Abstract
The neuroprotective ability of alkaloid-rich leaf extract of Dalbergiella welwitschii in streptozotocin-induced type 2 diabetic rats were investigated in this study. Dalbergiella welwitshii leaf alkaloid-rich extract was obtained using standard procedure. Streptozotocin was injected into the experimental animals intraperitoneally at a dose of 45 mg/mg body weight. Prior to this, the animals were given 20% (w/v) fructose for one week. The animals were grouped into five (n = 8), comprising of normal control (NC), diabetic control (DC), diabetic rats treated with low (50 mg/mg body weight) and high (100 mg/kg body weight) doses of Dalbergiella welwitschii alkaloid-rich leaf extracts (i.e., DWL and DWH respectively) and 200 mg/kg body weight of metformin (MET). The animals were sacrificed on the 21st day, blood and brain tissue were harvested and used for the determination of neurotransmitters, cholinesterase, some ATP activities, oxidative stress biomarkers and histological examination. The results show that diabetic rats placed on DWL, DWH and MET significantly (p < 0.05) reduced cholinergic, elevated some ATPase activities and ameliorated oxidative stress biomarkers. These were supported by the histological examination by improving neuroprotective effects in diabetic rats administered DWL, DWH and MET. Hence, it can be presumed that DWL and DWH could be beneficial in treating diabetic neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pharmaceutical companies have been paying more attention to drug discovery and development from natural food sources because most chemical and synthetic drugs currently available for treatments of various diseases have been connected to a number of undesirable side effects and consequences (Ghara et al. 2021). On the other hand, medications derived from plants, sometimes known as herbal medicines, are typically seen as safe, affordable, and having fewer adverse effects. Plant natural products that are secondary metabolites, including alkaloids, phenolics, flavonoids, and terpenoids, are highly significant. According to Zannini et al. (2013), they have demonstrated encouraging antioxidant properties and are being utilized to treat a number of illnesses, including cancer, high blood pressure, diabetes, neurological disorders, atherosclerosis, and cardiovascular disease.
Hyperglycemia is a hallmark of diabetes mellitus (DM), a metabolic chronic illness that is typically caused by either inadequate or faulty insulin secretion. Long-term elevation of blood glucose in diabetes has a number of negative effects, such as metabolic imbalance, elevated oxidative stress, and inflammatory processes (Abdollahi and Hosseini 2014). These effects can lead to problems in the cardiovascular, neurological, and renal systems, among other physiological systems (Mani et al. 2022). Furthermore, because of its high oxygen consumption, high lipid content, and low antioxidant levels, the brain is the tissue most susceptible to oxidative damage (Ibrahim 2017).
Retinopathy, nephropathy, and diabetic neuropathy are examples of microvascular problems, whereas stroke, peripheral artery disease, and coronary artery disease are considered macrovascular consequences of hyperglycemia (Fowler 2008). According to Adhikari (2021) and Ajebli et al. (2021), alkaloids derived from plants are utilized as therapeutic medications for diabetes since they have been shown to block the enzymes that raise blood glucose levels.
The shrub Dalbergiella welwitschii is also known as Elemosoo in Yoruba and Blackwood in West Africa. The components of D. welwitschii are utilized in traditional medicine. The entire plant is used to treat rheumatism, the roots are used to treat gastrointestinal disorders, and the leaves are used to treat skin infections (Burkill 1995). In traditional medicine, the leaf is also applied locally to manage or treat oxidative stress-related illnesses (Ajiboye et al. 2022). Although the neuroprotective properties of the alkaloid-rich extracts of D. welwitschi have not been proven, their therapeutic effect on oxidative stress-related illnesses have been documented by Ajiboye et al. (2022). Thus, the goal of this work was to assess whether D. welwitschi alkaloid-rich extracts could protect diabetic rats from streptozotocin (STZ)-induced neurodegeneration.
Materials and methods
Plant materials source, authentication and processing
Dalbergiella welwitschii leaves were collected in an open location at the Federal Research Institute of Nigeria in Ibadan, Oyo State, Nigeria. The leaves were dried for two weeks at room temperature. Then grind into powder form.
Chemicals, reagents and enzyme kits used
The chemicals used in this study were purchased from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany). Also, all the enzyme kits used were products of Randox Laboratory.
Alkaloid extracts of Dalbergiella welwitschii
Alkaloid extracts of Dalbergiella welwitschii were prepared according to Harbone’s (1998) technique, previously modified by Ademiluyi et al. (2016). Dalbergiella welwitschii was pulverized and defatted with n-hexane for 24 h. Thereafter, 10 g of defatted Dalbergiella welwitschii were extracted for 24 h with 100 mL of 10% acetic acid in ethanol. Filtration was then performed, first with muslin cloth and subsequently with filter paper. In a rotary evaporator, the clear filtrate was concentrated under a vacuum at 45oC. The concentrated ammonium hydroxide was added to precipitate the filtrate. Thereafter, the solution was allowed to settle, and the precipitate was collected and washed with dilute ammonium hydroxide. The obtained extract was collected and kept at 4 °C in the refrigerator.
Experimental animals
A total of 40 male Wistar rats of two months old, weighing 100–130 g were acquired from Animal Holding Unit of Ekiti State University, Ado-Ekiti, Ekiti State, Nigeria. The animals were divided into five groups and were kept in a standard laboratory environment. The animals were acclimatized for a week. The rats were fed with normal rat pellet chow and given 20% fructose and food for a week (except the normal control group) as previously described by Salau et al. (2021).
Induction of diabetes into experimental animals
Twelve hours before the induction of streptozotocin, foods were removed from the rat’s cage and were served with only water. STZ was dissolved in sodium citrate buffer of pH 4.5 to a concentration of 20 mg/ml and the rats were injected 5 min after freshly prepared STZ. 45 mg/kg body weight of STZ was injected into the experimental groups after which an equal volume of citrate buffer pH 4.5 were injected into the control rats.
Experimental groups
Rats with fasting blood glucose of greater than or equal to 250 mg/dL were used for the study (Ajiboye et al. 2018). The rats were placed into five groups, with eight rats in each group as follows:
-
Group I: Rats without induction (Normal Control);
-
Group II: Diabetic rats without treatment (Diabetic Control);
-
Group III: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich
extract of Dalbergiella welwitschi (DWL);
-
Group IV: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi (DWH); and.
-
Group V: Diabetic rats administered 200 mg/kg of metformin (MET).
The alkaloid-rich extract of Dalbergiella welwitschii leaf and metformin were given orally to the appropriate animals using a needle and intubator. This method of administration was chosen because of its safety, convenience of consumption, and ability to prevent pain. During the experimental period, the administration was done every day (between 10 and 11 a.m.) for 20 days.
Tissue collection and processing
The animals were sacrificed by cervical dislocation on the 21st day of oral administration. Each rat’s complete blood was collected through cardiac puncture and allowed to clot before being centrifuged at 3000 rpm for 15 min to obtain the serum from the blood. The serum was promptly refrigerated until it could be processed further. Each animal’s brain was collected, washed in normal saline, wiped using filter paper, weighed, and homogenized in 0.1 M potassium phosphate buffer at pH 6.5. The homogenized samples were centrifuged at 4000 rpm for 15 min before being analyzed.
Biochemical parameters studied
Determination of neurotransmitter levels
The levels of norepinephrine, dopamine, and serotonin in the rat brain homogenates were carried out using commercial enzyme-linked immunosorbent assay (ELISA) kits (Cusabio; Houston, TX, USA) method.
Determination of cholinesterase activity
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities were determined by using the procedure of Ellman et al. (1961). Briefly, 50 mL of brain homogenate, 50 mL of 2-nitrobenzene acid and 175 mL of 0.1 mol/L phosphate buffered saline (pH 8.0), mixed thoroughly and incubated at 250C for 20 min. Consequently, 25 mL of both acetylthiocholine iodide and butyrylthiocholine iodide solution was respectively added to the solution. The absorbance was read at 412 nm with the aid of microplate reader.
Determination of nitric oxide level
Briefly, 0.1 mL of brain homogenate was added to both sample and blank test tubes. Then, 0.1 mL of reagent R1 (sodium nitrite) of standard and block standard was added to the respective test tube. The reagent R2 (Sulphanilamide) at 0.1 mL was added to all the test tubes. Each test tube was mixed thoroughly and incubated for 5 min. Thereafter, 0.1 mL of reagent 3 (N – (1 – naphthyl) -ethylenediamine (NEDA) was added to both sample and standard test tubes, mixed thoroughly. The absorbance was read spectrophotometrically at 540 nm (Montgomery and Dymock 1961).
Determination of ATPase
The method of Bewaji et al. (1985) was employed in this assay.
Determination of Ecto-nucleoside triphosphate diphosphohydroase (ENTPDase)
Briefly, 20 µL of the reaction samples was incubated with a mixture containing 200 µL of the reaction buffer (1.5 mM CaCl2, 5 mM KCl, 0.1 mM EDTA, 10 mM glucose, 225 mM sucrose and 45 mM Tris-HCl) for 10 min at 37 °C. 20 µL of 50 mM ATP was then added to the reaction mixture and further incubated in a shaker for 20 min at 37 °C. Reaction was halted by adding 200 µL of 10% TCA, followed by 200 µL of 1.25% ammonium molybdate and a freshly prepared 9% ascorbic acid. The mixture was allowed to stand on ice for 10 min. Absorbance was read at 600 nm (Akomolafe et al. 2017).
Determination of Na+/K+-ATPase activity
For the test, 400 µL of 200 mM NaCl/40 mM KCl/60 mM Tris (pH 7.4) was added into test tube. Thereafter, 20 µL of MgCl2.6H2O (80 mM), 20µL of EGTA (20 mM), 240µL of distilled water and 20 µL of appropriately diluted tissue supernatant were added. This was mixed and incubated at 370C for 5 min. Then, 100 µL of ATP (8 mM) was added. This was mixed and incubated at 370C for 30 min. Thereafter, 200 µL of SDS (5%) and 2,000 µL of reagent C were added. The mixture was allowed to stand at room temperature for 30 min for colour development. The blank was similarly prepared but 20 µL of distilled water was used instead of the 20 µL of brain supernatant. The absorbance was read at 820 nm (Ronner et al. 1977).
Determination of oxidative stress and antioxidant markers
The oxidative stress and antioxidant biomarkers (such as GST, CAT, GPx, SOD and MDA levels) were determined using a procedure described in commercial enzyme kits (Randox, United Kingdom).
Brain histology examination
The was performed using the method reported by Sultana et al. (2016).
Data analysis
The results were of 8 replicates with standard deviation (SD). Graph Pad Prism 7 software was used to analyze the results. One-way ANOVA and the Tukey post hoc test were used to determine differences, and differences were considered significant at p < 0.05.
Results
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on neurotransmitter levels in streptozotocin-induced diabetic rats
Figure 1 reveals a significant (p < 0.05) increase in dopamine, norepinephrine and serotonin levels in streptozotocin-induced diabetic rats as compared to normal group. However, diabetic rats treated with alkaloid-rich extracts from Dalbergiella welwitschi leaf showed significantly (p < 0.05) decreased dopamine, norepinephrine and serotonin dose dependently when compared to the diabetic control.
Some neurotransmitter levels of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Each value is a mean of eight determinations ± SD. # p < 0.05 vs. NC, * p < 0.05 vs. DC. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, and MET: Diabetic rats administered 200 mg/kg of metformin
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on cholinesterase activities in streptozotocin-induced diabetic rats
Streptozotocin induction caused a significant (p < 0.05) increase in acetylcholinesterase and butyrylcholinesterase activities of diabetic rats when compared with the normal control. However, the effect of streptozotocin was reversed and decreased acetylcholinesterase and butyrylcholinesterase activities were observed when both low (DWL) and high (DWH) doses of alkaloid-rich extract from Dalbergiella welwitschi leaf was administered as depicted in Fig. 2. However, the effect of the extract on cholinesterase activities was dose-dependent with the high dose (DWH) insignificantly (p > 0.05) different when compared with the normal control, unlike the low dose and standard drug used.
Cholinesterase activities of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Each value is a mean of eight determinations ± SD. # p < 0.05 vs. NC, * p < 0.05 vs. DC. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, MET: Diabetic rats administered 200 mg/kg of metformin, AChE: Acetylcholinesterase, and BChE: Butyrylcholinesterase
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on nitric oxide level in streptozotocin-induced diabetic rats
Brain nitric oxide (Fig. 3) was elevated upon streptozotocin induction as evident in the diabetic control group. In the contrary, the nitric oxide level in the brain of a treated diabetic rat was significantly reduced (p < 0.05) when compared to the untreated group in a dose-dependent manner.
Brain nitric oxide level of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Each value is a mean of eight determinations ± SD. # p < 0.05 vs. NC, * p < 0.05 vs. DC. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, MET: Diabetic rats administered 200 mg/kg of metformin, and NO: Nitric oxide
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on ATPase activities in streptozotocin-induced diabetic rats
A significant (p < 0.05) decrease in ATPase, E-NTPDase and Na+/K+-ATPase (Fig. 4) activities of diabetic group when compared with control was observed. However, treatment with alkaloid-rich extract of Dalbergiella welwitschi leaf showed an increase in ATPase, E-NTPDase and Na+/K+-ATPase activities when compared with the diabetic control.
Some brain ATPase activities of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Each value is a mean of eight determinations ± SD. # p < 0.05 vs. NC, * p < 0.05 vs. DC. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, MET: Diabetic rats administered 200 mg/kg of metformin, and E-NTPDase: Ecto-nucleoside triphosphate diphosphohydrolase
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on brain oxidative stress biomarker in streptozotocin-induced diabetic rats
The activities of antioxidant enzymes (GST, CAT, GPx and SOD) were decreased in brains of STZ- induced diabetic rats as compared to those of normal control group. On the other hand, the activities of antioxidant enzymes in the brain of diabetic rats treated with low and high doses of alkaloid-rich extracts from Dalbergiella welwitschi leaf were dose dependently elevated as compared to those of diabetic control group as shown in Fig. 5. The MDA levels were greatly increased (p < 0.05) in brains of diabetic rats, as compared to those of normal control group. The treatment of diabetic rats with alkaloid-rich extracts from Dalbergiella welwitschi leaf decreased the elevated MDA levels in brains of diabetic rats.
Brain oxidative stress biomarkers of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Each value is a mean of eight determinations ± SD. #p< 0.05 vs. NC, * p < 0.05 vs. DC. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi,DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, MET: Diabetic rats administered 200 mg/kg of metformin, GST: Glutathione-S-Transferase, CAT: Catalase, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, and MDA: Malondialdehyde
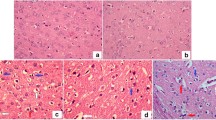
Effects of alkaloid-rich extracts from Dalbergiella welwitschi leaf on brain histoligical changes in streptozotocin-induced diabetic rats
Control group shows normal appearance of small blood vessels in yellow arrows. Surrounding the blood vessels, partly as an artifact of fixation, is a space without cells or parenchyma. In the diabetic control group, STZ induction causes edema and neuron cells shrinkage, demyelination and axonal degradation. After treatment with alkaloid-rich extracts from Dalbergiella welwitschi leaf, edema was still evident in DWL (low dose), which was absent in DWH (high dose) (Fig. 6).
Brain histological examination of alkaloid-rich extracts from Dalbergiella welwitschi leaf in streptozotocin-induced diabetic rats. Stained with H&E, Magnification: x800. Legend: NC: Normal Control, DC: Diabetic Control, DWL: Diabetic rats administered low dose (50 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, DWH: Diabetic rats administered high dose (100 mg/kg body weight) of alkaloid-rich extract of Dalbergiella welwitschi, MET: Diabetic rats administered 200 mg/kg of me tformin, Black arrow: neuronal cells, and Yellow arrow: blood vessel
Discussion
Diabetes mellitus is a lifelong chronic metabolic disorder that results from deficiency in insulin secretion, resistance to insulin action or both, leading to high blood sugar (Ibrahim and Abd El-Maksoud 2015). Continuously high blood sugar, however, harms the blood vessels that supply the nerves, this can result in damage to the central nervous system, which can then impair the organs' ability to function (Wang et al. 2012). The present study evaluates the neuroprotective role of alkaloid-rich extracts from Dalbergiella welwitschi leaf against STZ-induced diabetes in rats.
Dopamine is an endogenous catecholamine that was first recognized as a neurotransmitter in the central nervous system (Cools et al. 2011). Outside the nervous system, dopamine has several functions in the body including survival of pancreatic beta cells (Garcia Barrado et al. 2015). Dopamine, the feel good hormone, regulates insulin secretion through a heteromeric complex of receptors, thereby providing new targets for antidiabetic medication (Uefune et al. 2022). Serotonin is a monoamine that has various functions in both neuronal and non-neuronal systems. In the central nervous system, it regulates mood and feeding behaviors as a neurotransmitter. Determination of serotonin level has been suggested as a therapeutic target in diabetes (Oh et al. 2016). It was observed that there was a marked elevation in dopamine, norepinephrine and serotonin level in diabetic rats’ brain that is due to increased blood glucose level. On the other hand, treatment of diabetic rats with alkaloid-rich extracts from Dalbergiella welwitschi extract decreased the levels of dopamine, norepinephrine and serotonin.
Cholinesterases are a group of serine hydrolases that split the neurotransmitter acetylcholine (ACh) and terminate its action. There are two types of cholinesterase, butyrylcholinesterase (BChE) and acetylcholinesterase (AChE), however, AChE plays the key role in ending cholinergic neurotransmission. Cholinesterase inhibitors are substances that interfere with the break-down of ACh and prolong its action. Hence, the crucial role of the cholinesterases in neural transmission makes them a primary target of a large number of cholinesterase-inhibiting drugs used in the treatment of neurodegenerative disorders (Pohanka 2011). In this study, alkaloid-rich extracts from Dalbergiella welwitschi leaf was used as a cholinesterase inhibitor and it was observed that the level of AChE and BChE which was elevated in STZ-induced diabetic rats was reduced when treated with alkaloid-rich extracts from Dalbergiella welwitschi leaf, indicating an inhibition of the enzyme. This result is in accordance with the findings of Secnik et al. (2020) that cholinesterase inhibitors have positive effects on diabetic patient.
STZ-induced diabetes generates reactive oxygen species (ROS) like oxygen free radicals and leads to nerve damage (Archana et al. 2022), hence, in vivo antioxidant ability provides information about the antioxidant potential of the alkaloid-rich extracts from Dalbergiella welwitschi leaf. The scavenging potential of alkaloid-rich extracts from Dalbergiella welwitschi leaf against NO radicals as reported in the result indicates that the level of NO that was raised by STZ induction was significantly reduced after treatment. The NO free radical’s scavenging ability of flavonoid-rich extract of D welwitschii have been reported (Ajiboye et al. 2022).
ATPase is the activity of any enzyme’s ability to decompose ATP (adenosine triphosphate) into ADP (adenosine diphosphate) and Pi (phosphate) (Abad 2011). Ectonucleotidases control the levels of ATP and its hydrolysis products ADP, AMP and adenosine in the synaptic cleft. The major families of ectonucleotidases include CD39, ecto-nucleotide pyrophosphatase/ phosphodiesterases, alkaline phosphatases, and CD73. Na+/K+-ATPase uses the chemical energy released from the hydrolysis of a molecule of ATP (to ADP and Pi) to transport three Na+ out of and two K+ ions into the cell. This causes an electrochemical gradient across the cell membrane that is required for active transport and for the excitability of neuron cells (Buxbaum 2019). Generally, diabetes mellitus affects the morphology and plasticity of the brain, leading to cognitive and electrophysiological impairment (Imam-Fulani et al. 2016). The ability of alkaloid-rich extracts from Dalbergiella welwitschi leaf to reverse the effect of streptozotocin and increase ATPases activities indicate that the extract is capable of maintaining the brain morphology and hence, inhibit electrophysiological impairment.
Oxidative stress is an imbalance between oxidants, that is free radicals, and the antioxidants (enzymatic and non-enzymatic) system of the body which is associated with the pathogenesis of diabetes (Afrin et al. 2016). Antioxidant enzymes including catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST) and superoxide dismutase (SOD) are capable of neutralizing or stabilizing free radicals either by reducing the energy of the free radicals or by giving up some of their electrons (Vávrová et al. 2013), hence, investigation of antioxidant enzymes activities can reflect the level of oxidative stress in an organ. The results obtained from this study indicated that streptozotocin induction resulted in lipid peroxidation, an oxidative stress condition, in the brain as shown by the increased MDA content, a metabolic product of lipid peroxidation. Antioxidant markers (GSH, GPx, SOD and CAT) activities were decreased significantly (p < 0.05) when compared to the normal control, which is an indication that the antioxidant enzymes are being used to mop up the excessive free radicals caused by hyperglycemia. GSH is important in the enzymatic antioxidant system which acts as a cofactor for enzymes such as glutathione peroxidase (GPx) to neutralize hydrogen peroxide produced from free radical to water (Krishnamurthy and Wadhwani 2012), SOD is an antioxidant enzyme responsible for the dismutation of the unstable superoxide radicals into hydrogen peroxide, while catalase catalyses the decomposition of hydrogen peroxide into inert water and oxygen (Omodanisi et al. 2017). Treatment with alkaloid-rich extract from Dalbergiella welwitschi leaf, caused an obvious increase in the activities of antoxidant enzymes in the brain when compared to the diabetic controls, hence, reflecting an increased protection against oxidative stress. In addition, treatment of diabetic rats also significantly reduced the formation of by-products of lipid peroxidation, MDA. Ajiboye et al. (2022) reported the antioxidant activities of Dalbergiella welwitschi leaf falvonoid-rich extract.
High blood sugar, hyperglycemia, over time damages blood vessels in the brain that carry oxygen-rich blood. When the brain receives too little blood, brain cells can die. This is called brain atrophy and can cause problems with memory and thinking and eventually can lead to vascular dementia (Glaser et al. 2017). Streptozotocin induction caused edema and neuron cells shrinkage, demyelination and axonal degradation, which was reversed by treatment with alkaloid-rich extracts from Dalbergiella welwitschi leaf.
Conclusion
The results of this research reveal that the alkaloid-rich extract of Dalbergiella welwitschi leaf has therapeutic potential and neuroprotective effects against brain damage resulted from STZ-induced diabetes as evidenced by its ability to reduce oxidative stress, cholinergic dysfunction and lower neurotransmitter activities. As a result, Dalbergiella welwitschi alkaloid-rich leaf extract could be used as a pharmacological adjuvant in the treatment of diabetic neurodegenerative diseases.
Data availability
On special request from the corresponding author.
Code availability
Not applicable.
References
Abad JP (2011) Atpase. In: Gargaud M, Amils R, Quintanilla JC, Cleaves HJ, Irvine WM, Pinti DL, Viso M (eds) Encyclopedia of astrobiology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 123–127
Abdollahi M, Hosseini A (2014) Streptozotocin. In: Wexler P (ed) Encyclopedia of toxicology, vol 4, 3rd edn. Elsevier Inc., Academic Press, pp 402–404
Ademiluyi AO, Ogunsuyi OB, Oboh G (2016) Alkaloid extracts from Jimson weed (Datura stramonium L.) modulate purinergic enzymes in rat brain. Neurotoxicology 56:107–117
Adhikari B (2021) Roles of alkaloids from medicinal plants in the management of diabetes mellitus. Hindawi Journal of Chemistry 2021. https://doi.org/10.1155/2021/2691525
Afrin R, Arumugam S, Wahed MII, Pitchaimani V, Karuppagounder V, Sreedhar R, Harima M, Suzuki H, Miyashita S, Nakamura T, Suzuki K, Nakamura M, Ueno K, Watanabe K (2016) Attenuation of endoplasmic reticulum stress-mediated liver damage by mulberry leaf diet in streptozotocin-induced diabetic rats. Am J Chin Med 44(01):87–101
Ajebli M, Khan H, Eddouks M (2021) Natural alkaloids and diabetes mellitus: a review. Endocr Metab Immune Disord Drug Targets 21(1):111–130
Ajiboye BO, Ojo OA, Adeyonu O, Imiere O, Oyinloye BE, Ogunmodede O (2018) Ameliorative activity of ethanolic extract of Artocarpus heterophyllus stem bark on alloxan-induced diabetic rats. Adv Pharm Bull 8(1):141
Ajiboye BA, Oyinloye BE, Ojo O, Lawal O, Jokomba Y, Balogun B, Adeoye A, Ajuwon O (2022) Effect of flavonoid-rich extract from Dalbergiella welwitschii leaf on redox, cholinergic, monoaminergic, and purinergic dysfunction in oxidative testicular injury: ex vivo and in silico studies. Bioinform Biol Insights 16:117793222211155
Akomolafe SF, Akinyemi AJ, Ogunsuyi OB, Oyeleye SI, Oboh G, Adeoyo OO, Allismith YR (2017) Effect of caffeine, caffeic acid and their various combinations on enzymes of cholinergic, monoaminergic and purinergic systems critical to Neurodegeneration in Rat Brain-In Vitro. Neurotoxicology 62:6–13. https://doi.org/10.1016/j.neuro.2017.04.008
Archana J, Annapurna A, Devayani P (2022) Neuroprotective role of Tinospora cordifolia extract in streptozotocin induced neuropathic pain. Braz J Pharm Sci 58:e18501. https://doi.org/10.1590/s2175-97902020000118501
Bewaji CO, Olorunsogo OO, Bababunmi EA (1985) Comparison of the membrane-bound (Ca2+ + Mg2+)-ATPase in erythrocyte ghosts from some mammalian species. Comp Biochem Physiol 82B:117–123
Burkill HM (1995) The useful plants of west tropical Africa, vol 3, 2 edn. Families J-L, Royal Botanic Gardens, Kew
Buxbaum E (2019) Na/K-atpase activity and ketone body metabolism in long-term diabetic rats. https://doi.org/10.1101/567180
Cools R, Nakamura K, Daw ND (2011) Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology 36(1):98–113
Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fowler MJ (2008) Microvascular and macrovascular complications of diabetes. Clin Diabetes 26:77–82
Garcia Barrado MJ, Iglesias Osma MC, Blanco EJ, Hernandez C, Sánchez M, Robledo V, Iniesta C, Carrero LS, Carretero J (2015) Dopamine modulates insulin release and is involved in the survival of rat pancreatic beta cells. PLoS ONE 10(4):e0123197
Ghara AR, Ezzati GF, Hosseini SH, Piacente S, Cerulli A, Alizadeh A, Mirmahmoudi R (2021) Antioxidant and antidiabetic effect of capparis decidua edgew (forssk.) Extract on liver and pancreas of streptozotocin-induced diabetic rats. J Appl Biotechnol Rep 8(1):76–82. https://doi.org/10.30491/JABR.2020.222547.1194
Glaser N, Sasaki- Russell J, Cohen M, Little C, O’Donnell M, Sall J (2017) Histological and cognitive alterations in adult diabetic rats following an episode of juvenile diabetic ketoacidosis: evidence of permanent cerebral injury. Neurosci Lett 650:161–167
Harborne JB (1998) Phytochemical methods a guide to modern techniques of plant analysis. Springer Science and Business Media
Ibrahim DS (2017) Neuroprotective effect of cucumis melo var. Flexuosus leaf extract on the brains of rats with streptozotocin-induced diabetes. Metab Brain Dis 32(1):69–75
Ibrahim DS, Abd El-Maksoud MAE (2015) Effect of strawberry (fragaria× ananassa) leaf extract on diabetic nephropathy in rats. Int J Exp Pathol 96(2):87–93
Imam-Fulani AO, Bamikole OK, Owoyele BV (2016) Effects of caffeine administration on brain sodium-potassium atpase activity in healthy and streptozotocin-induced diabetic female wistar rats. J Caffeine Res 6(3):117–125
Krishnamurthy P, Wadhwani A (2012) Antioxidant enzymes and human health. Antioxid Enzyme 3:1–17
Mani V, Arfeen M, Sajid S, Almogbel Y (2022) Neuroprotective effect of aqueous extract of ajwa seeds via anti-inflammatory pathways in type-2 diabetic-induced rats. Int J Pharmacol 18(2):299–306
Montgomery HAC, Dymock JF (1961) Analyst 86:414
Oh C-M, Park S, Kim H (2016) Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J 40(2):89–98
Omodanisi EI, Aboua YG, Oguntibeju OO (2017) Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules 22(4):439
Pohanka M (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155(3):219–229
Ronner P, Gazzotti P, Carafoli E (1977) Lipid 'Requirement for the (Ca2+ + Mg2+)-activated ATPase of erythrocyte membranes. Arch Biochem Biophys 179: 578–583
Salau VF, Erukainure OL, Olofinsan KA, Islam MS (2021) Vanillin exerts therapeutic effects against hyperglycemia-altered glucose metabolism and purinergic activities in testicular tissues of diabetic rats. Reprod Toxicol 102(2021):24–34
Secnik J, Schwertner E, Alvarsson M, Hammar N, Fastbom J, Winblad B, Garcia-Ptacek S, Religa D, Eriksdotter M (2020) Cholinesterase inhibitors in patients with diabetes mellitus and dementia: an open-cohort study of ~ 23 000 patients from the Swedish dementia registry. BMJ Open Diabetes Res Care 8(1):e000833
Sultana T, Butt K, Sultana S, Al-ghanim KA, Mubashra R, Bashir N, Ahmed Z, Ashraf A, Mahboob S (2016) Histopathological changes in liver, gills and intestine of Labeo rohita inhabiting industrial waste contaminated water of river Ravi. Pakistan J Zool 48(4):1171–1177
Uefune F, Aonishi T, Kitaguchi T, Takahashi H, Seino S, Sakano D, Kume S (2022) Dopamine negatively regulates insulin secretion through activation of d1-d2 receptor heteromer. Diabetes 71(9):1946–1961
Vávrová L, Kodydková J, Zeman M, Dušejovská M, Macášek J, Staňková B, Tvrzická E, Zák A (2013) Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes Facts 6(1):39–47
Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY (2012) Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo ¹H MR spectroscopy at 9.4 T. J Neurochem 121(3):407–417. https://doi.org/10.1111/j.1471-4159.2012.07698.x
Zannini E, Mauch A, Galle S, Gänzle M, Coffey A, Arendt EK, Taylor JP, Waters DM (2013) Barley malt wort fermentation by exopolysaccharide-forming w eissella cibaria mg 1 for the production of a novel beverage. J Appl Microbiol 115(6):1379–1387
Funding
This study was self-funded.
Author information
Authors and Affiliations
Contributions
BOA, BEO and SAO designed the study. TEO, SAS and SAO conducted the experiment. BOA, HH and RM analyzed the data obtained. TEO, SAS and SAO drafted the manuscript. BAO, BEO, SAO, HH and RM revived the manuscript. All the authors read and approved the submission.
Corresponding author
Ethics declarations
Ethics approval
This study was carried out in line with FUOYE Faculty of Science Ethical clearance (FUOYEFSC 201122-REC2022/008).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare none.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajiboye, B.O., Omojolomoloju, T.E., Salami, S.A. et al. Effect of Dalbergiella welwitschi alkaloid-rich extracts on neuroprotective in streptozotocin-induced diabetic rats. Metab Brain Dis (2024). https://doi.org/10.1007/s11011-024-01386-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11011-024-01386-9