Abstract
Repeated doses of c-kit+ cardiac progenitor cells (CPCs) are superior to a single dose in improving LV function in rats with old myocardial infarction (MI). However, this concept needs testing in different species to determine whether it is generalizable. We used a new murine model of chronic ischemic cardiomyopathy whose unique feature is that cell therapy was started late (3 months) after MI. Mice received three echo-guided intraventricular infusions, 5 weeks apart, of vehicle, CPCs × 1, or CPCs × 3. Echocardiography demonstrated that the single-dose group exhibited improved LV ejection fraction (EF) after the 1st infusion (CPCs), but not after the 2nd and 3rd (vehicle). In contrast, in the multiple-dose group LVEF continued to improve, so that the final value was greater than in vehicle or single-dose groups (P < 0.05). Hemodynamic studies showed that compared with vehicle, both preload-dependent and preload-independent functional parameters were significantly increased in the multiple-dose group but not in the single-dose group. Thus, two independent methods of functional assessment (echocardiography and hemodynamic studies) consistently demonstrated the superiority of three doses of CPCs vs. one dose. Compared with the single-dose group, the multiple-dose group exhibited less LV hypertrophy, as evidenced by a greater reduction in LV/body weight ratio and cardiomyocyte cross-sectional area. Furthermore, unlike the single dose, three CPC doses reduced myocardial inflammatory cells in the risk region. This is the first study of echo-guided intraventricular infusion of CPCs in mice with chronic ischemic cardiomyopathy. The results demonstrate that the beneficial effects of three CPC doses are greater than those of one dose, supporting the concept that multiple treatments are necessary to properly evaluate cell therapy. Our findings indicate that this concept applies not only to rat models but also to murine models. The generalizability of this strategy greatly enhances its importance and provides a rationale for large animal studies.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent evidence supports a new paradigm that may fundamentally transform the field of cell therapy – the use of repeated cell administrations [2]. The rationale for repeated dosing stems from the recognition that exogenous cells, regardless of their nature, fail to engraft in the host myocardium and disappear quickly after transplantation [7, 8, 15, 20]. In previous studies in a rat model of ischemic cardiomyopathy, we have found that three repeated doses of c-kit+ cardiac progenitor cells (CPCs) are more effective than a single dose [18]. We have extended this concept to a mouse model of recent myocardial infarction (MI), in which cardiac mesenchymal cells (CMCs) were given 3 weeks after MI administration [7]. Whether the greater efficacy of multiple doses of CPCs is applicable to more than one species, however, is unknown since, at present, the superiority of repeated administrations of CPCs is supported only by results obtained in a rat model [16, 18]. The validity of this paradigm has not been tested in models of chronic ischemic cardiomyopathy in other species.
The implications of the repeated-doses paradigm are potentially vast. To date, almost all preclinical studies and virtually all clinical studies of cell therapy have used one cell administration, based on the assumption that one dose of cells is sufficient to achieve a therapeutic effect [2]. In contrast, the new paradigm implies that the efficacy of cell therapy cannot be adequately assessed on the basis of a single cell administration and that future preclinical and clinical studies of cell therapy need to incorporate the use of repeated cell doses. It is possible that previous “negative” studies may have been caused by inadequate dosing – i.e., that one dose of cells was insufficient to produce the desired effect.
Because of its vast implications, it is important that the repeated-doses paradigm be tested in different species and using different cell types in order to determine whether its validity depends on the specific animal model used or the specific cell type administered. Accordingly, the goal of the present study was to determine, using a new model of chronic ischemic cardiomyopathy in mice, whether three doses of CPCs are superior to one dose. The distinguishing feature of this model is that cell therapy was started 3 months after MI. We are not aware of any studies in murine models of ischemic cardiomyopathy that have used such a long interval between MI and treatment. The rationale for such a long interval is to ensure that the inflammatory response to MI has completely subsided and that the scar is in a stable state, a setting analogous to that of the large number of patients who develop chronic heart failure (HF) over the course of years as a result of old MIs. The present study differs importantly from our previous study in mice [7] in two important respects: i) in our previous study, we administered cell therapy at 3 weeks after MI, a time at which the healing of the infarct is not yet complete; and ii) in our previous study, we used a different cell type (CMCs instead of CPCs).
Materials and Methods
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 86–23) and with the guidelines of the Animal Care and Use Committee of the University of Louisville School of Medicine. All surgical procedures, measurements of LV function (echocardiography and hemodynamic studies), and pathological analyses were performed by investigators blinded to treatment allocation.
Isolation and Culture of c-kit Positive Cardiac Progenitor Cells (CPCs)
The isolation, characterization, and culture of lin−/c-kit+ murine CPCs have been previously described [11]. Lin−/c-kit+ CPCs (henceforth referred to simply as CPCs) were isolated from GFP transgenic mice, RFP transgenic mice, and wild-type (WT) mice (male, C57BL6, 8–10 weeks of age). Hearts were finely minced and cultured to establish cell outgrowth cultures over ~7 days using growth medium (F12 K medium supplemented with bFGF, LIF, and 10% FBS). CPCs were isolated from the cell outgrowth of the explants by sequential sorting procedures including depletion of mature hematopoietic lin + cells and sorting for c-kit with a specific anti-c-kit antibody (Santa Cruz) and magnetic immunobeads (Miltenyi). To maximize the purity of the preparation, the c-kit sorting procedure was repeated three consecutive times at 14-day intervals. The lin−/c-kit+ cells were cultured, and the purity of the sorted cells was confirmed quantitatively by flow cytometry and immunofluorescent staining before use. In all studies, the CPCs used for cell transplantation in vivo were passaged 4–6 times [11].
Mouse Model of Ischemic Cardiomyopathy
This study was performed in C57BL6 female mice (age, 16–19 wks; body weight, 20–25 g), purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mouse model of myocardial ischemia and reperfusion has been described in detail [5,6,7, 9, 11, 13]. Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and ventilated using carefully selected parameters. The chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the mid-left anterior descending coronary artery using an 8–0 nylon suture. To prevent hypotension, blood from a female donor mouse was transfused at serial times during surgery [6]. Rectal temperature was carefully monitored and maintained at 37.0 ± 0.2 °C throughout the experiment. In all groups, myocardial infarction (MI) was produced by a 60-min coronary occlusion followed by reperfusion. The development of MI-induced ischemic cardiomyopathy was confirmed by a decline in ejection fraction (EF) to ≤35%, as measured by echocardiography [7] (Vevo 2100 Imaging System) 3 months after MI. Only mice that exhibited an EF ≤35% at 3 months after MI were included in the study.
Treatment Protocol
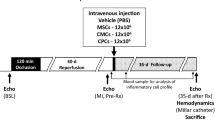
Three months after MI, mice were randomly allocated to three treatment groups: vehicle, single CPC dose, or multiple CPC doses (Fig. 1). All treatments (either CPCs or vehicle) were given via echo-guided injection into the left ventricular (LV) cavity [7]. The 1st, 2nd, and the 3rd doses were given at 5-week intervals. Mice in the vehicle group received three doses of vehicle (phosphate-buffered saline, PBS) (200 μl for each dose); mice in the single-dose group received GFP+ CPCs (1 × 106 cells in 200 μl of PBS) on the 1st injection and vehicle on the 2nd and 3rd injections; and mice in the multiple-dose group received three doses of CPCs (GFP+ CPCs on the 1st injection, WT CPCs on the 2nd, and RPF+ CPCs on the 3rd; 1 × 106 cells in 200 μl of PBS for each injection). To detect newly-formed cells, all mice received 5-bromo-2′-deoxyuridine (BrdU, Sigma; 33 mg/kg/day s.c.) continuously for 5 weeks starting right after the 1st treatment and 5-iodo-2′-deoxyuridine (IdU, Sigma) for 5 weeks right after the 3rd treatment. IdU was given in the drinking water at a final concentration of 0.1%. Neither BrdU nor IdU were administered after the 2nd dose (from week 5 to week 10). At 5 weeks after the 3rd dose, mice were subjected to final echocardiographic and hemodynamic studies and then euthanized for histologic studies (Fig. 1).
Experimental protocol. Mice were subjected to a 60-min coronary artery occlusion followed by reperfusion to induce myocardial infarction (MI). Three months later, mice received the 1st treatment (vehicle or CPCs) via echo-guided LV injection, followed by a 2nd and 3rd injection at 5-week intervals. Serial echocardiographic studies were performed before and 5 weeks after each injection. BrdU was given via mini-osmotic pumps right after the 1st injection until the 2nd injection. After the 3rd injection, IdU was given for 5 weeks. Mice then underwent hemodynamic studies and the hearts were fixed for histological studies
Echo-Guided Intraventricular Injection
We have previously developed a protocol for percutaneous, echo-guided injection of cells into the LV cavity of mice with MI [7]. All injections were performed using the Vevo 2100 Imaging System (VisualSonics, Inc.) equipped with a 30-MHz transducer, a Vevo Image Station with Injection Mount, and micro-manipulation controls. Briefly, mice were anesthetized with isoflurane (3% for induction, 1.5% for maintenance). The anterior chest was shaved and the animal placed on the imaging table in the right lateral decubitus position with the left lateral side facing the injection mount. After a good long-axis view of left ventricle was procured, the transducer was then turned 90 degrees clockwise. The LV was scanned in the short-axis view from apex to base to determine the optimal site for needle insertion. To prevent bleeding from the LV wall, it is crucial to find a site that avoids the infarct scar and the coronary arteries. Under real-time B-mode view, a 0.5-in. 30 G needle attached to a 1.0 ml syringe was carefully inserted from the left lateral side and advanced into the center of the LV cavity. Successful penetration of the LV cavity was indicated by a small reflux of bright red blood from the needle into the syringe tip. CPCs or PBS were infused at a steady rate over 100 s. After the infusion, the needle was quickly withdrawn from the left ventricle. The body temperature of the animal was controlled in the range of 37 ± 0.2 °C, and the electrocardiogram and respiration were monitored carefully during the whole procedure. Mice were allowed to recover in a temperature-controlled area.
Echocardiographic Studies
The echocardiographic studies were performed using the Vevo 2100 Imaging System (VisualSonics, Inc.) equipped with a 30-MHz transducer, as previously described [11]. Serial echocardiograms were obtained before treatment (3 months after MI, before the 1st injection) and 5 weeks after each treatment and were performed under isoflurane anesthesia (3% for induction, 1% for maintenance). Using a rectal temperature probe, body temperature was carefully maintained at 37 ± 0.2 °C throughout the study. The parasternal long axis and parasternal short-axis views were used to obtain 2D mode images for the measurement of LV mass, end-diastolic and end-systolic LV volume (LVEDV and LVESV), stroke volume (SV), and EF, as previously described [3, 11]. Digital images were analyzed off-line by blinded observers using the Vevo 2100 workstation software. Measurements were performed according to the American Society for Echocardiography [4, 13]. At least three measurements were taken and averaged for each parameter.
Hemodynamic Studies
At 5 weeks after the 3rd injection, hemodynamic studies were performed just before euthanasia as previously described [7, 13]. Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.), intubated, and ventilated with a positive pressure ventilator (Hugo-Sachs Electronik D-79232 [Germany]; ventilation rate, 105/min; tidal volume 10.3 μl/g). Rectal temperature was kept at 37 ± 0.2 °C. A 1.0 French pressure–volume (PV) catheter (PVR-1035, Millar Instruments) was inserted into the left ventricle via the right carotid artery. The position of the catheter was carefully adjusted until typical and stable PV loop signals were acquired. After 30 min of stabilization, the PV signals were recorded continuously with an MPVS ULTRA Pressure-Volume Unit (Millar Instruments) coupled with a Powerlab 16/30 converter (AD Instruments), stored, and displayed on a computer with LabChart 7.0 software (AD Instruments). Inferior vena cava occlusions were performed with external compression to produce variably loaded beats for determination of the end-systolic PV relation and other derived constructs of LV performance. Parallel conductance from surrounding structures was calculated by a bolus injection of 5 μl of 30% NaCl through the jugular vein. Echocardiography-derived SV was used as outside reference in alpha calibration for LV volume. All hemodynamic data analyses were performed off-line using LabChart 7.0 software by an investigator blinded to the treatment.
Histological Studies
The protocol for histologic analysis has been described [7, 19]. Briefly, at the end of the hemodynamic study, the heart was arrested in diastole by an i.v. injection of 0.15 ml of CdCl2 (100 mM), excised, and perfused retrogradely at 60–80 mmHg (LVEDP = 8 mmHg) with heparinized PBS followed by 10% neutral buffered formalin solution for 15 min. The heart was fixed in formalin for 24 h, then sectioned into three transverse slices (~2 mm thick) from apex to base, and subjected to tissue processing and paraffin embedding. Slices were sectioned at 4-μm intervals and stained with Masson’s trichrome (Sigma), picrosirius red, or antibodies against EGFP and cell-type-specific markers. Images were acquired digitally with Nikon Ni-E and Nikon TiE light microscopes and analyzed using Nikon Elements software [NIS, 64-bit version 3.22.00 (Build 710)]. From the Masson’s trichrome–stained images, morphometric parameters, including total LV area, risk region area, and scar area, were measured in each section [7]. Myocardial collagen content was quantitated on picrosirius red-stained heart images acquired under polarized light microscopy by determining collagen density (arbitrary unit) per mm2 of risk region or non-infarcted region with the NIS Elements software [7, 19]. Fluorescent stains and antibodies were used to identify specific cells, cell markers and compartments. Imaging was performed using Nikon Ni-E and Nikon TiE light microscopes; digitally acquired images were analyzed using NIS-Elements software.
Immunohistochemistry
Immunohistochemistry was performed in formalin-fixed, paraffin-embedded, 4-μm-thick heart sections as described previously [7, 16, 19]. Myocytes were stained with an anti-α-sarcomeric actin (α-SA) antibody (Sigma). Myocyte membranes were stained with Rhodamine conjugated wheat germ agglutinin (WGA) (Vector Labs) to facilitate the identification of individual myocytes for analysis of myocyte cross-sectional area and myocyte density by identification of cell borders. Double immunofluorescent staining was performed with specific anti- α-SA and anti-BrdU or anti-IdU (Abcam) antibodies for evaluation of proliferating myocytes. An anti-CD45 (Abcam) antibody was used to identify inflammatory cells. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole).
Statistical Analysis
Data are presented as mean ± SEM. Data were tested for normality using the Shapiro-Wilk test. Data that are normally distributed were analyzed with one-way or two-way ANOVA, followed by unpaired Student’s t-tests with the Bonferroni correction [1, 10, 12, 17]. The Kruskal–Wallis one-way ANOVA on ranks was used for data that are not normally distributed. A P value <0.05 was considered statistically significant. All statistical analyses were performed using the Sigma Stat software system [7].
Results
Exclusions and Gross Measurements
A total of 112 mice were initially enrolled for surgical induction of MI. Perioperative mortality (at 48 h after MI) was 19% (21 mice). The remaining 91 mice underwent pretreatment echocardiographic studies at 3 months after MI; of these, 13 were excluded because LV EF was >35% (prespecified exclusion criterion). The remaining 78 mice were randomly allocated to one of the three treatment groups (vehicle, single dose, or multiple doses). Seven mice died after the 1st injection, 2 died after the 2nd injection, and 1 died after the 3rd injection, all within 24 h after injections. Another 2 mice in the vehicle group died before the final echocardiographic study. Therefore, a total of 66 mice were included in the final analysis: 22 in the vehicle group, 24 in the single-dose group, and 20 in the multiple-dose group (Supplementary Table 1).
By experimental design, rectal temperature was kept within a narrow, physiologic range (36.8–37.2 °C) in all groups during all procedures, including coronary occlusion/reperfusion, echo-guided injections, echocardiographic studies, and hemodynamic studies (Supplementary Table 2). Heart rate was also similar in all three groups (Supplementary Table 2).
At baseline, body weight was similar in all groups (Supplementary Table 3). Compared with pretreatment body weight, the vehicle group exhibited a body weight loss (−1.3 ± 0.7 g) after the 15-week follow-up, while the cell-treated groups exhibited a weight gain of 0.3 ± 0.4 g and 0.4 ± 0.3 g in the single-dose and multiple-dose groups, respectively. Final body weight was greater in the multiple-dose group compared with the vehicle group (P < 0.05) (Supplementary Table 3A). In the vehicle group, both LV mass and LV weight/body weight ratio were significantly greater than in age-matched normal control (non-operated) mice (+35% for both, P < 0.05), indicating compensatory LV hypertrophy after MI (Supplementary Table 3B). Importantly, the LV weight/body weight ratio was significantly reduced in both the single-dose and multiple-dose groups vs. the vehicle group (4.9 ± 0.3 and 4.2 ± 0.1 vs. 5.8 ± 0.3 mg/g, respectively, P < 0.05) (Supplementary Table 3B).
Effect of CPCs on LV Function Measured by Echocardiography
The results obtained by serial echocardiographic studies are presented in Figs. 2 and 3 and in Table 1. Under our strict inclusion criteria, only animals with severe LV dysfunction (EF ≤ 35%) at 3 months after MI were included in the final analysis. In the vehicle group, a progressive deterioration in LV function was observed, with average EF decreasing from 29.3% to 27.3% in the 15-week interval between the 1st treatment and euthanasia (105 days later) (Fig. 3a and Table 1). The decline in LV function in the vehicle group further confirmed the presence of cardiomyopathy in this murine model.
Echocardiographic assessment of LV volumes. Echocardiographic studies were performed before each treatment and at the end of the study. a LV end-diastolic volume; b LV end-systolic volume; c LV stroke volume; d Changes in LV ESV after the 1st, 2nd, and 3rd treatments compared with the pre-treatment values; e Cumulative changes in LV ESV after the 1st, 2nd, and 3rd treatments compared with the values measured before the 1st treatment; f Changes in LV SV after the 1st, 2nd, and 3rd treatments compared with the pre-treatment values; g Cumulative changes in LV SV after the 1st, 2nd, and 3rd treatments compared with the values measured before the 1st treatment. Data are means ± SEM
Echocardiographic assessment of LV EF. a Values of LV EF in individual mice; b mean values of LV EF; c Changes in LV EF (absolute units) after the 1st, 2nd, and 3rd treatments compared with the respective pre-treatment values; d Cumulative changes in LV EF (absolute units) after the 1st, 2nd, and 3rd treatments compared with the values measured before the 1st treatment. Data are means ± SEM
At 3 months after MI, before the 1st injection (Pre-Rx), there were no significant differences among the three groups with respect to LVEDV, LVESV, SV, and EF (Figs. 2a, b, c, 3a and b), indicating that the severity of post-MI LV remodeling and dysfunction was comparable in all groups. As expected, at 35 days after the 1st injection, both cell-treated groups exhibited a significant improvement in LV EF compared with the vehicle group (Fig. 3b).
After the 2nd injection, however, the two treated groups exhibited a different course. In the single-dose group, the 2nd and 3rd vehicle administrations effected no further improvement in EF (Fig. 3). In contrast, in the multiple-dose group that received three CPC injections, the 3rd CPC injection produced a further increase in EF (Fig. 3c); as a result, in the multiple-dose group the total cumulative increase in EF from pre-treatment values was 3.3 ± 0.5% (absolute units) after the 1st treatment, 0.4 ± 0.5% after the 2nd treatment, and 2.4 ± 0.5% after the 3rd treatment (Fig. 3c), so that at the end of the protocol, LV EF (35.4 ± 0.5%) was significantly greater compared not only with the vehicle group (27.3 ± 0.2%) but also with the single dose group (32.1 ± 0.5%, P < 0.05) (Fig. 3b). The total cumulative increase in EF from baseline (before 1st injection) values was double in the multiple-dose vs. the single-dose group (6.1 ± 0.6% vs. 3.1 ± 0.6%, respectively, P < 0.01) (Fig. 3d).
In summary, a single dose of CPCs (1 × 106 cells) administered into the LV cavity was sufficient to improve LV function in a murine model of chronic ischemic cardiomyopathy. Administration of 3 doses of CPCs (1 × 106 cells each) at 5-week intervals produced a further increase in LV EF, resulting in greater LV functional improvement than a single dose.
Hemodynamic Measurements
The echocardiographic results were corroborated by the hemodynamic studies performed just before euthanasia (Fig. 4). Compared with the vehicle group, a significant improvement in hemodynamic parameters was noted in both cell-treated groups, but the improvement was more robust in the multiple-dose group than in the single-dose group. Thus, compared with vehicle, LVESV (a preload-dependent functional parameter) was significantly reduced and LV end-systolic elastance (a preload-independent functional parameter) was significantly increased in the multiple-dose group but not in the single-dose group (Fig. 4f). Furthermore, the multiple-dose group exhibited a greater LV EF than the single-dose group (37.6 ± 1.3 vs. 30.0 ± 1.2%, respectively, P < 0.05) (Fig. 4h, Table 2).
Hemodynamic assessment of LV function. Invasive hemodynamic studies were performed with a 1F Millar conductance catheter 5 weeks after the 3rd treatment, just before euthanasia. a through c Representative pressure-volume loops recorded during preload manipulation by brief inferior vena cava occlusions. d LV end-diastolic volume. e LV end-systolic volume. f LV stroke volume. g LV pressure dP/dt maximum and dP/dt minimum. h LV EF. i LV end-systolic elastance. Data are means ± SEM
In summary, two independent methods of functional assessment (echocardiography and hemodynamic studies) consistently demonstrated that the functional benefits of three doses of CPCs (106 cells/dose) are superior to those of a single dose of 106 cells.
Morphometric Analysis
In each heart, a detailed quantitative analysis was performed on two sections (one from each of two mid-ventricular slices); the results are summarized in Fig. 5. Consistent with the post-mortem measurements, both treated groups had a significantly smaller LV mass compared with the vehicle group (Fig. 5e). The scar mass was numerically smaller in the two treated groups compared with vehicle, but the differences were not statistically significant (Fig. 5f). The ratio of scar mass to LV mass was similar in all groups (Fig. 5g). Since scar mass was not significantly different, the reduction in total LV mass in the two cell-treated groups suggests reduced hypertrophy.
Morphological and histological assessments. Trichrome–stained images were used for LV morphological measurements. a Representative Masson trichrome-stained myocardial sections. Scar tissue and viable myocardium are identified in white/blue and red, respectively; b Risk region area as percentage of LV area; c Scar area as percentage of LV area; d Viable tissue area as percentage of LV area; e LV mass; f Scar mass; g Scar mass/LV mass ratio. Data are means ± SEM
LV Fibrosis
Quantitative analysis of collagen content was performed using picrosirius red staining and polarized light microscopy of LV sections (Fig. 6). Collagen content was evaluated with pixel density analysis and expressed as a percentage of total myocardial area. There was no statistically significant difference in collagen content among groups in either the risk region or the noninfarcted region (Fig. 6).
Cardiomyocyte Hypertrophy
Chronic ischemic cardiomyopathy induces cardiomyocyte hypertrophy [14]. To evaluate whether cell therapy affected cardiomyocyte hypertrophy, WGA staining was performed. As shown in Fig. 7a, WGA demarcates the membrane of individual cells. In the single-dose group, there was a trend towards a decrease in cardiomyocyte cross-sectional area vs. the vehicle group (−12% and − 7% in the risk and noninfarcted region, respectively) but the differences were not statistically significant (Fig. 7b). However, in the multiple-dose group cardiomyocyte area was significantly reduced (−25%) compared with the vehicle group in the risk region (Fig. 7b); in the noninfarcted region, cardiomyocyte area was numerically smaller (−16%) than in the vehicle group but the difference was not statistically significant (Fig. 7b).
Analysis of myocyte cross-sectional area. Myocyte cross-sectional area was assessed by immunostaining of cardiac myocytes with DAPI (blue) and WGA (green) to facilitate identification of cell membranes. a Representative microscopic images of WGA-stained LV sections; b quantitative analysis of myocyte cross-sectional area in the risk and noninfarcted regions. Data are means ± SEM
Myocardial Content of Inflammatory Cells
The number of CD45+ cells in the risk and noninfarcted regions was quantitated and expressed as a percent of total cells counted in the corresponding region (Fig. 8). As expected, in all groups the content of CD45+ cells was higher in the risk region than in the noninfarcted region (Fig. 8). Compared with the vehicle group, the CD45+ cell content in the risk region was significantly lower in the multiple-dose group but not in the single-dose group (Fig. 8b). In the noninfarcted region, the CD45+ cell content was numerically lower in the multiple-dose group vs. vehicle but the difference was not statistically significant (Fig. 8b).
Cell Engraftment and Proliferation
No BrdU positive cells were found. Occasional IdU positive cells were observed in the scar area; these cannot be described as cardiomyocytes and were mainly interstitial cells. These data indicate that transplanted cells did not engraft to any appreciable extent in any treatment group, consistent with our previous observations [7, 16, 18].
Discussion
To avoid any bias, in this study all surgical procedures, functional measurements, and pathologic analyses were performed by investigators blinded to treatment allocation. The most unique aspect of this study is that we used a murine model of chronic ischemic cardiomyopathy in which a long period (minimum of 3 months) was allowed to elapse between MI and treatment and a long follow-up (15 weeks) was used after treatment. Such a chronic model has rarely, if ever, been used in murine studies of heart failure because it is expensive and logistically challenging; however, this model is arguably more relevant to patients with old MI than the commonly used models in which the interval between MI and study intervention is 4 weeks or less.
Using this model, we compared the effects of one or three doses of CPCs injected into the LV cavity. Our salient findings can be summarized as follow: i) a single dose of CPCs (1 × 106 cells) produced a significant improvement in LV function 35 days after treatment, as demonstrated by both echocardiographic and hemodynamic parameters; this improvement persisted for at least 15 weeks after the initial treatment whereas in the vehicle group LV function deteriorated progressively; ii) repeating the 106 cell dose three times at 5-week intervals produced further functional improvement, particularly after the 3rd dose, such that at the end of the study parameters of LV performance, measured both by echocardiography and hemodynamic studies, were significantly greater than after a single dose; iii) the superior functional benefits of three CPC doses compared with one dose were associated with a reduction in LV hypertrophy, as reflected in a reduction in the LV weight/body weight ratio and in cardiomyocyte cross-sectional area; iv) unlike the single-dose group, the multiple-dose group exhibited a reduction in the density of inflammatory cells in the infarcted region and a trend toward a reduction in the noninfarcted region.
The reduction in LV weight, LV weight/body weight ratio, and cardiomyocyte cross-sectional area effected by a single, and even more so, by multiple CPC doses (Supplementary Table 3B; Fig. 7) is remarkable. Since we used a model of chronic ischemic cardiomyopathy where the scar was at least 3 months old, our results indicate that cell therapy partially reversed LV hypertrophy after it developed. This is an important beneficial action of CPCs that had not been emphasized in prior studies. Whether it reflects a primary action of CPCs on cardiomyocytes or is secondary to improved LV performance remains to be determined.
In a previous study in a murine model of subacute (3-week–old) MI [7], we have demonstrated that administration of three doses of cardiac mesenchymal cells (CMCs) (1 × 106 cells/dose at 2-week intervals) had significantly greater beneficial effects than a single dose. There are major differences between these two studies. In the present study we used CPCs, not CMCs. Unlike the subacute MI model used in the previous study, here we allowed a long interval (3 months) to elapse between MI and first treatment in order to mimic the common clinical setting of patients with chronic heart failure caused by old MIs. These patients appear to be one of the potential beneficiaries from cell therapy in the clinical arena [20]. The rationale for waiting 3 months after MI was to ensure complete resolution of post-MI inflammation and the formation of a stable scar, as the remodeling and inflammatory processes may still be ongoing at the time point used in our previous study (3 weeks after MI). Very few, if any, studies of cell therapy in mice have allowed such a long time between MI and treatment. Despite different cell types (CPCs vs. CMCs), different stages of ischemic cardiomyopathy (3-weeks-old MI vs. 3-months-old MI), and different dose frequency (every 2 weeks vs. every 5 weeks), our present results are generally consistent with our previous observations [7]. Thus, the present results further corroborate the concept that three repeated cell doses are superior to a single dose and extend this concept to the setting of chronic ischemic cardiomyopathy in murine models.
Using a rat model of chronic ischemic cardiomyopathy (1-month-old MI), we have previously demonstrated that repeated administrations of CPCs have cumulative beneficial effects and, therefore, are superior to a single administration [18]. Our present results in the mouse corroborate these findings, indicating that the repeated-treatment paradigm is not limited to one species. The generalizability of this strategy greatly enhances its importance and provides a rationale for testing it in larger animals and possibly, humans.
In conclusion, the present study adds further evidence to our previous studies [7, 16, 18] to support the concept that although increasing the number of cells in a single dose may not yield further benefit [16], greater therapeutic efficacy can still be achieved by adopting the repeated-treatment paradigm. The importance of our observations is that they suggest that although there may be a “ceiling” to the effects of a single dose of cells [16], a single dose does not exhaust the range of potential benefits that can be imparted to the failing heart. Repeated administrations can still improve cardiac function above the maximum effects achievable with a single dose. Our data further support the concept that the full therapeutic effects of cell therapy can only be properly evaluated by repeated doses [20]. This concept has important implications not only for the interpretation of previous results but also for the design of future studies [2].
Abbreviations
- CPC:
-
Cardiac progenitor cell
- CMC:
-
Cardiac mesenchymal cell
- MI:
-
Myocardial infarction
- LV:
-
Left ventricle
- LVEDV:
-
End-diastolic LV volume
- LVESV:
-
End-systolic LV volume
- SV:
-
Stroke volume
- EF:
-
Ejection fraction
- PV:
-
Pressure–volume
- WGA:
-
Wheat germ agglutinin
References
Bennett, W. R., Yawn, D. H., Migliore, P. J., Young, J. B., Pratt, C. M., Raizner, A. E., Roberts, R., & Bolli, R. (1987). Activation of the complement system by recombinant tissue plasminogen activator. Journal of the American College of Cardiology, 10(3), 627–632. https://doi.org/10.1016/s0735-1097(87)80206-1.
Bolli, R. (2017). Repeated cell therapy: A paradigm shift whose time has come. Circulation Research, 120(7), 1072–1074. https://doi.org/10.1161/CIRCRESAHA.117.310710.
Dawn, B., Guo, Y., Rezazadeh, A., Huang, Y., Stein, A. B., Hunt, G., Tiwari, S., Varma, J., Gu, Y., Prabhu, S. D., Kajstura, J., Anversa, P., Ildstad, S. T., & Bolli, R. (2006). Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circulation Research, 98(8), 1098–1105. https://doi.org/10.1161/01.RES.0000218454.76784.66.
Gottdiener, J. S., Bednarz, J., Devereux, R., Gardin, J., Klein, A., Manning, W. J., Morehead, A., Kitzman, D., Oh, J., Quinones, M., Schiller, N. B., Stein, J. H., Weissman, N. J., & American Society of, E. (2004). American Society of Echocardiography recommendations for use of echocardiography in clinical trials. Journal of the American Society of Echocardiography, 17(10), 1086–1119. https://doi.org/10.1016/j.echo.2004.07.013.
Guo, Y., Jones, W. K., Xuan, Y. T., Tang, X. L., Bao, W., Wu, W. J., Han, H., Laubach, V. E., Ping, P., Yang, Z., Qiu, Y., & Bolli, R. (1999). The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proceedings of the National Academy of Sciences of the United States of America, 96(20), 11507–11512. https://doi.org/10.1073/pnas.96.20.11507.
Guo, Y., Wu, W. J., Qiu, Y., Tang, X. L., Yang, Z., & Bolli, R. (1998). Demonstration of an early and a late phase of ischemic preconditioning in mice. The American Journal of Physiology, 275(4 Pt 2), H1375–H1387. https://doi.org/10.1152/ajpheart.1998.275.4.H1375.
Guo, Y., Wysoczynski, M., Nong, Y., Tomlin, A., Zhu, X., Gumpert, A. M., Nasr, M., Muthusamy, S., Li, H., Book, M., Khan, A., Hong, K. U., Li, Q., & Bolli, R. (2017). Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Research in Cardiology, 112(2), 18. https://doi.org/10.1007/s00395-017-0606-5.
Hong, K. U., Guo, Y., Li, Q. H., Cao, P., Al-Maqtari, T., Vajravelu, B. N., Du, J., Book, M. J., Zhu, X., Nong, Y., Bhatnagar, A., & Bolli, R. (2014). c-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One, 9(5), e96725. https://doi.org/10.1371/journal.pone.0096725.
Hong, K. U., Li, Q. H., Guo, Y., Patton, N. S., Moktar, A., Bhatnagar, A., & Bolli, R. (2013). A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Research in Cardiology, 108(3), 346. https://doi.org/10.1007/s00395-013-0346-0.
Li, Q., Bolli, R., Qiu, Y., Tang, X. L., Guo, Y., & French, B. A. (2001). Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation, 103(14), 1893–1898 https://www.ncbi.nlm.nih.gov/pubmed/11294809.
Li, Q., Guo, Y., Ou, Q., Chen, N., Wu, W. J., Yuan, F., O'Brien, E., Wang, T., Luo, L., Hunt, G. N., Zhu, X., & Bolli, R. (2011). Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Research in Cardiology, 106(5), 849–864. https://doi.org/10.1007/s00395-011-0180-1.
Li, R. C., Ping, P., Zhang, J., Wead, W. B., Cao, X., Gao, J., Zheng, Y., Huang, S., Han, J., & Bolli, R. (2000). PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. American Journal of Physiology. Heart and Circulatory Physiology, 279(4), H1679–H1689. https://doi.org/10.1152/ajpheart.2000.279.4.H1679.
Mehra, P., Guo, Y., Nong, Y., Lorkiewicz, P., Nasr, M., Li, Q., Muthusamy, S., Bradley, J. A., Bhatnagar, A., Wysoczynski, M., Bolli, R., & Hill, B. G. (2018). Cardiac mesenchymal cells from diabetic mice are ineffective for cell therapy-mediated myocardial repair. Basic Research in Cardiology, 113(6), 46. https://doi.org/10.1007/s00395-018-0703-0.
Pfeffer, M. A., & Braunwald, E. (1990). Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation, 81(4), 1161–1172. https://doi.org/10.1161/01.cir.81.4.1161.
Tang, X. L., Li, Q., Rokosh, G., Sanganalmath, S. K., Chen, N., Ou, Q., Stowers, H., Hunt, G., & Bolli, R. (2016). Long-term outcome of administration of c-kit (POS) cardiac progenitor cells after acute myocardial infarction: Transplanted cells do not become Cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circulation Research, 118(7), 1091–1105. https://doi.org/10.1161/CIRCRESAHA.115.307647.
Tang, X. L., Nakamura, S., Li, Q., Wysoczynski, M., Gumpert, A. M., Wu, W. J., Hunt, G., Stowers, H., Ou, Q., Bolli, R. (2018). Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J Am Heart Assoc, 7(4), 1–14. https://doi.org/10.1161/JAHA.117.007400.
Tang, X. L., Qiu, Y., Park, S. W., Sun, J. Z., Kalya, A., & Bolli, R. (1996). Time course of late preconditioning against myocardial stunning in conscious pigs. Circulation Research, 79(3), 424–434. https://doi.org/10.1161/01.res.79.3.424.
Tokita, Y., Tang, X. L., Li, Q., Wysoczynski, M., Hong, K. U., Nakamura, S., Wu, W. J., Xie, W., Li, D., Hunt, G., Ou, Q., Stowers, H., & Bolli, R. (2016). Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: A new paradigm in cell therapy. Circulation Research, 119(5), 635–651. https://doi.org/10.1161/CIRCRESAHA.116.308937.
Wysoczynski, M., Guo, Y., Moore, J. B. T., Muthusamy, S., Li, Q., Nasr, M., Li, H., Nong, Y., Wu, W., Tomlin, A. A., Zhu, X., Hunt, G., Gumpert, A. M., Book, M. J., Khan, A., Tang, X. L., & Bolli, R. (2017). Myocardial reparative properties of cardiac Mesenchymal cells isolated on the basis of adherence. Journal of the American College of Cardiology, 69(14), 1824–1838. https://doi.org/10.1016/j.jacc.2017.01.048.
Wysoczynski, M., Khan, A., & Bolli, R. (2018). New paradigms in cell therapy: Repeated dosing, intravenous delivery, Immunomodulatory actions, and new cell types. Circulation Research, 123(2), 138–158. https://doi.org/10.1161/CIRCRESAHA.118.313251.
Acknowledgements
This work was supported by National Institutes of Health grants P01 HL078825 and UM1 HL113530.
Author information
Authors and Affiliations
Contributions
All authors meet the International Committee for Medical Journal Editors (ICMJE) authorship criteria. Y.G., Y.N., A.T., and R.B. analyzed and interpreted the data and drafted the manuscript. Y.G., Y.N., A.T., and X.Z. performed surgery, cell injections and acquisition of the data. Y.G., A.T., and X.Z. prepared the figures and tables. Q.L. provided CPCs and vehicle, as well as IdU, BrdU and minipumps for BrdU administration. A.G. performed pathology studies. Q.L., Y.G., A.K., J.S. and R.B. made substantial contributions to the design and critically revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 147 kb)
Rights and permissions
About this article
Cite this article
Guo, Y., Nong, Y., Li, Q. et al. Comparison of One and Three Intraventricular Injections of Cardiac Progenitor Cells in a Murine Model of Chronic Ischemic Cardiomyopathy. Stem Cell Rev and Rep 17, 604–615 (2021). https://doi.org/10.1007/s12015-020-10063-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10063-0