Abstract
In the field of cell therapy for heart disease, a new paradigm of repeated dosing of cells has recently emerged. However, the lack of a repeatable cell delivery method in preclinical studies in rodents is a major obstacle to investigating this paradigm. We have established and standardized a method of echocardiography-guided percutaneous left ventricular intracavitary injection (echo-guided LV injection) as a cell delivery approach in infarcted mice. Here, we describe the method in detail and address several important issues regarding it. First, by integrating anatomical and echocardiographic considerations, we have established strategies to determine a safe anatomical window for injection in infarcted mice. Second, we summarize our experience with this method (734 injections). The overall survival rate was 91.4%. Third, we examined the efficacy of this cell delivery approach. Compared with vehicle treatment, cardiac mesenchymal cells (CMCs) delivered via this method improved cardiac function assessed both echocardiographically and hemodynamically. Furthermore, repeated injections of CMCs via this method yielded greater cardiac function improvement than single-dose administration. Echo-guided LV injection is a feasible, reproducible, relatively less invasive and effective delivery method for cell therapy in murine models of heart disease. It is an important approach that could move the field of cell therapy forward, especially with regard to repeated cell administrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite 2 decades of intensive research in cell therapy for heart disease, the optimal cell type, dosage, delivery method and frequency are still elusive, as is the mechanism behind the observed cardiac benefits of cell therapy [1, 2]. The available evidence supports two fundamental concepts: (i) the majority of cell-related effects do not result from direct cardiomyocyte differentiation but rather from paracrine mechanisms via the release of various factors that may favorably affect collagen deposition, cardiomyocyte apoptosis, local or systemic inflammation, immunomodulation, and/or angiogenesis [3, 4], and (ii) all cells, regardless of cell type or delivery approach, fail to engraft in the heart to a significant extent [4]. Therefore, expecting a single administration of short-lasting cells to produce long-term benefits may be unrealistic. A paradigm shift from single administration to repeated administrations of cells is underway [1, 3,4,5].
Typical cell delivery approaches in clinical studies include intracoronary infusion, transendocardial injection, and direct epicardial injection [1, 2]. The first two are catheter based, and can be repeated without opening the chest, although it would be practically difficult to perform repeated catheter-based deliveries in humans. However, in preclinical studies in rodents, repeated cell administrations can be difficult to perform. Most cell delivery methods used in rodents require open-chest surgery [6,7,8,9,10], and in general, rodents do not tolerate multiple (> 2) thoracotomies. Ultrasound-guided transthoracic intramyocardial injection is a less invasive approach [11] that has been used to deliver medications [12] or cells [13] in different disease models in mice, but the efficacy of this method in improving cardiac function remains unclear [13,14,15,16,17,18]. The uncertainty as to whether the needle tip remains inside the myocardium during the entire injection time (intermittent tip movements would result in cells being delivered into the pericardial space or the LV cavity), the limited targetable myocardial region, and the very limited injectable volume are major challenges that may prevent ultrasound-guided intramyocardial injection from being widely utilized for cell administration. We used a murine model not only because of the lower cost, but also because it provides relatively rapid testing and sufficient sample size to achieve adequate statistical power. Furthermore, this model makes it possible to dissect biological cellular mechanisms and molecular pathways with the availability of transgenic and gene-targeted animals. Since cell therapy-related research is still in an early stage and it is necessary to determine the safety, efficacy, fate, and mechanism of various cell types [1], it is appropriate to use rodents as screening models before moving to larger animal models or clinic trials [1].
The new research paradigm of repeated cell administrations requires a reliable cell delivery approach that can be used in small animals multiple times [1, 4, 19]. Among the available candidates, echocardiography-guided transthoracic intraventricular injection appears to be a feasible method [4]. Although this method has been successfully utilized as a systemic administration approach to deliver various agents [20, 21] and cancer cells [21, 22] into the systemic circulation of recipient animals, it is unclear whether it can be used to deliver cells that target the damaged heart. In our first study of repeated cell administrations in infarcted mice [23], we briefly discussed a method for echocardiography-guided, percutaneous injection into the LV cavity as a delivery approach for cell therapy, but the method has not been standardized to a methodological level that would allow other labs to reproduce it. In this paper, we build on that previous report [23], we summarize more than 700 injections that have been conducted in our lab since the method was established, and we address several important issues regarding the standardization of this delivery approach. First, we examine the feasibility of the method and the safe cardiac window for injection. Second, we test the reproducibility of the method and discuss how to handle complications. Third, we test whether cells delivered via this method improve cardiac function, both after a single injection and after repeated doses. Since our previous paper [23] was the only publication that administered more than 2 doses of cells via echo-guided injections in mice with a myocardial infarction, we reanalyzed the cardiac function data from our previous study [23] and present them as representative results in the current paper. We believe this method is an important technical advance that could help to move the field of cell therapy forward, especially with regard to repeated administrations.
Methods
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Louisville Institutional Animal Care and Use Committee (protocol number:14034). The general methods have been described previously [23, 24]. Here, we describe in detail the method of echocardiography-guided percutaneous left ventricular intracavitary injection (echo-guided LV injection) and summarize the other methods.
Ischemia/reperfusion mouse model
Adult, female, C57BL6/J mice were subjected to 60 min of regional myocardial ischemia followed by reperfusion as previously described [23, 25, 26]. In this model, an 8–0 nylon suture was passed under left coronary artery (LCA) approximately 2 mm below the left auricle and a nontraumatic balloon occluder was applied on the artery. Ischemia/reperfusion (I/R) was induced by tightening and inflating the occluder and then deflating and removing it. To minimize the impact of blood loss, blood from a donor mouse was given at serial times during surgery via intravenous injections into the jugular vein [25].
Determination of safe window for echo-guided LV injection in mice
The ideal safe windows for echo-guided LV injections should be able to target the LV cavity without damaging surrounding organs or vessels, especially major cardiac vessels. For this purpose, we utilized available anatomical and echocardiographic techniques in mice to visualize the unwanted targets and to reveal their locations on echocardiographic screens using cardiac structural landmarks as references. All echocardiography-related studies were done using a Vevo 2100 Imaging System (VisualSonics, Inc.) equipped with a 30 MHz transducer. Baseline echocardiography was performed on healthy blood donor mice. Parasternal long-axis and short-axis images were acquired (Fig. 1c and d).
The mouse heart has a wide window for intracavitary injection. Anterior view (a) and lateral view (b) of the thoracic cage are illustrated in the upper panel. Representative parasternal long-axis image (c) and short-axis image (d) from a healthy mouse are shown in the lower panels. The fan-shaped shadow indicates a safe window for intracavitary injection without damaging the lung
To visualize the major cardiac vessels, the ribcage of the donor mouse was immediately removed after blood was drained via transthoracic cardiac puncture. The heart and major vessels were exposed. Heparinized phosphate-buffered saline (PBS, 10 U/ml) was retrogradely infused into thoracic aorta via a 27 G needle attached to a 1 ml syringe. A 5-mm-long PE20 polyethylene tubing catheter was inserted into left anterior vena cava and secured by a 5–0 silk suture. The right anterior vena cava and posterior vena cava were then ligated. All three major venae cavae were then disconnected from distal ends. The aorta was then cannulated with a blunted 23 G needle. The heart was sequentially perfused via an aortic cannula with 1 ml saturated KCl, 3 ml heparinized PBS, and 10 ml 4% formalin solution. After fixation, 0.5-ml red latex dye was injected via the aortic cannula and then 0.5-ml blue latex dye was injected via the left anterior vena cava catheter. Digital photos of the heart with visualized coronary artery and cardiac vein were then acquired (Fig. 2a and b).
Determination of a safe injection window avoiding major cardiac vessels. Hearts from healthy blood donor mice were fixed and major cardiac vessels were visualized: a Anterior view of the heart; b Lateral view of the heart; c a heart slice was transversely cut at a level 2 mm away from the left auricle (indicated as yellow dashed line in a and b). Red arrows indicate the locations of left coronary artery, blue arrows indicate the left cardiac vein. d Locations of LCA (red dots) and LCV (blue ovals) from 6 donor mice. The blue shadow indicates a safe injection window. (Color figure online)
The fixed heart was frozen and then cut transversely 2 mm distal to the left auricle. The heart slices were photographed under a microscope. The location of coronary arteries and cardiac veins was visualized in the photo of those slices (Fig. 2c). The photos and the matched short-axis and long-axis echocardiographic images were used to determine the safe window for echo-guided LV injections.
Echo-guided LV injection in infarcted mice
Successfully infarcted mice that underwent ischemia/reperfusion were subjected to echo-guided LV injection at selected timepoints after infarction. Some mice underwent multiple injections at 2- or 5-week intervals. Cardiac mesenchymal cells (CMCs) or c-kit+ cardiac progenitor cells (CPCs) were used in these studies.
Preparations before starting
Materials: 30 G 0.5-inch needle, 1-ml syringe, sterile ultrasound transmission gel (Parker Laboratories, Inc.).
Mice were anesthetized with isoflurane (3% for induction and 1.5% for maintenance) and placed on the imaging table in supine position. The anterior chest was shaved and hair removal cream was applied to clean the rest of hair. 10% povidone-iodine and 70% isopropyl alcohol were applied to sterilize the skin. A thick layer of prewarmed sterile ultrasound gel was then applied to the chest wall.
Locating the injection window in infarcted mice
The ultrasound transducer was fixed to the mount system in a position such that the transducer was parallel to the forward–backward direction (Y) of the system. The position of the imaging table was adjusted to make sure that a good long-axis view image could be acquired. The transducer was then turned 90° clockwise to the short-axis view position.
Serial short-axis images, 1 mm stepwise from apex to base, were acquired by turning the fine Y direction control knob. In short-axis images, infarcted myocardium could be identified as a thinner wall and with less radial motion or even paradoxical motion (Fig. 3). An experienced echocardiographer can distinguish which level of the short-axis image is above the infarcted zone by carefully reviewing the images. A more accurate but time-consuming method would involve strain analysis to distinguish infarcted and noninfarcted regions (Fig. 4a and b).
Serial short-axis images from apex to base are shown to determine a safe level for injection. a Diagram of serial short-axis levels. b–i Representative short-axis images, 1 mm stepwise from apex to base, are shown. The yellow line indicates the infarcted zone defined by thinner wall and less radial motion. (Color figure online)
Echo-guided LV injection in infarcted mice. Strain analysis was performed in short-axis images from an infarcted mouse. Vector view at end-systole is shown in the upper panel. a Vector of radial motion indicates the infarcted zone with reduced or reversed motion. b Short-axis image above the suture level shows a synchronous myocardial radial motion, indicating a safe level for intracavitary injection. c Representative echo-guided LV injection image (before insertion). The green dashed line is the guidance line for injection. The white arrow indicates the tip of the needle. The fan-shape shadow indicates a safe window for injection. e Representative echo-guided LV injection image with injection needle in the cavity. d and f Setups for freehand and Vevo Injection System injections. (Color figure online)
A short-axis image above the site of the suture placed during ischemia/reperfusion surgery with synchronous myocardial radial motion was considered to be a safe injection level.
Injection
Echo-guided LV injection can be conducted by free hand or using the Vevo Injection system.
For a right-handed operator, the mouse was put on the imaging table with the tail toward the operator. The imaging table was then tilted 30–45° to the right of the animal and the transducer mount was accordingly turned 35–50° counterclockwise to give enough window to access the left lateral chest wall (Fig. 4d).
For a left-handed operator and the Vevo injection system, the imaging table was turned 180° with the head of the animal toward the operator. The table and transducer mount were adjusted accordingly to let the left lateral chest wall face the injection mount (Fig. 4f).
Re-acquired short-axis images were used to locate the safe injection window if the level had been changed during adjustment of the table. Under real-time B-mode imaging, an injection guidance line was drawn to guide the injection (Fig. 4c).
A syringe attached to a 30 G needle was filled with the desired volume of cell solution, making sure there was no air bubble in the system. The syringe was placed under the transducer with the needle exactly parallel to the scan head. The needle was seen in the real-time screen. Following the guidance line, the needle was then advanced into the LV cavity (Fig. 4e and S3 Video).
1 × 106 cells in 200 µl medium were injected into the LV cavity over 90 s. In some mice, a higher dose of 3 × 106 cells in 600 µl medium was infused over 200 s. The same volume of PBS served as vehicle control.
The depth of the needle and the physical status of the animal were monitored throughout the injection. After the delivery of cells, the needle was quickly withdrawn. A cotton tipped applicator was pushed against the skin at the insertion site for a few second to prevent bleeding. An experienced operator can finish the whole procedure in 20 min from preparing to completing the delivery.
After injection care
Mice were then removed from the imaging table and allowed to recover in a cage on a heating pad. Mice were monitored until able to move freely (typically within 15 min).
Cell preparation
CMCs [23, 24, 26, 27] and CPCs [7, 8] were isolated and cultured as previously described. All cells were from adult male mice with C57BL/6 J background, some were from GFP or RFP transgenic male mice. The rationale for using male donor cells was that the fate of transplanted cells can be tracked by detecting Y-chromosomepos cells in female recipient hearts. Cells were cultured in growth medium consisting of DMEM/F12 (Invitrogen), 10% FBS (Seradigm, VWR), bFGF (10 ng/ml), EGF (10 ng/ml), ITS (insulin/transferrin/selenium), glutamine, and Pen-Strep. Cells were passaged 4–6 times. On the day of injection, cells were harvested, counted and resuspended in PBS as previously described. All cells were injected within 15 min after the last centrifugation.
Echocardiography
Transthoracic echocardiography was performed to assess cardiac function before cell administration and 5 weeks after treatment. In the multiple injections studies, serial echocardiograms were obtained before each injection and at the end of the study. Parasternal long-axis images were used to measure cardiac functional parameters [23, 26].
Hemodynamic study
Mice were subjected to pressure–volume loop assessments right before euthanasia, as previously described [6, 23]. A Millar MPVS ULTRA Pressure–Volume System equipped with a PVR-1035 pressure–volume catheter was used.
Statistical analysis
Data are presented as mean ± SEM. All data were analyzed with Student’s t tests or one-way ANOVA for normally distributed data followed by unpaired Student’s t tests with the Bonferroni correction, or Kruskal–Wallis one-way analysis of variance on ranks for data that are not normally distributed, as appropriate, A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the Sigma Stat software system [28,29,30,31,32,33,34,35,36,37].
Results
Echo-guided LV injection is feasible in infarcted mice
Unlike the human heart, in the mouse, the majority of the LV anterior and lateral wall is not covered by the lung [38, 39]. This anatomical feature gives us a wide window for potential direct transthoracic injection into the LV cavity without damaging the lung (Fig. 1). To determine a safe window to avoid major cardiac vessels during injections, hearts from 6 mice were fixed and major cardiac vessels were visualized by colored latex dye [40]. On the heart slices cut at 2 mm from the left auricle, the locations of the LCA and left cardiac vein (LCV), including the angles and the distances from epicardium, were measured as illustrated in Fig. 2. Every coronary artery has its unique path and branch tree. However, at a distance of 2 mm from the left auricle, the LCA typically has one main trunk [41], and sometimes an early separate branch (1 in 6 observed mice). The distribution of the LCA on the heart slices is also variable but is usually within the LV anterior wall in a 70° arc as illustrated in Fig. 2d. The main trunk of the LCV has less variation and is within a 50° arc in the LV posterior wall (Fig. 2d). Our results suggested that the LV lateral wall between the anterior and posterior papillary muscles is safe to avoid major cardiac vessels.
In infarcted mice, serial short-axis images were acquired to determinate a safe injection level without puncturing the infarct scar. From apex to the suture level, ischemia-damaged myocardium was observed in short-axis images. It manifested thinner wall thickness compared with the remote walls (septal and posterior wall) and either akinesis or dyskinesis. In contrast, above the suture level, about 6 mm from the apex, the noninfarcted myocardium showed a synchronous myocardial radial motion. An experienced echocardiographer can distinguish between infarcted and noninfarcted zone by carefully reviewing the images (Fig. 3). For beginners, strain analysis can be used to determinate a safe injection level [42]. In vector/B-mode view of strain analysis window, the motion vector (direction and speed) of each wall segment is revealed; this made it possible to define the noninfarcted normal motion zone and the infarcted hypokinetic zone (Fig. 4a, b).
The integration of anatomy and echocardiography provided a feasible method for safe echo-guided LV injections in infarcted mice. The method was established during our first repeated cells administration project in mice.
Echo-guided LV injection is a reproducible, less invasive approach for cell delivery
Since its establishment [23, 24], echo-guided LV injection has been utilized in several projects in our lab. Those projects enrolled 435 mice; among them, 181 mice underwent a 3-injections protocol. As shown in Table 1, the overall survival rate after a total of 734 injections was 91.4%. Notably, the survival rate of 181 mice that completed the 3-injection protocol was 81.2%.
Autopsies of the 63 mice that died suggested that the major cause of death was bleeding (Table 2). Among 34 bleeding cases, 23 were acute bleeding and the mice died within 5 min after the injections; another 11 mice survived the first 4 h but eventually died 12 h after injections. The majority of the bleeding cases (25 out of 34, 74%) occurred during the first 100 injections, a phenomenon that reflected a “learning curve” for this procedure in our lab. After the method was standardized to avoid major cardiac vessels and infarction scars, only 9 cases of bleeding occurred among the remaining 634 injections.
Another cause of death was considered to be cell-related because it occurred only in cell-treated mice but not in vehicle groups. Those animals manifested acute respiratory and circulatory failure when the infusion started and most of them died before the total volume of cells could be delivered. Those cases typically occurred at the beginning of a new project. The complications were fed back to the cell preparation lab for quality control. To reduce cell clumping, vials containing cells were inverted several times or gently vortexed before cells were placed into the injection syringe. The injection duration was extended to 90 s instead of the original 60 s. Those changes were sufficient to keep mortality rate under control.
No cardiac perforation or rupture was observed.
In our lab, a new operator with experience in echocardiography has been trained to perform echo-guided LV injections through a training course that required 10–15 mice.
Cells delivered via echo-guided LV injection improve cardiac function
To determine whether CMCs delivered via echo-guided LV injection are beneficial in chronic heart failure, data from all the studies using the same CMCs and vehicle control were pooled together. As shown in Fig. 5a, echocardiographic assessments were performed 5 weeks after infarction (before treatment) and 35 days after cell administration. Mice were subjected to administration of 1 × 106 CMCs or vehicle via echo-guided LV injection. There was no difference in LV end-diastolic volume (LVEDV) and EF between the 2 groups before treatment, indicating similar degrees of cardiac dysfunction. While LVEDV did not change after treatment in both groups, CMCs increased stroke volume (SV), and therefore, improved ejection fraction (EF) (Fig. 5b–d). Hemodynamic data also showed that compared to vehicle, the CMC-treated group manifested better cardiac function in both load-dependent parameters such as EF and dP/dt (Fig. 5e, f), and load-independent parameter such as end-systolic elastance (Fig. 5g). Raw data can be found in the supporting information S1 Dataset; the full set of echocardiographic and hemodynamic results can be found in S2 Table1 and S2 Table2.
CMCs delivered via echo-guided LV injection improve cardiac function in infarcted mice. Mice with prior MI received CMCs or vehicle, n = 36 for each group. Echocardiograms were acquired before and 5 weeks after treatment. a Schematic protocol of the experiments performed in b–g. Green arrows indicate echocardiographic assessments. Red arrow indicates cells/vehicle injection. b LV end-diastolic volume; c stroke volume; and d EF. Vehicle group in dark grey and CMC group in blue. Hemodynamic assessment was performed before euthanasia: e EF; f dP/dt maximum and dP/dt minimum; g end-systolic elastance. Data are mean ± SEM. #p < 0.05 compared with vehicle group. (Color figure online)
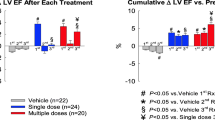
To examine whether repeated administrations of CMCs via echo-guided LV injection can produce additional beneficial effects compared with a single administration, we reanalyzed the cardiac function results from our previous study in mice with subacute infarction (3 weeks after infarction) [23]. The results presented here are the same as those published in our previous paper [23] but are presented in a different format. Three repeated doses of 1 × 106 CMCs were delivered via echo-guided LV injection at 2-week intervals (Fig. 6a). As reported previously [23], each dose of CMCs resulted in a net increase in EF, such that the cumulative EF gain was greater after multiple doses than after a single dose (Fig. 6b). Therefore, the final EF in the repeated-doses group was significantly higher than in the vehicle or single-dose group [23] (Fig. 6c). Raw data can be found in the supporting information S1 Dataset; the full set of echocardiographic results can be found in S2 Table3.
Repeated administration of CMCs or CPCs via echo-guided LV injection is superior to single administration. a Schematic protocol of the subacute infarction experiment. Green arrows indicate echocardiographic assessments. Red arrow indicates cells/vehicle injection. EF was measured by echocardiography before treatment (Pre-Rx) and after each treatment (1st Rx, 2nd Rx and 3rd Rx). b Time course of changes of EF (delta-EF) compared with the EF value of Pre-Rx. c Time course of EF. Data are mean ± SEM. #p < 0.05 compared with vehicle group, §p < 0.05 compared with single-dose group. The results presented here are the same as those published in our previous paper [23] but are presented in a different format. (Color figure online)
Discussion
After 2 decades of intensive research in the field of cell therapy for heart disease, conclusive demonstration of therapeutic efficacy of the cells is still lacking, and a fundamental understanding of how cell therapy works is still elusive [1, 3, 4, 19, 43, 44]. This has led to discussions of changes in the field and search for new paradigms for future research. A paradigm of repeated dosing of cells has become one of the investigational priorities [4, 43, 45]. However, the lack of a reliable, repeatable cell delivery approach in preclinical studies using small animals hinders progress. We have reported briefly on the method of echo-guided LV injection previously [23, 24]. Here, we standardize the method and describe it in detail in an effort to provide sufficient reproducibility for other labs.
Direct intramyocardial and intracoronary injection are commonly used in preclinical studies in rodents. Both of these cell delivery approaches require a thoracotomy, which limits their applicability in studies requiring repeated cells administrations. Some researchers have proposed to use ultrasound-guided intramyocardial [13,14,15,16] or intrapericardial injection [46] to deliver therapeutic agents including cells. However, in animal models of infarction, the pericardium is not intact and it is uncertain whether the injection needle can be kept in the pericardial space without stabbing the myocardium in a beating heart. Furthermore, pericardial delivery does not allow for cells to infiltrate the myocardium. Echo-guided intramyocardial injections could be repeated in mice. However, in an infarcted murine heart, there is limited viable myocardium that could be targeted for injection. Furthermore, because of the thinness of the myocardial wall, the volume that can be delivered is also limited. Finally, it is difficult to be certain that the injection needle remains inside the targeted myocardium during the entire injection without entering the LV cavity. These issues could be the reason why some cells delivered via echo-guided intramyocardial injection have been reported not to yield cardiac functional improvement [14]. On the other hand, echo-guided LV intracavitary injection allows a larger injectable volume and a larger safe zone without penetrating into the myocardium. The coronary blood flow route also enables cells to reach all cardiac regions. Although this method may require a higher number of cells than direct intramyocardial injection, this disadvantage can be easily overcome. Therefore, we chose echo-guided LV intracavitary injection as a cell delivery approach in infarcted mice.
We first examined the feasibility of this method. Unlike in humans, in rodents, the majority of the LV anterior and lateral wall (including the infarcted zone, border zone and remote zone) is not covered by lungs or other organs. By combining anatomical and echocardiographic considerations, we have established strategies to locate a safe window for intracavitary injection and avoid major cardiac vessels and infarct scars. The fact that this method had been successfully utilized in multiple projects in our lab proves its feasibility. A similar method has also been used in a rat model [47, 48] in our group, suggesting its generalizability in preclinical studies of rodents.
Next, we examined the reproducibility of this method. We have used this method in more than 700 injections. As summarized in Table 1, the overall survival rate was 91.4% and the survival rate after 3 injections was 81.2%. The deaths during our “learning curve” were included in the mortality count. With the strategies of locating a safe window and the quality control of cells preparations, bleeding and cell-related complications are expected to be reduced even more in future experiments. Compared to open-chest surgeries that require an experienced surgeon, ventilation, and hours of procedure time, echo-guided LV injection can be conducted by an echocardiographer without using ventilation, and only require about 20 min. In our lab, a new operator with experience in echocardiography can be trained with 10–15 mice. We believe that with the details described herein, it is not difficult for other groups to reproduce this method in their lab.
For echo-guided LV injection, a dose of cells tenfold higher than with intramyocardial or intracoronary approaches was chosen. In our previous study [23], to measure cell retention, female mice underwent echo-guided LV injection with 1 × 106 male CMCs; 5 hearts were harvested at 5 min after injection and another 5 hearts at 24 h after injections. The number of male cells in the female heart was calculated as described [49] and compared with our previous studies of 1 × 105 CPCs delivered via intracoronary or intramyocardial injection [7, 49]. Considering that coronary flow is ~ 5% of cardiac output [50] and that cell doses > 1 × 106 may be difficult to produce for repeated injections, we used a 10 times higher dose (1 × 106 cells) for intracavitary injection to achieve retention comparable with direct intramyocardial injection or intracoronary injection (1 × 105 cells). The results of our previous study suggested that intracavitary injection of a tenfold higher cell dose yielded an almost twofold greater cell retention in the heart at 5 min after injection, and similar retention at 24 h compared with the other two approaches [23]. These results in mice are consistent, and corroborate those in our previous rat study using a similar echo-guided intraventricular injection method. In infarcted rats, the number of cells retained 24 h after intraventricular injection of a 12 × 106 dose (112 983 ± 56 300 cells/heart, n = 6) was similar to that retained 24 h after intracoronary infusion of 1 × 106 CPCs (118 924 ± 24 458, n = 3) [48]. These previous results support the generalizability of using a tenfold higher dose of cells with LV intracavitary injection in small rodents. Although it is still unclear whether engraftment or retention of transplanted cells is necessary for their cardiac beneficial effects, there is evidence linking the beneficial effects to better retention [51, 52]. These considerations may help assuage the concern as to whether sufficient numbers of cells delivered with this method are retained in the heart.
Finally, the efficacy of this cell delivery approach was examined in the settings of both a single injection and repeated injections. Compared with vehicle treatment, CMCs delivered via this method improved cardiac function assessed both by echocardiography and hemodynamic measurements. Furthermore, the results from previous subacute infarction experiment [23] suggested that repeated injections of CMCs via this method yielded superior cardiac functional improvement than single-dose administration. A similar echo-guided LV injection approach was also utilized in rats. As previously reported, repeated administrations of CPCs in rats also produce a cumulative beneficial effect on LV function and structure [48]. Notably, result from rat studies suggested that three repeated doses of cells were superior to one combined dose even though the total number of cells infused was the same [47]. All these findings support the concept that echo-guided LV injection is an effective cell delivery approach.
It is now well established that the beneficial effects of transplanted cells in the heart do not result from direct cardiomyocyte differentiation (transplanted cells do not engraft) but rather from paracrine mechanisms [4, 53]. Transplanted cells probably act by releasing a complex array of factors including growth factors, cytokines, miRNAs, and bioactive lipids [1], as well as extracellular vesicles/exosomes [4, 54]. These factors are thought to act in a paracrine fashion on adjacent cells, influencing in a favorable manner a variety of processes such as cell proliferation, apoptosis, remodeling of extracellular matrix, inflammation, immunomodulation and angiogenesis [4]. Although we do not have direct evidence of a link between better cell retention and greater secretion of paracrine factors, our previous studies in mice [23] and rats [48] showed that hearts that received multiple injections of CMCs or CPCs manifested less collagen deposition compared with hearts that received a single injection. These data suggest that the greater number of cells delivered via multiple injections might have produced more paracrine factors, such as metalloproteinases [1], interleukin-6 [1, 4], indoleamine 2,3-dioxygenase [1, 4] and exosomes [1, 4], which could have reduced collagen deposition and contributed, at least in part, to the improvement in cardiac function.
The current study corroborates and expands some of the findings of our first study of repeated cell administration in mice [23]. However, there are major differences between the two studies. Our previous paper [23] addressed the question as to whether repeated doses of CMCs are superior to one single dose, while the current paper addresses the question as to whether echo-guided LV injection is a reliable and reproducible method for cell delivery. The focus of the present paper is the standardization of this method; accordingly, here, we provide details regarding the procedure protocol and handling of complications that were not provided in the previous paper but are essential for other labs to reproduce this method. Importantly, the findings of our previous paper were based on a study of 77 mice with 3-weeks-old infarctions that were treated with one type of cells (CMCs), while the present paper, being a methodological study, summarizes studies of 435 mice, different cell types (CMCs and CPCs), and different stages of infarction (subacute and chronic). Thus, this report substantially expands our previous report [23] and should be useful to investigators interested in adopting this methodology.
Multiple deliveries of cells would be easier in larger animal models. Compared with larger animals, it is relatively difficult to administer cells to the hearts of small rodents, especially with multiple deliveries. However, small animal models, especially mouse models, offer significant advantages in preclinical research of cell therapy for heart disease. First, the mouse model is less expensive than larger animals such as pigs and sheep (~ 1/10 to 1/100 of the cost of larger animals) [1]. Second, this model enables relatively rapid testing and adequate sample sizes to achieve sufficient statistical power [1, 43]. Third, the availability of genetically engineered mice provides unique opportunities to explore molecular and cellular mechanisms [43]. Despite 2 decades of intensive research in the field of cell therapy for heart disease, the optimal cell type, dosage, delivery method and frequency of treatment are still elusive, as is the mechanism behind the beneficial effects of cell therapy [1]. Furthermore, the results of clinical trials performed to-date are not conclusive [3]. Therefore, preclinical studies are critical to advance the field [1, 4, 19, 43, 44]. To evaluate the safety and efficacy of new cell types, to explore new paradigms, and to dissect the underlying mechanisms, it is necessary to use rodents as screening models before moving to larger animals and eventually to clinical trials. One of the most important new therapeutic paradigms is repeated dosing [4, 45]. It is difficult to perform multiple cell deliveries in small animals via a thoracotomy; hence, the importance of the approach described herein, which makes it possible to perform repeated cell injections with low mortality.
In summary, our results suggest that echo-guided LV injection is a feasible, reproducible, relatively noninvasive and effective delivery approach for cell therapy in murine models of heart disease. It can be utilized in mice or rat models, at any time point, at any interval, with a wide injection volume capacity and an almost unlimited number of possible repetitions. For example, no other method would enable more than three cell administrations in the same animal. It is an important approach that could move cell therapy forward, especially with regard to repeated cell administrations.
Data availability
All data and material are available from the corresponding author on reasonable request.
References
Tompkins BA, Balkan W, Winkler J, Gyongyosi M, Goliasch G, Fernandez-Aviles F, Hare JM (2018) Preclinical studies of stem cell therapy for heart disease. Circ Res 122:1006–1020. https://doi.org/10.1161/CIRCRESAHA.117.312486
Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moye L, Simari RD, Wolf A, Hare JM, Cardiovascular Cell Therapy Research N (2016) Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med 5:186–191. https://doi.org/10.5966/sctm.2015-0101
Vrtovec B, Bolli R (2019) Potential strategies for clinical translation of repeated cell therapy. Circ Res 124:690–692. https://doi.org/10.1161/CIRCRESAHA.118.314653
Wysoczynski M, Khan A, Bolli R (2018) New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res 123:138–158. https://doi.org/10.1161/CIRCRESAHA.118.313251
Sanganalmath SK, Bolli R (2013) Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113:810–834. https://doi.org/10.1161/CIRCRESAHA.113.300219
Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, Zhu X, Wang XL, Du J, Wu WJ, Xie W, Hong KU, Li Q, Bolli R (2015) Preconditioning human Cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells 33:3596–3607. https://doi.org/10.1002/stem.2198
Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R (2014) c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE 9:e96725. https://doi.org/10.1371/journal.pone.0096725
Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R (2011) Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol 106:849–864. https://doi.org/10.1007/s00395-011-0180-1
Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R (2010) Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121:293–305. https://doi.org/10.1161/CIRCULATIONAHA.109.871905
Tang XL, Rokosh DG, Guo Y, Bolli R (2010) Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circ J 74:390–404
Prendiville TW, Ma Q, Lin Z, Zhou P, He A, Pu WT (2014) Ultrasound-guided transthoracic intramyocardial injection in mice. J Vis Exp. https://doi.org/10.3791/51566
Maeda K, Seymour R, Ruel M, Suuronen EJ (2017) Echocardiography-guided intramyocardial injection method in a murine model. Methods Mol Biol 1553:217–225. https://doi.org/10.1007/978-1-4939-6756-8_17
Springer ML, Sievers RE, Viswanathan MN, Yee MS, Foster E, Grossman W, Yeghiazarians Y (2005) Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol 289:H1307–H1314. https://doi.org/10.1152/ajpheart.00164.2005
Deddens JC, Feyen DA, Zwetsloot PP, Brans MA, Siddiqi S, van Laake LW, Doevendans PA, Sluijter JP (2017) Targeting chronic cardiac remodeling with cardiac progenitor cells in a murine model of ischemia/reperfusion injury. PLoS ONE 12:e0173657. https://doi.org/10.1371/journal.pone.0173657
Yeghiazarians Y, Gaur M, Zhang Y, Sievers RE, Ritner C, Prasad M, Boyle A, Bernstein HS (2012) Myocardial improvement with human embryonic stem cell-derived cardiomyocytes enriched by p38MAPK inhibition. Cytotherapy 14:223–231. https://doi.org/10.3109/14653249.2011.623690
Toeg HD, Tiwari-Pandey R, Seymour R, Ahmadi A, Crowe S, Vulesevic B, Suuronen EJ, Ruel M (2013) Injectable small intestine submucosal extracellular matrix in an acute myocardial infarction model. Ann Thorac Surg 96:1686–1694. https://doi.org/10.1016/j.athoracsur.2013.06.063 (discussion 1694)
Angeli FS, Zhang Y, Sievers R, Jun K, Yim S, Boyle A, Yeghiazarians Y (2012) Injection of human bone marrow and mononuclear cell extract into infarcted mouse hearts results in functional improvement. Open Cardiovasc Med J 6:38–43. https://doi.org/10.2174/1874192401206010038
Inaba Y, Davidson BP, Kim S, Liu YN, Packwood W, Belcik JT, Xie A, Lindner JR (2014) Echocardiographic evaluation of the effects of stem cell therapy on perfusion and function in ischemic cardiomyopathy. J Am Soc Echocardiogr 27:192–199. https://doi.org/10.1016/j.echo.2013.10.011
Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Luscher TF, Martin JF, Menasche P, Pinto FJ, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC, Group TW (2018) Global overview of the transnational alliance for regenerative therapies in cardiovascular syndromes (TACTICS) recommendations: a comprehensive series of challenges and priorities of cardiovascular regenerative medicine. Circ Res. https://doi.org/10.1161/CIRCRESAHA.117.312099
Dave M, Menghini P, Sugi K, Somoza RA, Lee Z, Jain M, Caplan A, Cominelli F (2017) Ultrasound-guided intracardiac injection of human mesenchymal stem cells to increase homing to the intestine for use in murine models of experimental inflammatory bowel diseases. J Vis Exp. https://doi.org/10.3791/55367
Balathasan L, Beech JS, Muschel RJ (2013) Ultrasonography-guided intracardiac injection: an improvement for quantitative brain colonization assays. Am J Pathol 183:26–34. https://doi.org/10.1016/j.ajpath.2013.03.003
Salamon J, Peldschus K (2014) Ultrasound-guided intracardial injection and in vivo magnetic resonance imaging of single cells in mice as a paradigm for hematogenous metastases. Methods Mol Biol 1070:203–211. https://doi.org/10.1007/978-1-4614-8244-4_15
Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R (2017) Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 112:18. https://doi.org/10.1007/s00395-017-0606-5
Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP (2016) A new method to stabilize C-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol 4:78. https://doi.org/10.3389/fcell.2016.00078
Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R (2015) The NHLBI-sponsored consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 116:572–586. https://doi.org/10.1161/CIRCRESAHA.116.305462
Mehra P, Guo Y, Nong Y, Lorkiewicz P, Nasr M, Li Q, Muthusamy S, Bradley JA, Bhatnagar A, Wysoczynski M, Bolli R, Hill BG (2018) Cardiac mesenchymal cells from diabetic mice are ineffective for cell therapy-mediated myocardial repair. Basic Res Cardiol 113:46. https://doi.org/10.1007/s00395-018-0703-0
Wysoczynski M, Guo Y, Moore JB, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL, Bolli R (2017) Myocardial reparative properties of cardiac mesenchymal cells isolated on the basis of adherence. J Am Coll Cardiol 69:1824–1838. https://doi.org/10.1016/j.jacc.2017.01.048
Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA (2001) Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation 103:1893–1898
Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R (2000) PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol 279:H1679–H1689. https://doi.org/10.1152/ajpheart.2000.279.4.H1679
Tang XL, Qiu Y, Park SW, Sun JZ, Kalya A, Bolli R (1996) Time course of late preconditioning against myocardial stunning in conscious pigs. Circ Res 79:424–434. https://doi.org/10.1161/01.res.79.3.424
Bennett WR, Yawn DH, Migliore PJ, Young JB, Pratt CM, Raizner AE, Roberts R, Bolli R (1987) Activation of the complement system by recombinant tissue plasminogen activator. J Am Coll Cardiol 10:627–632. https://doi.org/10.1016/s0735-1097(87)80206-1
Li XY, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R (1993) Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest 92:1025–1041. https://doi.org/10.1172/JCI116608
Triana JF, Li XY, Jamaluddin U, Thornby JI, Bolli R (1991) Postischemic myocardial “stunning”. Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res 69:731–747. https://doi.org/10.1161/01.res.69.3.731
Takano H, Bolli R, Black RG Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA (2001) A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res 88:520–528. https://doi.org/10.1161/01.res.88.5.520
Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R (2008) Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells 26:1646–1655. https://doi.org/10.1634/stemcells.2007-0715
Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A (2002) Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res 91:240–246. https://doi.org/10.1161/01.res.0000029970.97247.57
Ping P, Zhang J, Huang S, Cao X, Tang XL, Li RC, Zheng YT, Qiu Y, Clerk A, Sugden P, Han J, Bolli R (1999) PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol 277:H1771–H1785. https://doi.org/10.1152/ajpheart.1999.277.5.H1771
Doevendans PA, Daemen MJ, de Muinck ED, Smits JF (1998) Cardiovascular phenotyping in mice. Cardiovasc Res 39:34–49. https://doi.org/10.1016/s0008-6363(98)00073-x
Constantinescu GM, Cynthia Besch-Williford DVMPD (2018) Comparative anatomy of the mouse and the rat: a color atlas and text. Taylor Francis Group, Milton Park
Weyers JJ, Carlson DD, Murry CE, Schwartz SM, Mahoney WM Jr (2012) Retrograde perfusion and filling of mouse coronary vasculature as preparation for micro computed tomography imaging. J Vis Exp. https://doi.org/10.3791/3740
Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ (2017) Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313:H275–H282. https://doi.org/10.1152/ajpheart.00127.2017
Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R (2011) Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108:908–916. https://doi.org/10.1161/CIRCRESAHA.110.239574
Grigorian Shamagian L, Madonna R, Taylor D, Climent AM, Prosper F, Bras-Rosario L, Bayes-Genis A, Ferdinandy P, Fernandez-Aviles F, Izpisua Belmonte JC, Fuster V, Bolli R (2019) Perspectives on directions and priorities for future preclinical studies in regenerative medicine. Circ Res 124:938–951. https://doi.org/10.1161/CIRCRESAHA.118.313795
Chamuleau SAJ, van der Naald M, Climent AM, Kraaijeveld AO, Wever KE, Duncker DJ, Fernandez-Aviles F, Bolli R, Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes G (2018) Translational research in cardiovascular repair: a call for a paradigm shift. Circ Res 122:310–318. https://doi.org/10.1161/CIRCRESAHA.117.311565
Bolli R (2017) Repeated cell therapy: a paradigm shift whose time has come. Circ Res 120:1072–1074. https://doi.org/10.1161/CIRCRESAHA.117.310710
Laakmann S, Fortmuller L, Piccini I, Grote-Wessels S, Schmitz W, Breves G, Kirchhof P, Fabritz L (2013) Minimally invasive closed-chest ultrasound-guided substance delivery into the pericardial space in mice. Naunyn Schmiedebergs Arch Pharmacol 386:227–238. https://doi.org/10.1007/s00210-012-0815-2
Tang XL, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu WJ, Hunt G, Stowers H, Ou Q, Bolli R (2018) Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.007400
Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R (2016) Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res 119:635–651. https://doi.org/10.1161/CIRCRESAHA.116.308937
Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R (2013) A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 108:346. https://doi.org/10.1007/s00395-013-0346-0
Ngo C, Dahlmanns S, Vollmer T, Misgeld B, Leonhardt S (2018) An object-oriented computational model to study cardiopulmonary hemodynamic interactions in humans. Comput Methods Prog Biomed 159:167–183. https://doi.org/10.1016/j.cmpb.2018.03.008
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ (2019) Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun 10:3123. https://doi.org/10.1038/s41467-019-11091-2
Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R Jr, Gude NA, Sussman MA (2015) Cardiac stem cell hybrids enhance myocardial repair. Circ Res 117:695–706. https://doi.org/10.1161/CIRCRESAHA.115.306838
Bolli R, Kahlon A (2020) Time to end the war on cell therapy. Eur J Heart Fail 22:893–897. https://doi.org/10.1002/ejhf.1767
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114:333–344. https://doi.org/10.1161/CIRCRESAHA.114.300639
Acknowledgements
This work was supported in part by National Institutes of Health Grants P01 HL078825 (RB) and UM1 HL113530 (RB).
Funding
This work was supported in part by National Institutes of Health Grants P01 HL078825 (RB) and UM1 HL113530 (RB).
Author information
Authors and Affiliations
Contributions
RB: conceived, designed, supervised the study and the manuscript; YN: performed the experiments, analyzed the data, and provided the manuscript; YG: supervised the findings; AT and XZ: helped in carrying out the experiments. MW and QL: provided cells. All the authors reviewed and contributed to revisions and finalized the drafts.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All the authors have approved that the submitted works are original and the paper has not been published and is not being considered for publication elsewhere.
Research involving human and animal rights
All the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Louisville Institutional Animal Care and Use Committee (protocol number:14034).
Informed consent
All the participants for the study have signed the written informed consent. All the authors have approved the final version of this manuscript and have consented to the submission of this manuscript to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 8518 KB)
Rights and permissions
About this article
Cite this article
Nong, Y., Guo, Y., Tomlin, A. et al. Echocardiography-guided percutaneous left ventricular intracavitary injection as a cell delivery approach in infarcted mice. Mol Cell Biochem 476, 2135–2148 (2021). https://doi.org/10.1007/s11010-021-04077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04077-6