Abstract
The present study was carried out to investigate the protective effect of Physalis peruviana L. (family Solanaceae) against cadmium-induced neurotoxicity in rats. Adult male Wistar rats were randomly divided into four groups. Group 1 was used as control. Group 2 was intraperitoneally injected with 6.5 mg/kg bwt of cadmium chloride for 5 days. Group 3 was treated with 200 mg/kg bwt of methanolic extract of Physalis (MEPh). Group 4 was pretreated with MEPh 1 h before cadmium for 5 days. Cadmium treatment induced marked disturbances in neurochemical parameters as indicating by significant (p < 0.05) reduction in dopamine (DA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in cerebellum, hippocampus, and cerebral cortex and enhanced significantly (p < 0.05) the levels of lipid peroxidation and nitric oxide in the brain. Cadmium treatment also decreased the amount of nonenzymatic and enzymatic antioxidants significantly (p < 0.05). Pretreatment with MEPh resulted in significant (p < 0.05) decreases in lipid peroxidation and nitric oxide levels and restored the amount of glutathione successfully. Although, preadministration of MEPh also brought the activities of cellular antioxidant enzymes, namely superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase significantly (p < 0.05) to the control levels, as well as the levels of Ca2+, Cl−, DA, 5-HT, and serotonin metabolite, 5-HIAA. These data indicated that Physalis has a beneficial effect in ameliorating the cadmium-induced oxidative neurotoxicity in the brain of rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a major toxic metal and represents an increasing risk for cancer as a pollutant of the environment at large. Inhalation of Cd fumes or dust is the main route in occupational exposure to Cd. It is widely used in industry as anticorrosive in plating metals and manufacturing storage batteries, alloys, stabilizer, and pigments. Also, it is absorbed in significant quantities from cigarette smoke. Therefore, Cd concentration has been widely increased in the environment [1, 2].
Cadmium accumulation in the human body is dangerous and may also be linked to osteomalacia, infertility, hepatotoxicity, and nephrotoxicity [1]. Due to its high blood-brain barrier permeability [3], neurological disorders such as learning disabilities and hyperactivity in children may occur after Cd exposure [4]. Parkinsonism has been noticed by Okuda et al. [5] after acute exposure to Cd. The mechanism by which Cd induces neurological damage is partially known. Cd induces oxidative stress, which produces protein damage in neuronal cells [4] and subsequently neurodegeneration [5]. Cd is also known to enhance the production of free radicals in the brain and affects the cellular defense mechanism [6]. However, the toxic effects of Cd are rather complex and still debated [3].
Strong interest in the antioxidant properties of fruits is growing rapidly during the last few years [7, 8]. Physalis peruviana Linnaeus is belonging to the family Solanaceae and genus Physalis. Many medicinal properties are attributed to Physalis such as antispasmodic, diuretic, antiseptic, sedative, analgesic, helping to fortify the optic nerve, throat trouble relief and elimination of intestinal parasites and amoeba. There are studies indicating that eating the fruit of Physalis reduces blood glucose after 90 min postprandial in young adults, causing a greater hypoglycemic effect after this period [8]. The calyces of Physalis are widely used in folk medicine for its properties as anticancer, antimicrobial, antipyretic, diuretic, and anti-inflammatory immunomodulator [9]. For examples, in different regions of Colombia, some of its medicinal properties are to purify blood of kidneys, decrease albumin, clean the cataract, and calcify and control amebiasis [8]. In Peruvian traditional medicine, the fruit of Physalis is used empirically to treat cancer and other diseases like hepatitis, asthma, malaria, and dermatitis; however, their properties have not been scientifically proven [10].
In this study, we evaluated the use of Physalis against cadmium-induced neurotoxicity in rats. Such investigation will explore the potential therapeutic or preventive approaches that can be developed in future studies by blocking or minimizing the destructive effects of Cd to the brain.
Materials and Methods
Animals
Twenty-eight adult male Wistar albino rats weighing 200–250 g (10–12 weeks) were obtained from The Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). The animals were kept in wire-bottomed cages in a room under standard condition of illumination with a 12-h light-dark cycle at 25 ± 1 °C for 1 week until the beginning of treatment. They were provided with tap water and balanced diet ad libitum. All animals have received human care in compliance with the state authorities following the Egyptian rules of animal protection.
Plant Material
P. peruviana L. fresh fruits were collected from the market of East Cairo, Egypt, in the months of February–March 2012. The plant material was authenticated in the Botany Department, Faculty of Science, Helwan University, Cairo, Egypt, on the basis of taxonomic characters and by direct comparison with the herbarium specimens available at the herbarium of the Botany Department.
Physalis Juice Preparation and Stability
The methanolic extract of Physalis (MEPh) was prepared as described [11]. Briefly, the fresh fruits of P. peruviana L. (10 kg) were separated from their calyces and homogenized. The pulp was consecutively macerated for 1 day in petroleum ether, ethyl acetate, chloroform, and methanol, respectively. On the basis of the preliminary phytochemical tests conducted, the methanol extract was found to be rich in terms of chemical constituents and therefore was selected for the experiment. The methanol was removed under reduced pressure to obtain a semisolid mass of MEPh. The MEPh was then stored in −20 °C until used.
Experimental Protocol
To study the protective effects of Physalis on Cd-mediated neurological toxicity, adult male rats were randomly allocated to four groups of seven rats of each. Group 1 (Con) served as control and received 300 μl of saline by oral administration route every day for 5 days. Group 2 (Cd) received daily 300 μl of saline by oral administration, and after 1 h, they received intraperitoneal injection of 6.5 mg CdCl2/kg bwt for 5 days. Group 3 (MEPh) received daily oral administration of 200 mg MEPh/kg bwt for 5 days, and the animals of group 4 (MEPh + Cd) administrated with 200 mg MEPh/kg bwt 1 h before cadmium injection and daily for 5 days. The level of the orally administered dosage of MEPh (200 mg/kg bwt) was based on the previous work of Abdel Moneim and El-Dieb [12] and the dose of CdCl2 (6.5 mg/kg bwt) selected on the basis of the previous studies [13, 14].
After 24 h of the last injection of CdCl2, the animals of all groups were sacrificed by cervical dislocation under diethyl ether anesthesia. Brains of rats were carefully removed. Dissection of brains was performed on an ice-cold glass plate for the separation of three brain regions (cerebellum, hippocampus, and cerebral cortex) according to the method described by Glowinski et al. [15]. The three regions from seven rats in each group were longitudinally divided into two equal parts; the first parts were stored frozen for further determination of monoamines and ions levels. The second parts were weighed and homogenized immediately to give 50 % (w/v) homogenate in ice-cold medium containing 50 mM Tris-HCl, pH 7.4, and centrifuged at 500 g for 10 min at 4 °C. The supernatant was used for the various biochemical determinations.
The total protein content of the homogenized brain was determined by the method of Lowry et al. [16] using bovine serum albumin as a standard.
Biochemical Estimations
Monoamines and Ion Levels in Brain Regions
Dopamine (DA), serotonin (5-HT), and serotonin metabolites, 5-hydroxyindoleacetic acid (5-HIAA), were extracted and measured in the three regions of each brain using fluorometric technique according to the modified method of Ciarlone [17]. The fluorescence was measured in Jenway 6200 fluorometer. The brain ion (Ca2+ and Cl−) levels were also determined according to the method of Murphy [18]. Briefly, tissue samples of the brain regions were dried in oven at 60 °C and combusted at 450 °C for 24 h. Thereafter, the combusted samples were dissolved in hot solution of 1 M HNO3. The samples were transferred into 50-ml volumetric flasks and adjusted with the deionized water to this volume. The appropriately diluted and digested tissue samples were analyzed using flame atomic absorption spectrophotometer (Perkin-Elmer, 3100).
Oxidative Stress
Nitrite/nitrate (NO) and lipid peroxidation (LPO) were assayed colorimetrically in the brain homogenates according to the methods of Green et al. [19] and Ohkawa et al. [20], respectively, where LPO was determined by using 1 ml of trichloroacetic acid 10 % and 1 ml of thiobarbituric acid 0.67 % which were then heated in a boiling water bath for 30 min. Thiobarbituric acid reactive substances (TBARS) were determined by the absorbance at 535 nm and expressed as malondialdehyde (MDA) formed. Nitric oxide was determined by optimized acid reduction method at 540 nm using UV-Vis spectrophotometer (Jasco, V-630, Tokyo, Japan).
In addition, the neuronal glutathione (GSH) was determined by the methods of Ellman [21]. This method is based on the reduction of Elman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid) “DTNBˮ) with GSH to produce a yellow compound. The reduced chromogen was directly proportional to GSH concentration, and its absorbance can be measured at 405 nm (UV-Vis spectrophotometer, Jasco, V-630, Tokyo, Japan).
Enzymatic Antioxidant Status
The activities of neuronal antioxidant enzymes as superoxide dismutase (SOD) activity were assayed by the method of Nishikimi et al. [22]. This assay relies on the ability of the enzyme to inhibit the phenazine methosulfate-mediated reduction of nitroblue tetrazolium (NBT) dye. NBT method is adequate to determine both forms of SOD, MnSOD, and Cu/ZnSOD.
Brain catalase (CAT) activity was assayed by the method of Aebi [23]. Briefly, 50 μl of brain homogenates were added to 30 mM H2O2 in 50 mM of potassium phosphate buffer (pH 7.8), and the consumption of H2O2 was measured at 340 nm for 120 s at 20-s intervals. CAT activity was expressed in units per gram tissue (U/g tissue).
The activity of GSH peroxidase was determined by the method described in the study of Smith and Levander [24]. The reagent mixture was modified for analysis to be conducted in 1-ml reagent using a quartz cuvette with 1-cm path length. It contained 50 mM phosphate buffer, pH 7.0, 25 mM tBOOH, 0.0025 % sodium azide, and 0.5 mM GSH; and 0.1 mM NADPH and 2 units/ml GSH reductase. Approximately 100 μl of sample was used for each determination. After the reaction was started by addition of GSH reductase, the decrease in NADPH level was measured at a wavelength of 340 nm by taking a reading every minute for up to 15 min. The decrease should be linear during the entire period, and the activity of the enzyme was calculated from the rate of change in NADPH using ɛ = 6,270 M/cm.
The activity of GSH reductase was measured by the method described by Dringen and Gutterer [25] in a 1-ml reagent containing 50 mM phosphate buffer, pH 7.0, 1 mM EDTA, 10 mM GSSG, and 0.1 mM NADPH. Activity of the enzyme was determined by the time-dependent change in NADPH (ɛ = 6,270 M/cm) after the addition of the sample.
Statistical Analysis
Results were expressed as the mean ± standard error of the mean (SEM). Data for multiple variable comparisons were analyzed by one-way analysis of variance (ANOVA). For the comparison of significance between groups, Duncan’s test was used as a post hoc test according to the Statistical Package for the Social Sciences (SPSS version 17.0), and figures were drawn with Origin (version 8). All p values are two-tailed, and p < 0.05 was considered as significant for all statistical analysis in this study.
Results
It is clear from the data in Table 1 that the daily intraperitoneal injection of 6.5 mg CdCl2/kg bwt for five consecutive days induced a significant (p < 0.05) reduction in DA, 5-HT, and 5-HIAA levels on all examined brain regions versus the control group. On the other hand, the MEPh administration to Cd-intoxicated rats caused a significant (p < 0.05) increment in the levels of DA, 5-HT, and 5-HIAA as compared to Cd-injected group.
To evaluate the effect of Cd on brain ion homeostasis, Ca2+ and Cl− were measured in the brain tissues. As shown in Table 2, Ca2+ levels increased significantly (p < 0.05) in the cerebral cortex, hippocampus, and cerebellum upon Cd treatment. The levels of Ca2+ were slightly recovered to normal levels after treatment with MEPh for five consecutive days. Additionally, there was a significant (p < 0.05) reduction in Cl− level in all brain regions under investigation of Cd-intoxicated rats. However, the oral administration of MEPh induced a significant (p < 0.05) increase in Cl− level in the cerebral cortex, hippocampus, and cerebellum as compared to the Cd group.
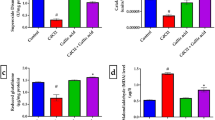
To examine the effect of Cd on oxidative stress markers, lipid peroxidation induction in the brain homogenates of rats treated with CdCl2 was measured, and its levels were shown in Fig. 1. CdCl2 administration caused a significant (p ˂ 0.05) increase in the level of LPO in brain compared to the control group. MEPh caused a significant (p < 0.05) reduction in LPO level that increased by CdCl2 administration. Although, the production of nitrite/nitrate (nitric oxide; NO) in the brain was significantly (p < 0.05) increased in the Cd group when compared to the control group (Fig. 2). However, treatment with MEPh caused a significant decrease in the level of NO in the brain homogenates when compared to the Cd inoculated group.
Effects of Physalis on lipid peroxidation induction in the brain of rats treated with cadmium chloride for 5 days, data expressed as malondialdehyde formed. Values are means ± SEM. Significant change at p < 0.05 with respect to the control group (a). Significant change at p < 0.05 with respect to Cd group (b)
To examine the major nonenzymatic antioxidant component involved in the clearance of substances formed during oxidative stress, GSH was measured in the brain of all the rat groups. As shown in Fig. 3, GSH substance was significantly (p < 0.05) reduced in the brain homogenates of the Cd group compared to the control group. This reduction was largely prevented by MEPh treatment.
To study the effect of neurotoxicity of cadmium in rats on production of cellular oxidants and modulation of antioxidant defense system, several parameters of oxidative stress were examined, including the activity of SOD, CAT, GSH peroxidase (GPx), and GSH reductase (GR) enzymes. As shown in Fig. 4 and Table 3, Cd administration led to modulation of all studied parameters of oxidative stress relative to the control animals. After 5 days of CdCl2 administration, SOD, CAT, GPx, and GR activities in the brain homogenates decreased significantly (p < 0.05) compared to the controls. On the other hand; MEPh pretreatment elevated the activities of SOD, CAT, GPx, and GR significantly (p < 0.05) compared to the Cd group. However, MEPh treatment alone decreased the activity of SOD in the brain homogenates when compared with the control value (Fig. 4).
Discussion
Environmental Cd pollution may accumulate in human body through direct exposure or by food chain, resulting in neurodegeneration as well as many other diseases. Cd has a number of biological toxicities, including genotoxicity, reproductive toxicity, and teratogenic effects. Additionally, Cd may induce Parkinson’s disease, Alzheimer’s disease, and other neurodegenerative disorders primarily by triggering neuronal cell death [1, 2, 26]. In the current study, the decreases in monoamines levels as well as the disturbance in homeostasis of ions in the brain induced by Cd detoxification were prevented by MEPh pretreatment, demonstrating that neurotoxicity of the brain was improved by this treatment.
Several reports studied the effect of cadmium accumulation in the brain as a whole brain tissue or in discrete brain regions. Cd administration is associated with changes in several neurotransmitter systems, such as DA, norepinephrine, serotonin, and serotonin metabolite, 5-HIAA. The changes in the severity are distinct in different brain regions depending on route, dose, and timing of exposure [27–29].
Pillai et al. [30] deduced that rat exposure to Cd associated with reduction of 5-HT and its metabolite 5-HIAA level in the brain tissues, due to changes on neurotransmitter metabolism in the central nervous system. It was confirmed by Andersson et al. [31], who attributed the reduction of 5-HIAA level to the blocking effect of Cd on 5-HT uptake which prevents 5-HT from metabolization by intraneuronal monoamine oxidase (MAO). In agreement with our observations, Lafuente et al. [28] and Pillai et al. [30] also indicated that rats treated with CdCl2 showed a marked reduction of DA content in each investigated region.
Changes in the homeostasis of cytosolic Ca2+ concentration can affect the regulation of many neuronal functions. Our data revealed that injection of CdCl2 for five consecutive days disrupted brain ion (Ca2+ and Cl−) homeostasis. Cd may block the Ca2+ influx through membrane channels into the nerve terminal following the action potential, and this reduction may be associated with an altered transmitter release [32]. In addition, Cd acts as a competitive ion to Ca2+ at the voltage-dependent Ca2+ channels, and it is a potent blocker of the Ca2+-dependent neurotransmitter release [33].
On the other hand, our neurochemical estimations revealed that P. peruviana extract modulated the disturbances in DA, 5-HT, and 5-HIAA levels in selected brain regions, indicating its ameliorative role in Cd-intoxicated rats. P. peruviana has an anxiolytic activity, through its flavonoids and endogenous benzodiazepines which acts as a potential central nervous system depressant and analgesic actions [34, 35]. In agreement with our results, Bopaiah et al. [36] found that plant extract formulation containing Withania somnifera (Solanaceae) induced a significant increase in the levels of DA in the frontal cortex, hypothalamus, and hippocampus and the 5-HT in the hypothalamus.
Brain has a high rate of oxidative metabolism, consuming ~20 % of the cardiac output. At the same time, the brain compared to lung, liver, and other organs contains relatively low levels of enzymatic and nonenzymatic antioxidants and high amounts of peroxidizable unsaturated lipids, rendering it more vulnerable to oxidative stress compared to other tissues [37]. Increasing evidences suggested that excessive production-free radicals in the brain and the imbalance between oxidative species and antioxidant defenses are related to the pathogenesis of neurodegenerative diseases. Recent studies have shown that administration of Cd produces reactive oxygen species (ROS), resulting in an increased lipid peroxidation, depletion of sulfhydryls, altered calcium homeostasis, impairment of antioxidant defenses, and finally DNA damage [38–40]. Our results show elevated LPO level in the brain due to Cd exposure, indicating that Cd-induced neurotoxicity might be due to the overproduction of free radicals and lipid peroxidative products.
In mitochondria, cadmium enters in structure of phosphorilation-oxidative enzymes and disrupts producing energy cycles. Also, it can replace many metal nutrients such as Ca2+ and reduce Ca2+ entering to the cell [41, 26]. Cd induces LPO by stimulating the production of superoxide anions and, due to inhibition of antioxidants, such as GPx and SOD, accumulates free radicals, damage to the cell, and produce chronic diseases [42].
It is also possible that Physalis extract induced protection from Cd by impairing Cd-mediated lipid peroxidation, through decreased production of free radical derivatives, as evident from the ameliorated LPO level. The group that received the Cd treatment alone was prone to high lipid peroxidation, whereas the group that received the coadministration of Physalis exhibited significant protection. This also suggests the defense mechanism against the ROS and thereby the antioxidant potential of Physalis. Antioxidant effect of flavonoids that was found in Physalis enhanced the process of regeneration. This might be due to destruction of free radicals, supplying a competitive substrate for unsaturated lipids in the membrane and/or accelerating the repair mechanism of damaged cell membrane [11].
The detoxification of ROS in the brain involves the cooperative action of the intracellular antioxidant enzymes, SOD, CAT, and GPx. The decreased activity of these antioxidant enzymes in the brain of Cd-intoxicated rats was also observed in the present study. The diminished activities of these antioxidant enzymes might be due to binding of Cd with the sulfhydryl group of some enzymes, replacement of essential metals from their active sites, and oxidative modification of amino acid side chains, which alters these enzyme structure and leads to the inactivation or impaired activity of these enzymes [38].
In the present study, GPx activity was inhibited. When Cd accumulates in the brain, it can combine with the active center (Se-Cys) of GPx and lead to structural changes that inactivate GPx, an effect which has also been mentioned in some reports [43]. The reduction of SOD activity may be due to the replacement of zinc and/or manganese of the SOD molecules by Cd as it has been shown that zinc protects the cadmium-induced neuronal toxicity. Again, the results of the present study were in accordance with the previous study that Cd depletes GSH and protein-bound sulfhydryl groups, resulting in enhanced production of ROS such as superoxide ion, hydroxyl radicals, and hydrogen peroxide [44]. In our study, CAT, which catalyzes the conversion of hydrogen peroxide to water and molecular oxygen, was found to be decreased in the brain of the Cd group. It is possible that the inhibited activity of CAT may be correlated with the displacement of endogenous metals (cofactors from the active sites) by cadmium [45]. Depletion in the activity of GR in the brain of Cd-treated rats may be due to decreased synthesis of enzyme or oxidative inactivation of enzyme protein [46]. In the present study, increased lipid peroxidation, associated with decreased antioxidant status in Cd-treated rats, can therefore be related to insufficient antioxidant potential.
In our study, the activity of antioxidant enzymes such as SOD, CAT, GPx, and GR decreased in Cd-treated group was recovered by treatment of Physalis. The protective effects of Physalis in maintaining the GSH level toward control have increased the capacity of endogenous antioxidant defense and increased the steady state of GSH and/or its rate of synthesis. Our study confirmed the protective effects of Physalis against the Cd-induced oxidative stress and speculated that high level of polyphenols and other antioxidants like flavonoids could attribute to this protective effect.
It was found that quercetin is the main polyphenols in P. peruviana L., followed by myricetin and kaempferol [47]. Quercetin, for example, has various biological activities such as antioxidant and anti-inflammatory functions [48, 49]. Recently, it was reported that quercetin prevented the neurotoxic effect of Cd [49]. Moreover, Physalis extracts contain many withanolide glycosides [47]. Withanolides are natural steroidal lactones produced mainly by plants in the Solanaceae that often have many health benefits such as anti-inflammatory activity [50].
In conclusion, exposure to cadmium could generate free radicals, which resulted in the elevation of neuronal lipid peroxidation and nitric oxide as well as a reduction in the enzymatic and nonenzymatic antioxidant components. That neurotoxicity induced by Cd is associated with alternation in monoamines. However, Physalis could protect from the Cd-induced neurotoxic effects. This protective effect of Physalis extract may be due to the radical scavenging activity and its phenolics and flavonoids contents that maintain an adequate level of nonenzymatic and enzymatic antioxidant defense against Cd neurotoxicity. These interesting results show that P. peruviana could be used as a supplementary extract to provide a novel dietary therapeutic strategy against acute cadmium neurotoxicity.
References
Othman MS, Nada A, Zaki HS et al (2014) Effect of Physalis peruviana L. On cadmium-induced testicular toxicity in rats. Biol Trace Elem Res 159:278–287
El-Habit OH, Abdel Moneim AE (2014) Testing the genotoxicity, cytotoxicity, and oxidative stress of cadmium and nickel and their additive effect in male mice. Biol Trace Elem Res 159:278–287
Lopez E, Figueroa S, Oset-Gasque MJ et al (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol 138(5):901–911
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034
Okuda B, Iwamoto Y, Tachibana H et al (1997) Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg 99(4):263–265
Shukla GS, Hussain T, Srivastava RS et al (1989) Glutathione peroxidase and catalase in liver, kidney, testis and brain regions of rats following cadmium exposure and subsequent withdrawal. Ind Health 27(2):59–69
Vijaya Kumar Reddy C, Sreeramulu D, Raghunath M (2010) Antioxidant activity of fresh and dry fruits commonly consumed in india. Food Res Int 43(1):285–288
Puente LA, Pinto-Muñoz CA, Castro ES et al (2011) Physalis peruviana linnaeus, the multiple properties of a highly functional fruit: a review. Food Res Int 44(7):1733–1740
Franco LA, Matiz GE, Calle J et al (2007) Actividad antinflamatoria de extractos y fracciones obtenidas de cálices de Physalis peruviana L. Biomedica 27:110–115
Zavala D, Mauricio Q, Pelayo A et al (2006) Citotoxic effect of Physalis peruviana (capuli) in colon cancer and chronic myeloid leukemia. Anales de la Facultad de Medicina 67(4):283–289
Al-Olayan E, Elkhadragy MF, Aref A, et al. (2014) The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of mmp-9 expression. Oxid Med Cell Longev 2014:381413
Abdel Moneim AE, El-Deib KM (2012) The possible protective effects of Physalis peruviana on carbon tetrachloride-induced nephrotoxicity in male albino rats. Life Sci J 9(3):1038–1052
Kyriakou LG, Tzirogiannis KN, Demonakou MD et al (2013) Gadolinium chloride pretreatment ameliorates acute cadmium-induced hepatotoxicity. Toxicol Ind Health 29(7):624–632
Fouad AA, Qureshi HA, Yacoubi MT et al (2009) Protective role of carnosine in mice with cadmium-induced acute hepatotoxicity. Food Chem Toxicol 47(11):2863–2870
Glowinski J, Axelrod J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. IV. Effects of drugs on the disposition and metabolism of h3-norepinephrine and h3-dopamine. J Pharmacol Exp Ther 153(1):30–41
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Ciarlone A (1978) Further modification of a fluoromertric method for analyzing brain amines. Microchem J 23:9–12
Murphy VA (1987) Method for determination of sodium, potassium, calcium, magnesium, chloride, and phosphate in the rat choroid plexus by flame atomic absorption and visible spectroscopy. Anal Biochem 161(1):144–151
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Smith AD, Levander OA (2002) High-throughput 96-well microplate assays for determining specific activities of glutathione peroxidase and thioredoxin reductase. Methods Enzymol 347:113–121
Dringen R, Gutterer JM (2002) Glutathione reductase from bovine brain. Methods Enzymol 348:281–288
Unsal C, Kanter M, Aktas C, et al. (2013) Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol Ind Health. doi:0748233713486960
Lafuente A, Gonzalez-Carracedo A, Romero A et al (2005) Toxic effects of cadmium on the regulatory mechanism of dopamine and serotonin on prolactin secretion in adult male rats. Toxicol Lett 155(1):87–96
Lafuente A, Gonzalez-Carracedo A, Romero A et al (2003) Effect of cadmium on 24-h variations in hypothalamic dopamine and serotonin metabolism in adult male rats. Exp Brain Res 149(2):200–206
Lafuente A, Marquez N, Pazo D et al (2000) Effects of subchronic alternating cadmium exposure on dopamine turnover and plasma levels of prolactin, gh and acth. Biometals 13(1):47–55
Pillai A, Priya L, Gupta S (2003) Effects of combined exposure to lead and cadmium on the hypothalamic-pituitary axis function in proestrous rats. Food Chem Toxicol 41(3):379–384
Andersson H, Petersson-Grawe K, Lindqvist E et al (1997) Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol Teratol 19(2):105–115
Antonio MT, Corredor L, Leret ML (2003) Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol Lett 143(3):331–340
Antonio MT, Peinado V, Gonzalez JC et al (2010) Effects of maternal cadmium administration on development of monoaminergic, gabaergic and glutamatergic systems. Environ Toxicol Pharmacol 29(1):87–90
Giorgetti M, Negri G (2011) Plants from Solanaceae family with possible anxiolytic effect reported on 19th century’s brazilian medical journal. Revista Brasileira de Farmacognosia 21:772–780
Giorgetti M, Negri G, Rodrigues E (2007) Brazilian plants with possible action on the central nervous system: a study of historical sources from the 16th to 19th century. J Ethnopharmacol 109(2):338–347
Bopaiah CP, Pradhan N, Venkataram BS (2000) Pharmacological study on antidepressant activity of 50 % ethanol extract of a formulated ayurvedic product in rats. J Ethnopharmacol 72(3):411–419
Abdel Moneim AE (2012) Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol Trace Elem Res 150(1–3):328–336
Shagirtha K, Muthumani M, Prabu SM (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 15(9):1039–1050
Abdel Moneim AE (2014) Citrus peel extract attenuates acute cyanide poisoning-induced seizures and oxidative stress in rats. CNS Neurol Disord Drug Targets 13(4):638–646
Karaca S, Eraslan G (2013) The effects of flaxseed oil on cadmium-induced oxidative stress in rats. Biol Trace Elem Res 155(3):423–430
Martelli A, Rousselet E, Dycke C et al (2006) Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88(11):1807–1814
Amara S, Douki T, Garrel C et al (2011) Effects of static magnetic field and cadmium on oxidative stress and DNA damage in rat cortex brain and hippocampus. Toxicol Ind Health 27(2):99–106
Zhang Y, Sun G, Yang M et al (2011) Chronic accumulation of cadmium and its effects on antioxidant enzymes and malondialdehyde in Oxya chinensis (orthoptera: Acridoidea). Ecotoxicol Environ Saf 74(5):1355–1362
Kara H, Cevik A, Konar V et al (2007) Protective effects of antioxidants against cadmium-induced oxidative damage in rat testes. Biol Trace Elem Res 120(1–3):205–211
Lacorte LM, Seiva FR, Rinaldi JC et al (2013) Caffeine reduces cadmium accumulation in the organism and enhances the levels of antioxidant protein expression in the epididymis. Reprod Toxicol 35:137–143
Renugadevi J, Prabu SM (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62(5):471–481
Ahmed LA (2014) Renoprotective effect of Egyptian cape gooseberry fruit (Physalis peruviana L.) against acute renal injury in rats. ScientificWorldJournal 2014:273870
Kalender Y, Kaya S, Durak D et al (2012) Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ Toxicol Pharmacol 33(2):141–148
Abdalla FH, Schmatz R, Cardoso AM, et al. (2014) Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na(+), K(+) - ATPase activities. Physiol Behav 135:152–167
Ahmad S, Malik A, Afza N et al (1999) A new withanolide glycoside from Physalis peruviana. J Nat Prod 62(3):493–494
Acknowledgments
This research project was supported by a grant from the Research Center of Female Center for Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel Moneim, A.E., Bauomy, A.A., Diab, M.M.S. et al. The Protective Effect of Physalis peruviana L. Against Cadmium-Induced Neurotoxicity in Rats. Biol Trace Elem Res 160, 392–399 (2014). https://doi.org/10.1007/s12011-014-0066-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0066-9