Abstract
Objective Inconsistent findings of associations between gestational diabetes mellitus (GDM) and birth defects suggest unaccounted confounders may underlie the actual basis for such associations. We conducted a systematic review to assess observed associations between GDM and birth defects and the extent to which these could be explained by pre-pregnancy obesity. Methods Using a combination of search terms for GDM and birth defects, we searched PubMed, Scopus, CINAHL, and ClinicalTrials.gov for human-based studies published through September 2013. Studies were eligible for inclusion if they included information on maternal diabetes status, method of diagnosis of GDM, and assessment of birth defects. Twenty-four of 768 potential articles were included. We collected information on study design, location and period, method of determination of diabetes status, types of birth defects, and measures of association reported. Results There was no evidence for consistent association of GDM with birth defects, with the exception of a weak association between GDM and congenital heart defects. When stratified by maternal pre-pregnancy BMI, an association between GDM and congenital heart defects and between GDM and neural tube defects was evident only in women with both GDM and pre-pregnancy obesity. Conclusions for Practice Our findings suggest reported associations between GDM and birth defects may be due, in part, to undiagnosed metabolic disorders associated with obesity, such as pregestational diabetes mellitus, rather than GDM. These findings highlight the need for increased efforts for pre-pregnancy screening for undiagnosed diabetes and awareness of the importance of weight management among women of childbearing age with obesity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Significance
An increasing number of recent studies have reported possible associations between gestational diabetes mellitus (GDM) and birth defects. Inconsistent findings suggest unaccounted confounders may underlie the actual basis for such associations. Our findings suggest that reported associations between GDM and birth defects may be due, in part, to undiagnosed metabolic disorders associated with obesity, such as pregestational diabetes, rather than GDM. Given the increasing prevalence of obesity and high number of unplanned pregnancies, these findings highlight the need for increased pre-pregnancy screening for undiagnosed diabetes and awareness of the importance of weight management among women of childbearing age with obesity.
Introduction
Pregestational diabetes mellitus (PGDM) is known to be associated with an increased risk for birth defects among offspring (Correa et al. 2008; Inkster et al. 2006; Sheffield 2002). Unlike most teratogens that have some organ specificity, PGDM is associated with defects spanning multiple organ systems, including the central nervous system (CNS), cardiovascular, renal, gastrointestinal, and skeletal systems, among others. Although mechanisms underlying these associations have not been elucidated, these observations suggest that metabolic disorders associated with hyperglycemia lead to disturbances in morphogenetic processes of embryogenesis, as noted in animal models of diabetic pregnancy (Reece et al. 2002, 2005).
In recent years, an increasing number of studies have examined possible associations between gestational diabetes mellitus (GDM)—glucose intolerance that begins during pregnancy—and birth defects but have produced inconsistent findings. Given that GDM occurs and is diagnosed after the development of most structural malformations, it is unclear whether studies reporting associations of GDM with birth defects are, in fact, reporting effects of uncontrolled confounders such as undiagnosed PGDM and/or pre-pregnancy obesity.
Obesity, a known risk factor for type 2 diabetes mellitus, has been reported to be associated with some birth defects. For instance, consistent associations have been observed between obesity and neural tube defects or selected cardiac defects (Correa and Marcinkevage 2013; Stothard et al. 2009); however, findings for other birth defects have been less consistent. Possible reasons for such inconsistencies include differences in case ascertainment and classification, obesity classification methods, prevalence of pre-pregnancy obesity phenotypes that may be associated with birth defects, and prevalence of undiagnosed PGDM.
Objective
The purpose of this study was to conduct a systematic review of the literature on possible associations between GDM and birth defects, and to assess the extent to which any associations observed might be explained by maternal pre-pregnancy obesity.
Methods
Information Sources and Search Strategy
We followed the guidelines in the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement” (Moher 2009) for development, implementation, and reporting of results. We conducted a comprehensive search of the PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus, and ClinicalTrials.gov databases in September 2013 for published studies in humans without language or time restrictions. Studies without an English translation were translated at the time of review. Combinations of search terms for “GDM” and “birth defects” were used to identify eligible articles. The full list of search terms is shown in Table 1. Reference lists of screened articles were reviewed to identify additional potential articles.
Eligibility Criteria and Study Selection
Articles were included if study participants were pregnant women, diabetes status of the women was reported and information on the presence of birth defects in the offspring was available. Articles were excluded if there was no differentiation in diabetes status (gestational vs pre-gestational), if no information was available on the methods of determination of diabetes status, or if the ascertainment and classification of birth defects was not reported. Articles using data from the same source were excluded if there was significant overlap in data period and birth defect type.
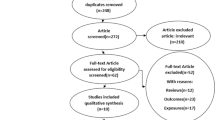
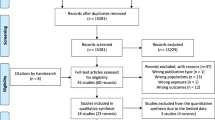
Search results from four separate databases returned a total of 768 potential articles for inclusion, 93 of which were duplicate articles, leaving 675 articles for review. After title and abstract review, 625 articles were excluded. The remaining 50 articles were reviewed in their entirety, 26 of which were excluded after full review, leaving 24 articles for inclusion. Reference lists of screened articles revealed 12 additional potential articles, all of which were excluded after review.
Data Extraction
Article review and selection was performed by two reviewers who agreed fully on the final 24 articles selected for inclusion. A single reviewer performed data extraction using a data extraction form to obtain information on study design and characteristics, data source, method of determination of diabetes status, and type and method of identification of birth defects. For a single article written in Spanish and requiring translation, a second reviewer/co-author fluent in Spanish performed data extraction.
Information on BMI Collection
Of the 24 studies included, 14 included information on BMI of participants. The method of BMI determination was not specified in one study and was measured directly in one study. In 4 studies, BMI values were obtained from medical records. In the remaining eight studies, BMI information was obtained through maternal self-reports.
Results
Study Selection and Characteristics
Twenty-four articles met inclusion criteria for the systematic review. Figure 1 displays the steps involved in selection of studies for review. Of 24 studies that met inclusion criteria, 10 were case-control and 14 were cohort studies.
Of the ten case-control studies, two were published before the year 2000, four between 2005 and 2008, and four between 2010 and 2013 (Table 2 ). Eight studies were performed in the United States and two in Spain. Maternal self-report was the most common method of assessment of diabetes status, followed by birth certificates and birth registries. Six studies included only live births, while four included live births, stillbirths, and elective terminations. Four studies grouped together all offspring with any type of birth defect, while six focused on specific types of defects. Two studies excluded offspring with defects recognized to have known associations with a chromosomal anomaly or genetic syndrome, and one excluded defects unlikely to be secondary to a neural tube defect.
Fourteen cohort studies were included in the review (Table 3 ). Two were published before the year 2000, six between 2000 and 2009, and six between 2010 and 2013. Three were from the United States, two from Sweden, and one each from Spain, Mexico, Italy, Iran, India, Saudi Arabia, Bangladesh, Malta, and the United Arab Emirates. Universal screening was the most common method of determination of maternal diabetes status. Other methods included self-report, birth registry, and birth certificates. All of the cohort studies included multiple types of birth defects. Seven included only live births, and four included live births and stillbirths. Three studies included live births, stillbirths, and terminations. One study excluded multiple gestation pregnancies and breech presentation, and another excluded infants with Trisomy 21.
Synthesis of Results
The overall findings of the systematic review are displayed for case-control studies in Table 4 and for cohort studies in Table 5.
Case-Control Studies
Of the ten case-control studies included in the systematic review, two were cases comprising multiple types of births defects, and eight focused on cases involving a selected subset of defects (Table 4 ). The two most commonly evaluated types of birth defects were cardiac and CNS defects.
Multiple Types of Defects Combined as a Group
Ramos-Arroyo et al. (1992) reported a significant association between GDM and any type of birth defect (OR 1.6 [CI 1.2, 2.2]). In this study, a stronger association between GDM and birth defects was reported in women using insulin when evaluating any minor or major birth defect (OR 1.9 [CI 1.1, 3.4]), and when evaluating major defects only (OR 1.9 [CI 1.0, 3.7]). Correa et al. (2008) reported a significant, although weak, association between GDM and isolated non-cardiac defects (OR 1.3 [CI 1.05, 1.6]), and a similar association with the presence of multiple non-cardiac defects, although the confidence interval included the null value in this group (OR 1.31 [CI 0.92, 1.80]).
Cardiac Malformations
A weak association between GDM and cardiac defects was reported in four case-control studies. Ferencz, Rubin, McCarter, and Clark (1990) evaluated multiple types of cardiac defects and reported a slight increase in the proportion of women with GDM in the group of offspring with cardiac defects, compared to those without (OR 1.45, [99.5% CI 0.94, 2.23]). No association was found between GDM and specific types of cardiac defects. Ramos-Arroyo et al. (1992) found a significant association between GDM and cardiac defects (OR 5.0 [95% CI 1.2, 17.8]). When analyzed by type of cardiac defect, they reported a significant association between GDM and transposition of the great arteries (OR 22.5 [95% CI 1.2, 170.30]). Correa et al. (2008) found an association between GDM and multiple types of cardiac defects evaluated as a group (OR 1.65 [95% CI 1.14, 2.39]), as well as an association between GDM and isolated cardiac defects (OR 1.59 [95% CI 1.27, 1.99]). Analysis by subtype of cardiac defect demonstrated an association between GDM and three specific types of cardiac defects: atrial septal defects (OR 2.16 [95% CI 1.46, 2.31]), tetralogy of Fallot (OR 1.8 [95% CI 1.12, 2.87]), and isolated pulmonary valve stenosis (OR 2.41 [95% CI 1.59, 3.64]). Gilboa et al. (2010) found an increase in the proportion of women with GDM in the group containing infants with cardiac defects, as compared to those without (OR 1.43 [95% CI 1.21, 1.70]).
Central Nervous System Defects
Three case-control studies demonstrated associations between maternal GDM and CNS defects. CNS defects types evaluated varied between studies, and included neural tube defects, anencephaly, holoprosencephaly, hydrocephaly, craniorachischisis, and microcephaly, among others.
Ramos-Arroyo et al. (1992) reported a significant association between GDM and CNS defects of any type (OR 4.1 [95% CI 1.4, 11.7]). When individual CNS defects were evaluated, they found significant associations between GDM and both neural tube defects and anencephaly (OR 5.1 [95% CI 1.6, 15.8] and OR 7.0 [95% CI 1.2, 31.3], respectively). Encephalocele, holoprosencephaly, and microcephaly demonstrated no association.
Correa et al. (2008) found no association between GDM and select types of CNS defects (including spina bifida, encephalocele, holoprosencephaly, hydrocephaly, anencephaly and craniorachischisis).
Anderson et al. (2005) evaluated the association between GDM and select types of CNS defects. Holoprosencephaly showed the strongest association with GDM (adjusted OR 2.9 [95% CI 1.0, 8.4]). For anencephaly, spina bifida, and hydrocephaly, no association was seen.
Agopian et al. (2013) described the association between GDM and spina bifida or anencephaly. Neither demonstrated an association with GDM when analyzed alone nor when analyzed as a group.
Stronger associations were reported by the Ramos-Arroyo group for both anencephaly and neural tube defects (as a combined group), as compared to either Anderson or Agopian when evaluating anencephaly or spina bifida, either individually or combined.
Genitourinary Defects
Two case-control studies evaluated specific defects of the genitourinary system. Porter et al. (2005) found no association between GDM and hypospadias. Congenital urinary tract anomalies of any type were reported by Shnorhavorian et al. (2011) to have a weak association with GDM (OR 1.25 [CI 1.06, 1.48]). When these defects were divided into isolated kidney defects or lower urinary tract defects, there was a stronger association between GDM and kidney defects (OR 1.42 [CI 1.09, 1.85]) than between GDM and lower urinary tract defects (OR 1.25 [CI 1.01, 1.56]).
Miscellaneous Defects
A few studies reported weak associations between maternal GDM and individual birth defect types that were less commonly a focus of the other studies reviewed (e.g., skeletal defects and microtia). Ramos-Arroyo et al. (1992) reported an association between GDM and pre-axial polydactyly (OR 9.0 [95% CI 1.7, 40.5]). Von Bennekom, Mitchell, Moore, and Werler (2013) reported a weak association between GDM and microtia; however, this did not meet statistical significance (OR 1.4 [CI 0.9, 2.2]).
Cohort Studies
Of the 14 cohort studies included in the review, three demonstrated weak but significant associations (OR 1.0–1.5) between GDM and birth defects as a group (Table 5 ).
Abolfazl, Hamidreza, Narges, and Maryam (2008) reported a relative risk of 7.28 (95% CI 1.59, 33.32) for the development of birth defects in offspring of women with GDM. Using data from a study reported by Lapolla et al. (2009), a crude odds ratio of 2.37 (95% CI 1.87, 2.99) was calculated for birth defects in offspring of women with GDM. Fadl et al. (2010) also reported a weak association (adjusted OR 1.19 [95% CI 1.02, 1.39]) between “major” birth defects (i.e., potentially life threatening or likely to lead to serious handicap or cosmetic defect if not surgically corrected) and GDM.
Janssen et al. (1996) reported no association between GDM and birth defects, whether evaluating the presence of multiple defects or single defects. When looking at specific defect types, they reported slightly stronger associations, however, none met statistical significance.
Agopian et al. (2012) reported an association between GDM and complete atrioventricular canal defect (CAVC) (adjusted prevalence ratio 1.7 [CI 1.0, 2.8]). In those with CAVC and heterotaxy syndrome, the association with GDM was stronger with an adjusted prevalence ratio of 2.0 [CI 1.0, 4.0]. The remaining eight cohort studies, summarized in Table 5, did not demonstrate associations between GDM and birth defects. (Aberg et al. 2001; Aryasinghe 2012; Gasim 2012; Mannan 2012; Moore et al. 2000; Pablo Velazquez 2010; Ramachandran et al. 1998; Savona-Ventura and Gatt 2004).
GDM Stratified by Pre-pregnancy BMI and Birth Defects
Several studies evaluated the association between GDM stratified by pre-pregnancy BMI category and birth defects. Most showed no association between GDM and birth defects in the normal pre-pregnancy BMI category, but did suggest an association for the higher pre-pregnancy BMI categories (Table 6 ).
Garcia-Patterson et al. (2004) divided women with GDM into BMI tertiles, and evaluated the association with major birth defects. They found that, within the higher BMI tertiles, there was a stronger association between GDM and offspring having at least one major birth defect (2nd tertile OR 2.54 [CI 1.28, 5.02]; 3rd tertile OR 2.67 [CI 1.36, 5.27]). When specific defect types were evaluated in sub-groups, there was a strong association between GDM and renal/urinary defects in women in the 3rd (highest) BMI tertile (OR of 5.22 [CI 1.15, 23.74]).
Anderson et al. (2005) stratified women with GDM by pre-pregnancy BMI (obese versus non-obese, using a BMI of >30 kg/m2 for obese). They reported a significant association between obese GDM women and both spina bifida (adjusted OR 4.5 [CI 1.5, 13]) and holoprosencephaly (adjusted OR 6.5 [CI 1.3, 31]), as compared to normal weight women without GDM. Anencephaly and hydrocephaly showed a weak association with obese GDM women when stratified by BMI category, but did not reach statistical significance (adjusted OR 1.5 [CI 0.3, 7.6] and 2.7 [CI 0.6, 11], respectively).
Two studies evaluating cardiac defects demonstrated a stronger association with GDM when stratified by pre-pregnancy BMI category. Martinez-Frias et al. (2005) reported a significant association between cardiac defects and women with both GDM and BMI ≥ 30 kg/m2 (OR 3.47 [CI 1.71, 7.03]). There was no association between cardiac defects and GDM in women with lower BMI categories (reference group included women without GDM in the same BMI category). When only women with GDM were considered (reference group consisting of those with GDM and BMI ≤ 20.9 kg/m2), there was a significant association between women with a BMI ≥ 30 kg/m2 and cardiac defects (OR 2.82 [1.13, 7.04]). When a broader group of defects was considered, they found a significant association between GDM women with a BMI ≥ 30 kg/m2 and the presence of any of a selected group of birth defects, as compared to the reference group of women without diabetes in the same BMI category (OR 2.76 [CI 1.49, 5.11]).
In 2010, Gilboa et al. evaluated the association between GDM, stratified by pre-pregnancy obesity status, and cardiac defects in the offspring and found an association between cardiac defects and maternal GDM and either overweight (BMI ≥ 25 and <30 kg/m2; OR 1.46 [CI 1.04, 2.05]) or obese status (BMI > 30 kg/m2; 1.82 [CI 1.36, 2.44]). When specific types of cardiac defects were evaluated, a significant association was noted between maternal GDM in the obese BMI category and the presence of either tetralogy of Fallot (OR 2.38 [CI 1.37, 4.14]) or left ventricular outflow tract defects (OR 1.87 [CI 1.15, 3.05]).
Comments
Study Strengths and Limitations
There are several strengths to our study design. Utilizing the strategies outlined in The PRISMA Statement (Moher 2009), we adhered to guidelines aimed at improving quality of reporting and accuracy and transparency of publications. Not limiting our search by language or date of publication allowed us to review a more robust group of available studies, decreasing the potential of omitting important studies due to search strategy and, thereby, strengthening our results.
Another strength is that we considered associations between GDM and multiple types of birth defects (combined as a group), as well as associations between GDM and specific defect types. This is important because evaluating the consistency of findings across studies depends on the comparability of case groups from individual reports, which is more likely to be achieved for specific types of birth defects than for birth defects combined as a group as such groups are likely to vary in inclusion criteria among studies. Lastly, by examining associations of GDM with birth defects stratified by maternal pre-pregnancy obesity, it was possible to determine that the associations were present only among offspring of women with GDM and pre-pregnancy obesity or overweight status, and not among offspring of women with GDM but no pre-pregnancy obesity or overweight status.
One limitation of our study is that GDM status was obtained using multiple methods, all of which are not likely to be directly comparable. For example, in eight of fourteen cohort studies, GDM was diagnosed using universal screening. Of these, seven utilized oral glucose tolerance testing (OGTT) and followed recommended cut-off values provided by well-established published guidelines, and one utilized a combination of fasting blood capillary glucose testing and OGTT during the study. Six cohort studies used birth registry data, birth certificate data, or a questionnaire completed by the patient or healthcare provider as the source of information on GDM.
Of the ten case-control studies, eight obtained GDM status through maternal self-report and two through birth registry data or a combination of birth certificate data and ICD-9 (International Classification of Diseases, Ninth Revision) coding from maternal medical records. Variation in data source and method of diagnosis probably resulted in some misclassification of GDM status within and among studies, and in lack of comparability regarding GDM status among studies. If GDM misclassification was non-differential with respect to case-control status, the net effect of such misclassification would be of attenuation of a true association between GDM and birth defects towards the null or of no effect in the absence of a true association.
Another limitation of this review is that the composition of phenotypes of birth defects included likely varied across studies, as such composition depends on the population under surveillance, case ascertainment and classification methods, and inclusion and exclusion criteria, all of which tend to vary across studies. Given the heterogeneity in methods and composition of case groups across studies, we did not attempt to pool the results from multiple studies into summary measures as such an approach would not provide a valid assessment of the relationship between GDM and birth defects.
Discussion
Our findings regarding the association between GDM and birth defects were inconsistent. However, when analyses were stratified by maternal pre-pregnancy BMI category, there was a significant association between GDM and birth defects, but only among offspring of women with pre-pregnancy obesity. In the four studies where these stratified analyses were reported, moderate (OR 1.5–2.0) to strong (OR > 2.0) associations were evident for selected birth defects (i.e., cardiac and neural tube defects) and GDM in the setting of obesity and with pre-pregnancy obesity in the absence of GDM. Furthermore, in studies with more than two BMI categories, there seemed to be a monotonic relationship between higher pre-pregnancy BMI and higher prevalence of birth defects, independent of the presence of GDM.
Conclusions and Implications
Our review of the literature indicates no consistent evidence of an association between GDM and birth defects in women with GDM but no pre-pregnancy obesity. However, there was consistent evidence of an increased risk of selected birth defects (i.e., cardiac and neural tube defects) among offspring of women with both pre-pregnancy obesity and GDM. These findings suggest that previously reported associations between GDM and birth defects that develop early in pregnancy may be due, in part, to undiagnosed metabolic disorders associated with obesity, such as PGDM, rather than to GDM that develops and is diagnosed later in pregnancy.
These findings are of public health concern given the increasing prevalence of obesity among women of childbearing age, particularly among minority populations (Correa and Marcinkevage 2013), and that about 40% of all pregnancies are unplanned (Finer and Zolna 2011; Mosher et al. 2012). These findings also highlight the need for increased efforts to screen for undiagnosed PGDM among women of childbearing age with obesity, and for referral of women with newly diagnosed PGDM for diabetes management, family counseling, and, when indicated, preconception care. Our findings also highlight the need for increasing awareness among women of childbearing age about the importance of appropriate weight management before and during pregnancy for their reproductive health and the health of their offspring.
References
Aberg, A., Westbom, L., & Kallen, B. (2001). Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Human Development, 61(2), 85–95.
Abolfazl, M., Hamidreza, T. S., Narges, M., & Maryam, Y. (2008). Gestational diabetes and its association with unpleasant outcomes of pregnancy. Pakistan Journal of Medical Sciences, 24(4), 566–570.
Agopian, A. J., Moulik, M., Gupta-Malhotra, M., Marengo, L. K., & Mitchell, L. E. (2012). Descriptive epidemiology of non-syndromic complete atrioventricular canal defects. Paediatric and Perinatal Epidemiology, 26(6), 515–524. doi:10.1111/ppe.12006.
Agopian, A. J., Tinker, S. C., Lupo, P. J., Canfield, M. A., & Mitchell, L. E. (2013). Proportion of neural tube defects attributable to known risk factors. Birth Defects Research Part A, 97(1), 42–46. Retrieved, from http://www.ncbi.nlm.nih.gov/pubmed/23427344.
Anderson, J. L., W, D. K., Canfield, M. A., Shaw, G. M., Watkins, M. L., & Werler, M. M. (2005). Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology, 16(1), 87–92.
Aryasinghe, L., M, D., Ansari, T. A., Khoury, R., Mathew, E., Sharbatti, S. A., & Shaikh, R. B. (2012). Congenital anomalies at birth: A hospital based study in UAE. Journal of Nepal Paediatric Society, 32(2), 105–112.
Correa, A., Gilboa, S. M., Besser, L. M., Botto, L. D., Moore, C. A., Hobbs, C. A., … Reece, E. A. (2008). Diabetes mellitus and birth defects. American Journal of Obstetrics and Gynecology, 199(3), 237.e231–239. doi:10.1016/j.ajog.2008.06.028.
Correa, A., & Marcinkevage, J. (2013). Prepregnancy obesity and the risk of birth defects: An update. Nutrition Reviews, 71(Suppl 1), S68–77. doi:10.1111/nure.12058.
Fadl, H. E., Ostlund, I. K., Magnuson, A. F., & Hanson, U. S. (2010). Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabetic Medicine, 27(4), 436–441. doi:10.1111/j.1464-5491.2010.02978.x.
Ferencz, C., Rubin, J. D., McCarter, R. J., & Clark, E. B. (1990). Maternal diabetes and cardiovascular malformations: Predominance of double outlet right ventricle and truncus arteriosus. Teratology, 41(3), 319–326. doi:10.1002/tera.1420410309.
Finer, L. B., & Zolna, M. R. (2011). Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. doi:10.1016/j.contraception.2011.07.013.
Garcia-Patterson, A., Erdozain, L., Ginovart, G., Adelantado, J. M., Cubero, J. M., Gallo, G., … Corcoy, R. (2004). In human gestational diabetes mellitus congenital malformations are related to pre-pregnancy body mass index and to severity of diabetes. Diabetologia, 47(3), 509–514. doi:10.1007/s00125-004-1337-3.
Gasim, T. (2012). Gestational diabetes mellitus: maternal and perinatal outcomes in 220 saudi women. Oman Medical Journal, 27(2), 140–144. doi:10.5001/omj.2012.29.
Gilboa, S. M., Correa, A., Botto, L. D., Rasmussen, S. A., Waller, D. K., Hobbs, C. A., … Riehle-Colarusso, T. J. (2010). Association between prepregnancy body mass index and congenital heart defects. American Journal of Obstetrics and Gynecology, 202(1), 51.e51–51.e10. doi:10.1016/j.ajog.2009.08.005.
Inkster, M. E., Fahey, T. P., Donnan, P. T., Leese, G. P., Mires, G. J., & Murphy, D. J. (2006). Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: Systematic review of observational studies. BMC Pregnancy and Childbirth, 6, 30. doi:10.1186/1471-2393-6-30.
Janssen, P. A., Rothman, I., & Schwartz, S. M. (1996). Congenital malformations in newborns of women with established and gestational diabetes in Washington State, 1984–91. Paediatric and Perinatal Epidemiology, 10(1), 52–63. Retrieved, from http://www.ncbi.nlm.nih.gov/pubmed/8746431.
Lapolla, A., Dalfra, M. G., Bonomo, M., Parretti, E., Mannino, D., Mello, G., & Di Cianni, G. (2009). Gestational diabetes mellitus in Italy: A multicenter study. European Journal of Obstetrics, Gynecology, and Reproductive Biology. doi:10.1016/j.ejogrb.2009.04.023.
Mannan, M. A., Rahman, M. H., Ara, I., & Afroz, H. (2012). Prevelence and pregnancy outcome of gestational diabetes mellitus among Bangladeshi urban pregnant women. Journal of Medicine (Bangladesh), 13(2), 147–151.
Martinez-Frias, M. L., Frias, J. P., Bermejo, E., Rodriguez-Pinilla, E., Prieto, L., & Frias, J. L. (2005). Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabetic Medicine, 22(6), 775–781. doi:10.1111/j.1464-5491.2005.01492.x.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., The Prisma Group (2009). Preferred reporting items for systemic reviews and meta-analyses: The Prisma Statement. Journal of Clincal Epidemiology, 62, 1006–1012.
Moore, L. L., Singer, M. R., Bradlee, M. L., Rothman, K. J., & Milunsky, A. (2000). A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology, 11(6), 689–694.
Mosher, W. D., Jones, J., & Abma, J. C. (2012). Intended and unintended births in the United States: 1982–2010. National Health Statistics Reports, 55, 1–28. Retrieved, from http://www.ncbi.nlm.nih.gov/pubmed/23115878.
Pablo Velazquez, G., Genero, V. M., & Martha Leticia Martinez, M. (2010). Neonatal morbidity and mortality associated with gestational diabetes. Revista Chilena de Obstetricia y Ginecologia, 75(1), 35–41.
Porter, M. P., Faizan, M. K., Grady, R. W., & Mueller, B. A. (2005). Hypospadias in Washington State: Maternal risk factors and prevalence trends. Pediatrics, 115(4), e495–e499. doi:10.1542/peds.2004-1552.
Ramachandran, A., Snehalatha, C., Clementina, M., Sasikala, R., & Vijay, V. (1998). Foetal outcome in gestational diabetes in south Indians. Diabetes Research and Clinical Practice, 41(3), 185–189.
Ramos-Arroyo, M. A., Rodriguez-Pinilla, E., & Cordero, J. F. (1992). Maternal diabetes: the risk for specific birth defects. European Journal Epidemiology, 8(4), 503–508. Retrieved, from http://www.ncbi.nlm.nih.gov/pubmed/1397216.
Reece, E. A., Ma, X. D., Wu, Y. K., & Dhanasekaran, D. (2002). Aberrant patterns of cellular communication in diabetes-induced embryopathy. I. Membrane signalling. The Journal of Maternal-Fetal & Neonatal Medicine, 11(4), 249–253. doi:10.1080/jmf.11.4.249.253.
Reece, E. A., Ma, X. D., Zhao, Z., Wu, Y. K., & Dhanasekaran, D. (2005). Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. American Journal of Obstetrics and Gynecology, 192(3), 967–972. doi:10.1016/j.ajog.2004.10.592.
Savona-Ventura, C., & Gatt, M. (2004). Embryonal risks in gestational diabetes mellitus. Early Human Development, 79(1), 59–63. Retrieved, from http://www.ncbi.nlm.nih.gov/pubmed/15449398.
Sheffield, J. S., Butler Koster, E.L., Casey, B.M., McIntire, D.D., Leveno, K.J. (2002). Maternal diabetes mellitus and infant malformations. Obstetrics and Gynecology, 100(5), 925–930.
Shnorhavorian, M., Bittner, R., Wright, J. L., & Schwartz, S. M. (2011). Maternal risk factors for congenital urinary anomalies: Results of a population-based case-control study. Urology. doi:10.1016/j.urology.2011.04.022.
Stothard, K. J., Tennant, P. W., Bell, R., & Rankin, J. (2009). Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA, 301(6), 636–650. doi:10.1001/jama.2009.113.
Van Bennekom, C. M., Mitchell, A. A., Moore, C. A., & Werler, M. M. (2013). Vasoactive exposures during pregnancy and risk of microtia. Birth Defects Research. Part A, 97(1), 53–59. doi:10.1002/bdra.23101.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Parnell, A.S., Correa, A. & Reece, E.A. Pre-pregnancy Obesity as a Modifier of Gestational Diabetes and Birth Defects Associations: A Systematic Review. Matern Child Health J 21, 1105–1120 (2017). https://doi.org/10.1007/s10995-016-2209-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-016-2209-4