Rey et al. 2015). Physalin, an active ingredient in physalis extract, can cause inhibition of glucagon while enhancing the insulin secretion from β-cells of the pancreas (Wang et al. 2018).
Another mechanism for explaining the STZ-induced damage of β-cells can be due to its nitrosourea moiety that may enhance the production of NO which acts as a highly reactive free radical, leading to the destruction of the genetic material (Mohamed et al. 2018). It is worth mentioning that STZ can activate nitric acid synthases to enhance the production of NO, leading to a substantial downregulation of SOD, CAT, and GPx (Dinçel et al. 2018). These suggestions were ensured by the elevated levels of NO and MDA associated with significant declines in activities of SOD, CAT, GR, and GPx, as well as the level of GSH, in the cerebral cortex of STZ-induced diabetic rats. MDA is a byproduct of LPO that attacks biomolecules causing β-pancreatic cell damage and subsequently altering glucose metabolism (Sivaraman et al. 2013). Also, the excessively produced reactive oxygen species (ROS) can downregulate the gene expression levels of these endogenous antioxidants (Ali et al. 2021). This explanation can be evidenced by the reduced mRNA expression levels of SOD, CAT, GPx, and GR, in the present study.
On the other hand, the administration of PJ following STZ-induction of diabetes caused remarkable elevations in the activities of SOD, CAT, GPx, and GR, whereas a significant decline in the levels of MDA in the cerebral cortex of rats in the present study. These findings can be attributed to the active ingredients of physalis extract like carotenoids, flavonoids, and vitamins, which can scavenge free radicals and boost the synthesis of the endogenous antioxidants (El-Beltagi et al. 2019). These outcomes were supported by the recorded upregulations of gene expression levels of mRNA of SOD, CAT, GPx, and GR of rats administered PJ following STZ-induced diabetes. Additionally, Dong et al. (2019) isolated five new Withanolides, naturally produced steroids, from the Physalis peruviana. They reported that these isolated Withanolides can act as NO inhibitors. This suggestion explains the reported depletion in the NO levels in the STZ + PJ group.
The process of programmed cell death “apoptosis” is characterized by some biochemical and morphological alterations like blebbing of plasma membranes, loss of cell volume, chromatin condensation, and nuclear decay. Cas-3 is a critical protein in the apoptotic signaling pathway that is encoded by the Cas-3 gene. The activation of Cas-3 induces apoptosis (Yin et al. 2016). Both Bax and Bcl-2 are proteins that belong to the Bcl-2 family, which regulates apoptosis. Bax protein acts as an apoptotic protein that initiates the cell apoptosis, whereas Bcl-2 acts as an anti-apoptotic protein that inhibits the apoptosis (He et al. 2018). In the present data, as compared to the controls, a remarkable elevation in the Bax protein concentration, whereas a marked decline in Bcl-2 protein level, in STZ-injected rats were reported. These findings can be due to the STZ-induced production of free radicals leading to significant alterations in the genes encoding for the apoptotic proteins. This explanation was ensured by the reported alterations in the expression levels of Bax, Bcl-2, and Cas-3 in the brain cortex of STZ-injected rats, in the present data. Furthermore, in the present study, apoptosis was ensured by the histopathological investigation of the brain cortex. He et al. (2018) reported similar findings in the levels of apoptotic proteins in the brain hippocampus of STZ-induced diabetic rats.
On the other hand, administering PJ after induction of diabetes caused significant elevations in the protein and mRNA expression levels of Bcl-2, whereas remarkable declines in those of Bax and Cas-3, as compared to those of STZ-injected rats. The reported PJ-induced changes may be due to its scavenging power toward the excessively generated free radicals involved in apoptosis (Çakir et al. 2014).
In the current work, the brain cortex of rats of STZ-treated groups showed significant elevations in the TNFα and IL-1β, as compared to the controls. TNF-α and IL-1β are two critical cytokines that appear early during inflammation (Zhang et al. 2017). Saperstein et al. (2009) suggested that IL-1β can enhance the TNF-receptors expression leading to the modulation of TNFα-mediated inflammatory responses. Similarly, Samarghandian et al. (2017) reported that STZ caused an elevation in the serum levels of glucose, MDA, IL-6, and TNF-α with a reduction in the GSH content. They suggested that the increased levels of TNF-α can alter the phosphorylation of insulin receptors leading to downregulation of the insulin signaling pathway. TNF-α can induce the lipolysis process, resulting in the liberation of more free fatty acids available for lipid peroxidation (Samarghandian et al. 2017).
In contrast, after 28 days of treatment with PJ, the brain cortex of STZ-induced diabetic rats showed significant declines in the levels of TNFα, IL-1β, and NO towards the control values, as compared to the STZ-injected rats. These results may be due to Physalin E that targets the nuclear factor-κappa Beta signaling pathway, leading to the inhibition of inflammatory cytokines production (Yang et al. 2017).
In the STZ-induced diabetic groups, the levels of BDNF, dopamine, norepinephrine, and serotonin were meaningfully lower than the controls. BDNF, one of the growth factors of the neurotrophin family that regulates neuronal survival and proliferation in the brain (Martinowich and Lu 2008). The reported reductions in the levels of BDNF can be linked to the diabetes-associated alterations in the glucocorticoid level (Prabhakar et al. 2015). It is noteworthy that BDNF is implicated in regulating the brain serotonin levels (Martinowich and Lu 2008). Down-regulation of monoamines in the brain was considered one of the most accepted mechanisms for diabetes-induced depression (Prabhakar et al. 2015). Insulin secretion was implicated in regulating norepinephrine production and secretion (Robertson et al. 2010). Accordingly, the observed reduction in the levels of norepinephrine can be attributed to STZ-induced hypoinsulinemia.
On the other hand, in the rats administered STZ + PJ, the levels of BDNF and the studied monoamine neurotransmitters were significantly higher than those of other STZ-injected groups. Accordingly, PJ substantially reduced the diabetes-associated neuropathy. It is documented that Physalis contains flavonoids and endogenous benzodiazepines that have analgesic effects on CNS (Giorgetti and Negri 2011). Flavonoids can inhibit monoamine oxidases hampering the breakdown of monoamines (Wang et al. 2019b). Similarly, Moneim et al. (2014) reported significant elevations in the levels of serotonin and dopamine in the brain of rats intoxicated with cadmium after treatment with Physalis extract.
Conclusions
In summary, we can conclude that STZ-induced diabetes caused remarkable alterations in the levels of all the studied biochemical, oxidative stress biomarker, apoptotic proteins, histopathology of the brain cortex of rats. On the other hand, PJ administration substantially ameliorated all the above-mentioned alterations in a way better than the applied traditional drug Metformin. The present study can pave the way for further therapeutic studies using PJ to alleviate diabetes-associated neurological implications.
Recommendations
Further neurobehavioral studies are recommended for confirming the present results.
Data availability
All data are available upon request.
Code availability
It is not applicable.
References
Abdel-Ghaffar O, Ali AA, Soliman SA (2018) Protective effect of naringenin against isoniazid-induced adverse reactions in rats. Int J Pharmacol 14(5):667–680. https://doi.org/10.3923/ijp.2018.667.680
Albasher G, Alsaleh AS, Alkubaisi N, Alfarraj S, Alkahtani S, Farhood M, Alotibi N, Almeer R (2020) Red beetroot extract abrogates chlorpyrifos-induced cortical damage in rats. Oxid Med Cell Longev 2020:2963020. https://doi.org/10.1155/2020/2963020
Ali AA, Mansour AB, Attia SA (2021) The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ Sci Pollut Res. https://doi.org/10.1078/0944711041495254
Al-Quraishy S, Dkhil MA, Abdel Moneim AE (2015) Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomed 10:6741–6756
Baty RS, Hassan KE, Alsharif KF, El-Hennamy RE, Elmahallawy EK, Hafez MM, Moneim AA, Kassab RB (2020) Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum Exp Toxicol 39(9):1200–1212. https://doi.org/10.1177/0960327120913094
Bouafir Y, Ait-Lounis A, Laraba-Djebari F (2017) Improvement of function and survival of pancreatic beta-cells in streptozotocin-induced diabetic model by the scorpion venom fraction F1. Toxin Rev 36(2):101–108
Çakir Ö, Pekmez M, Çepni E, Candar B, Fidan K (2014) Evaluation of biological activities of Physalis peruviana ethanol extracts and expression of Bcl-2 genes in HeLa cells. Food Sci Technol 34(2):422–430
Cho NH, Shaw JE, Karuranga S, Huang Y, Da RFJ, Ohlrogge AW, Malanda B (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 18:271–281
Dalise S, Cavalli L, Ghuman H, Wahlberg B, Gerwig M, Chisari C, Ambrosio F, Modo M (2017) Biological effects of dosing aerobic exercise and neuromuscular electrical stimulation in rats. Sci Rep 7(1):1–13
Dewi L, Sulchan M (2018) Potency of cape gooseberry (Physalis Peruviana) juice in improving antioxidant and adiponectin level of high fat diet streptozotocin rat model. Rom J Diabetes Nutr Metab Dis 25(3):253–260. https://doi.org/10.2478/rjdnmd-2018-0029
Dinçel GU, Yildirim S, Oğuz KU (2018) Role of nitric oxide and oxidative stress in pathophysiology of liver injury in streptozotocin-induced type 1 diabetic rats. Ankara Üniversitesi Veteriner Fakültesi Dergisi 65(1):39–50
Dong B, An L, Yang X, Zhang X, Zhang J, Tuerhong M, Jin DQ, Ohizumi Y, Lee D, Xu J, Guo Y (2019) Withanolides from Physalis peruviana showing nitric oxide inhibitory effects and affinities with iNOS. Bioorg Chem 87:585–593
El-Beltagi HS, Mohamed HI, Safwat G, Gamal M, Megahed BM (2019) Chemical composition and biological activity of Physalis peruviana L. Gesunde Pflanz 71(2):113–122
Giorgetti M, Negri G (2011) Plants from Solanaceae family with possible anxiolytic effect reported on 19th century’s Brazilian medical journal. Rev Bras Farmacogn 21:772–780
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal biochem 126(1):131–138
He X, Sun J, Huang X (2018) Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp Therap med 15(1):873–887. https://doi.org/10.3892/etm.2017.5438
Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O (2015) Epidemiology of and risk factors for type 2 Diabetes in Egypt. Ann Glob Health 81(6):815–816. https://doi.org/10.1016/j.aogh.2015.12.011
Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C (2017) Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int 2017:7839101. https://doi.org/10.1155/2017/7839101
Kang YM, Kim YJ, Park JY, Lee WJ, Jung CH (2016) Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovas Diabetol 15(1):131
Kim HJ, Jung BH, Yoo KY, Han JW, Um HS, Chang BS, Lee JK (2017) Determination of the critical diabetes duration in a streptozotocin-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration. J Periodontal Implant Sci 47(5):339–350
Martinowich K, Lu B (2008) Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 33:73–83
Mohamed AA, Ali MM, Dorrah MA, Bassal TT (2018) Mediation of inducible nitric oxide and immune-reactive lysozymes biosynthesis by eicosanoid and biogenic amines in flesh flies. Int J Trop Insect Sci 38(1):93–104. https://doi.org/10.1017/S1742758417000315
Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF (2014) The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol Trace Elem Res 160(3):392–399
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Onaolapo AY, Ayeni OJ, Ogundeji MO, Ajao A, Onaolapo OJ, Owolabi AR (2019) Subchronic ketamine alters behaviour, metabolic indices and brain morphology in adolescent rats: Involvement of oxidative stress, glutamate toxicity and caspase-3-mediated apoptosis. J Chem Neuroanat 96:22–33. https://doi.org/10.1016/j.jchemneu.2018.12.002
Pagel P, Blome J, Wolf HU (2000) High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J Chromatogr B Biomed Sci Appl 746(2):297–304
Prabhakar V, Gupta D, Kanade P, Radhakrishnan M (2015) Diabetes-associated depression: the serotonergic system as a novel multifunctional target. Indian J Pharmacol 47(1):4–10
Rey DP, Ospina LF, Aragón DM (2015) Inhibitory effects of an extract of fruits of Physalis peruviana on some intestinal carbohydrases. Rev Colomb Cienc Quím Farm 44(1):72–89
Robertson SD, Matthies HJ, Owens WA, Sathananthan V, Christianson NS, Kennedy JP, Lindsley CW, Daws LC, Galli A (2010) Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J Neurosci 30(34):11305–11316
Rorsman P, Ashcroft FM (2018) Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 98(1):117–214
Samarghandian S, Borji A, Farkhondeh T (2017) Attenuation of oxidative stress and inflammation by Portulaca oleracea in streptozotocin-induced diabetic rats. J Evid Based Complement Alternat Med 22(4):562–566
Saperstein S, Chen L, Oakes D, Pryhuber G, Finkelstein J (2009) IL-1beta augments TNF-alpha-mediated inflammatory responses from lung epithelial cells. J Interferon Cytokine Res 29(5):273–284
Sayed AA, Ali AA, Mohamed HR (2018) Fertility enhancing efficacy of Cicer arietinum in male albino mice. Cell Mol Biol 64(4):29–38. https://doi.org/10.14715/cmb/2018.64.4.6
Simeonova R, Vitcheva V, Kondeva-Burdina M, Popov G, Shkondrov A, Manov V, Krasteva I (2019) Alcesefoliside protects against oxidative brain injury in rats. Rev Bras Farm 29(2):221–227
Sivaraman K, SenthilKumar GP, Sankar P, Bobby Z (2013) Attenuation of oxidative stress, inflammation and insulin resistance by Allium sativum in fructose-fed male rats. J Clin Diagn Res 7:1860–1862. https://doi.org/10.7860/jcdr/2013/6924.3334
Vadivelu R, Vijayvergiya R (2018) Panvascular risk factor – Diabetes. Cor Vasa 60(1):18–29
Waisundara VY, Hsu A, Huang D, Tan BKH (2008) Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chin Med 36(3):517–540
Wang A, Wang S, Zhou F, Li P, Wang Y, Gan L, Lin L (2018) Physalin B induces cell cycle arrest and triggers apoptosis in breast cancer cells through modulating p53-dependent apoptotic pathway. Biomed Pharmacother 101:334–341. https://doi.org/10.1016/j.biopha.2018.02.094
Wang C, Liu Y, Wang Y, Wei Z, Suo D, Ning G, Wu Q, Feng S, Wan C (2019a) Low–frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury. Mol Med Rep 19(3):1687–1693
Wang J, Cheng C, Xin C, Wang Z (2019b) The antidepressant-like effect of flavonoids from Trigonella foenum-graecum seeds in chronic restraint stress mice via modulation of monoamine regulatory pathways. Molecules 24(6):1105. https://doi.org/10.3390/molecules24061105
Wang Y, Wang D, Jin Z (2019c) miR–27a suppresses TLR4–induced renal ischemia–reperfusion injury. Mol Med Rep 20(2):967–976
Yang YJ, Yi L, Wang Q, Xie BB, Dong Y, Sha CW (2017) Anti-inflammatory effects of physalin E from Physalis angulata on lipopolysaccharide-stimulated RAW 264.7 cells through inhibition of NF-κB pathway. Immunopharmacol Immunotoxicol 39(2):74–79
Yin C, Huang GF, Sun XC, Guo Z, Zhang JH (2016) Tozasertib attenuates neuronal apoptosis via DLK/JIP3/MA2K7/JNK pathway in early brain injury after SAH in rats. Neuropharmacology 108:316–323
Zhang F, Si M, Wang H, Mekhemar MK, Dörfer CE, Fawzy El-Sayed KM (2017) IL-1/TNF-α inflammatory and anti-inflammatory synchronization affects gingival stem/progenitor cells’ regenerative attributes. Stem Cells Int. 2017:1349481. https://doi.org/10.1155/2017/1349481
Zhang D, Yu YJ, Xu FS, Yuan JH, Wang R, Zhang CS, Wang LX, Liu Y, Song LM, Liu JL, Dong J (2019) Recombinant betatrophin (Angptl8/lipasin) ameliorates streptozotocininduced hyperglycemia and βcell destruction in neonatal rats. Mol Med Rep (5):4523–4532
Funding
This research work was not funded.
Author information
Authors and Affiliations
Contributions
Atef Abdel-Moneem Ali contributed to the experimental design, statistical analysis, manuscript writing and editing. Ehab Abdel-Raouf Essawy contributed to the experimental design and experimental procedures. Heba Salah El-Din Fathy Hamed contributed to the animal handling and biochemical analysis. Ahmed E. Abdel Moneim contributed to the molecular analysis and experimental procedures. Fawzy Ali Attaby contributed to the experimental design and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The applied procedures and techniques, in the present study, were approved by Cairo University - Institutional Animal Care and Use Committee (CU-IACUC), Giza, Egypt. The approval number is CU/I/F/21/19. Handling of the animals was performed according to the international guidelines of the laboratory animal care and use.
Consent to participate
It is not applicable.
Consent for publication
The data provided here is original.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A.AM., Essawy, E.AR., Hamed, H.S.ED.F. et al. The ameliorative role of Physalis pubescens L. against neurological impairment associated with streptozotocin induced diabetes in rats. Metab Brain Dis 36, 1191–1200 (2021). https://doi.org/10.1007/s11011-021-00730-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00730-7