Abstract
Context
Land-use change is a key driver of pollinator declines worldwide. Plantation forests are a major land use worldwide and are likely to expand substantially in the near term, especially with projected cellulosic biofuel production. But little is known about the potential local and landscape-scale impacts of plantation forestry on bees, the most important group of pollinators worldwide.
Objectives
We studied the effects of local management, landscape context, and their interaction on bee abundance and species richness in the southeastern US, in pine plantations and other nearby land uses.
Methods
We sampled bee communities using aerial netting and pan trapping in 85 sites over 3 years.
Results
We found that both landscape composition and configuration are important factors for bee diversity and abundance at the landscape scale, though interestingly many landscape factors showed contrasting directional responses for diversity versus abundance. Removing the four most common species, all in the genus Lasioglossum (and which comprised ~ 45% of all specimens) largely harmonized the results between diversity and abundance. In addition, we found several interactions between local management and landscape factors, all consistent with the idea that compositional heterogeneity and configurational complexity are more important for bee communities in poorer-quality local habitat.
Conclusions
Our results underscore the importance of considering (1) both landscape configuration and composition in analyses, and (2) interactions between local management and landscape factors. The interactions in particular highlight the need to maintain landscape compositional heterogeneity and configurational complexity, particularly in heavily managed landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bees (Hymenoptera: Apidae) are the most important single taxon of pollinators worldwide (e.g. IPBES 2016; Rader et al. 2016) and play a key functional role in agricultural crop production and the reproduction of wild plants, underscoring the need to better understand how they are affected by a range of anthropogenic environmental changes. Land-use change is among the most important environmental changes impacting communities of wild bees (Potts et al. 2010; IPBES 2016). Plantation forestry is a land-use type that currently occupies a large land area that is very likely to grow substantially in the near future (FAO 2012). But we know little about how bee communities might respond to expansion of plantation forestry systems, particularly in terms of landscape-scale patterns.
Understanding biodiversity effects of forest plantation expansion is particularly important given: (1) growing demands for wood, wood products, and pulp and paper products (FAO 2012); and (2) the potential for trees to be used as biofuel and bioenergy feedstocks (i.e. plants that can be converted into bioenergy in whole or in part). Recent technological developments—particularly focused on cellulosic bioethanol production—are a critically important driver of growth in biofuel feedstock land use. As the name implies, cellulosic biofuels are derived from cellulose and other carbon sources from plants that are more recalcitrant in terms of conversion to ethanol than the starches and sugars (primarily from edible crops like corn) that are other current sources for ethanol conversion (e.g. Carroll and Somerville 2009). While methods currently exist for conversion of cellulose to ethanol, these technologies are not currently economically scalable, but will be in the near future if technological advances in conversion efficiency continue at the current pace (Langholtz et al. 2016). In addition to technology developments, there are a range of policies from local to multi-national that support or even mandate biofuel feedstock cultivation worldwide (Sorda et al. 2010; Timilsina and Shrestha 2010; Huang et al. 2011). Among the largest mandates are those in the US, where the Energy Independence and Security Act of 2007 (EISA) mandates that the US produce 21 billion gallons of biofuel by 2022.
The southeastern US is a key region for plantation forestry generally, and specifically for future expansion of forestry-based biofuel feedstock cultivation. In particular, pine plantations in the southeastern US are currently cultivated for conventional timber products, and cover 13 million hectares, with ~ 600,000 hectares planted each year (Kline and Coleman 2010). Existing well-developed forestry operations and the rapid growth rate of native pine species in southeastern climates make this region ideal for biofuel production (Kline and Coleman 2010). Increasing pine cultivation to produce biofuel feedstocks will necessarily change large-scale land-use patterns, including very likely expansions of the current extent of pine plantations (Fargione et al. 2009).
We continue to have a poor understanding of how plantation forestry expansion will affect biodiversity generally (e.g. Fletcher et al. 2011), and bee communities specifically, at both local and landscape scales. At local scales, we know little about bee communities in tree plantations, or how suitable such plantations are for providing bee life-history requirements, particularly relative to other land uses like annual cropping systems (Bennett and Isaacs 2014; Campbell et al. 2016; Saunders 2016). On the one hand, such land use could have positive effects on bee communities relative to some alternate land uses. Perennial crops are often associated with less disturbance than annual crops, including soil disturbance, which could allow for greater potential nesting habitat for bees, many of which are ground-nesting (e.g. Cane 1991). Perennial crops also typically have lower chemical inputs, including pesticides, which can disrupt bee communities over large scales (e.g. Rundlöf et al. 2015) and herbicides, which can hypothetically reduce flowering plant resources (Bretagnolle and Gaba 2015). On the other hand, such land use change could also have negative effects. Densely-planted timber forests tend to support only sparse herbaceous flowering plant understories and fewer pollinators compared to more open habitat types (e.g. Hanula et al. 2016), and bee diversity and abundance can be much greater in urbanized habitats compared to relatively intact forested systems (Winfree et al. 2007). At landscape scales, while some studies have examined effects of land cover on bee communities (e.g. Steffan-Dewenter et al. 2002; Brosi et al. 2007, 2008, 2009; Steffan-Dewenter and Westphal 2007), few clear patterns have emerged. In addition, very few of those studies have separated out the effects of landscape composition versus configuration (Fahrig 2003; Hadley and Betts 2012), and even fewer have examined potential interactions between local and landscape factors (Holzschuh et al. 2007; but see Bourke et al. 2014).
To address these gaps, we studied the effects of both local and landscape factors associated with the cultivation of pine plantations on bee communities in the southeastern coastal plain of the US. We sampled bee communities across three important pine producing states (Alabama, Florida, and Georgia) in four land use classes: plantations, clearcuts, reference forest (longleaf pine) and an alternative land use (corn cultivation). We sampled in 85 sites, generating one of the largest systematically-collected datasets of bee communities. We assessed landscape context in terms of both composition and configuration, at a range of spatial scales, and specifically assessed interactions between local management and landscape factors. At the local scale, we hypothesized that we would find higher bee diversity and abundance in reference forest relative to production land uses (forestry and agriculture), and in forestry land uses relative to annual agriculture. At the landscape scale, we hypothesized that we would find effects of both landscape configuration and composition on bee communities, including finding greater bee diversity and abundance in landscapes with more compositional heterogeneity and configurational complexity (Fahrig 2003; Hadley and Betts 2012; Reynolds et al. 2018). In addition, we expected to find either a unimodal or monotonic positive relationship between tree cover and bee diversity and abundance. A unimodal relationship could result from tree-covered habitats contributing to landscape-level heterogeneity. Locally, tree covered habitats may support more bee diversity and abundance than many alternative land uses in our study region, particularly row-crop agriculture, which largely involves very high agrochemical inputs (e.g. conventional corn production). In terms of interactions between local and landscape factors, we hypothesized that landscape-level complementarity—for example, acquisition of different resources by bees in different land-uses surrounding ones in which they nest or spend the bulk of their time—would drive two patterns. First, we hypothesized that tree cover would have a stronger positive relationship on bee communities in local land uses that were not tree-covered, and second, that habitats that were generally lower-quality for bees (in particular, cornfields) would benefit more from greater compositional heterogeneity and configurational complexity.
Methods
Study sites
Sites
We sampled bees in 85 sites in the southeastern coastal plain of eastern Alabama, northern Florida, and southern Georgia (Fig. 1), an area with a substantial extent of pine plantations. Our sampling effort was part of a larger study which included sampling for birds (Gottlieb et al. 2017) as well as reptiles, amphibians, and bats. We sampled between April and July in three years, 2013–2015. In 2013, we sampled Newton County, GA. In 2014, we sampled Alabama (Butler, Conecuh, Escambia, and Monroe counties), Florida (Jefferson, Liberty, and Wakulla counties), and Georgia (Decatur and Thomas counties). In 2015, we sampled bees in Alabama (Butler and Covington counties), Florida (Alachua, Columbia, Gilchrist, Levy, Marion, and Suwannee counties), and Georgia (Mitchell county). All sites were spaced at least 2.5 km apart to ensure sample independence.
Local management
We sampled bees in sites with four general classes of local management (Table 1), henceforth referred to as ‘land use types’. Two of these land use types were focused on forestry (plantation forests and clearcuts), and two provided alternative reference conditions, an annual row-crop (cornfields) and the historical landcover in the region, longleaf pine (Pinus palustris) savannahs. These remnant ecosystems are managed to maintain some degree of the natural fire regime needed for maintenance of these systems (Christensen 2000). This study is part of a larger research project on pine biofuel production in the US (Gottlieb et al. 2017), and includes contrasts within plantation forest sites designed to compare forest management practices between biofuel feedstock and traditional timber production. A previous study emerging from this research project found no substantial differences in bee abundance, richness, and community composition among different management practices in standing plantations (Gruenewald 2014), so we have aggregated these sites. As such, this study has many more sites representing standing plantation forests than in the other land use types.
Transect design
We set up two 200 m × 2 m transects, one “interior” and one “edge”, in each site. The edge transect was placed 50 m from the edge of the tree stand, bordering non-tree land use (row-crop, grass, or urban). In cornfield sites, we placed the edge transect 50 m from the edge of the field, which bordered land not used for corn cultivation (forest, grass, urban, or non-corn row-crop). We placed the interior transect so that it was surrounded with a minimum radius of 150 m of the management type being studied. Interior transects were placed using ArcGIS and satellite data from National Land Cover Database 2011.
Bee sampling
Overview
We collected bees using both passive (pan traps) and active (aerial netting) sampling methods. We sampled bees at each site four times within the same year, from both the edge and interior transects, once every 3–4 weeks. Sites were sampled on a rotation such that samples from each site were temporally spread out to minimize any effects of flowering phenology across the growing season. We used both passive and active sampling in tandem for each rotation cycle. Sites were not sampled across multiple years.
Passive sampling
We used pan traps to passively sample bees. Pan traps comprised 104 mL plastic Solo cups (Model P325) painted with blue, white, or yellow UV reflective paint to attract bees (Kearns and Inouye 1993; Westphal et al. 2008). We filled the painted cups with ~ 75 mL of soapy water, which has reduced surface tension so that upon contact bees are quickly immersed and drown (Kearns and Inouye 1993). We set pan traps approximately 40 cm above the ground using Vigoro plant props (Model #611872). Fifteen pan traps were evenly distributed along the middle 100 m of each transect. We alternated pan trap colors, for a total of five blue, five yellow, and five white traps (Westphal et al. 2008). We collected bees from pan traps ~ 24 h after they had been set. We washed, pinned, and labeled bees the same day they were collected.
Active sampling
We actively sampled bees using aerial netting. A field team member walked along the 200 m transect line searching for bees for 30 min. We paused timers while handling bees. Sampling was conducted between 10 a.m. and 11 a.m. We pinned and labeled bees the same day they were collected.
Bee identification
We identified bees to the species level or lowest possible taxonomic category based on morphological characteristics. We used interactive keys from DiscoverLife (www.discoverlife.com) to identify bees. Particularly difficult specimens were determined with assistance from Sam Droege (USGS) and Ismael Hinojosa (UNAM).
Landscape metrics
Our landscape metrics were based on LANDSAT remote sensing data (30-m spatial resolution) from 2011 with automated classification from the National Land Cover Database (“NLCD”; Homer et al. 2015). The NLCD classifies land cover into sixteen landscape classes, which we aggregated into nine: water, tree-covered, row crop, grassland, urban, barren, shrub, pasture, and wetland. These classes are coarse and do not differentiate between land use distinctions that are very likely important for bee communities, for example different row crop types with wind versus insect-pollinated flowers. Still, this scale of classification matches our interest in focusing on general trends and patterns in land use rather than dissecting fine-scale differences.
We used this classification to calculate seven landscape metrics surrounding each site (Table 2), reflecting landscape composition [(1) % tree cover, (2) landscape richness, and (3) landscape Shannon diversity] as well as landscape configuration [(4) aggregation index, (5) mean patch shape, (6) mean core patch area, and (7) mean effective mesh size). We calculated metrics using SDMTools (VanDerWal et al. 2014) at four buffer radii around each site: 500, 1000, 2000, and 5000 m. All metrics except % tree cover were calculated at the landscape level, rather than the class level, i.e. each metric includes all land cover classes, rather than just a single class. Mean core patch area was calculated with an edge depth of one 30-m pixel (i.e., pixels at the very edge of a landscape class were not included in the core area calculation). We chose this edge depth because we felt it was most reflective of how the bulk of bee species would sense the environment relative to their flight distances. In that context, 60 m (across a landscape class transition on both sides) is a relatively substantial distance for bee flight between the core areas of two landscape classes. We re-scaled mean core patch area, mean effective mesh size, and aggregation index from 0 to 1 (based on the maximum and minimum values we observed) to allow better model fitting.
The landscape composition variables in this study describe the variety of grid cells of different types potentially available to bees within a landscape. We selected % tree cover to understand in part how pine plantation expansion may affect bees, though the NLCD classification does not distinguish between tree plantations and natural forests. Land cover richness quantifies the number of land classes in a landscape and is the simplest measure of landscape composition. Shannon’s Diversity Index here is focused on the landscape, rather than on species; it is a metric of landscape heterogeneity that takes both land cover type richness and evenness into account.
In addition to landscape composition variables, we use landscape configuration variables to describe attributes of constituent patches, such as shape, core area, subdivision, and dispersion. We selected one landscape metric for each of these factors. We selected mean shape index to describe patch shape because it is normalized to prevent a size dependency problem (e.g., circles of differing area have different edge-to-area ratios; this metric corrects for that) and it is not overly sensitive to sites with only a few patches (McGarigal and Marks 1995). We used mean core patch area because core area can be a better predictor of habitat quality than total area (Temple 1986). We chose effective mesh size to describe the subdivision of the landscape because it takes into account all patches according to their size, and it is more sensitive to fragmentation than other subdivision metrics (Jaeger 2000). We described dispersion of the different land classes in the landscape with the aggregation index. This metric tells us how dispersed the land classes are, and it is scaled to account for the maximum possible number of like adjacencies given the abundance of land classes (He et al. 2000).
Data analysis
Overview
We analyzed how bee abundance and species richness changed with local management (whether a study site was in a natural (longleaf) forest remnant, pine plantation, clearcut or cornfield) as well as various landscape context metrics (Tables 1, 2), specifically including both landscape configuration and composition. We also assessed interactions between local and landscape metrics. We used a model-selection framework (Burnham and Anderson 2002) to select parsimonious models from our set of explanatory variables. We conducted all statistical analyses in R (R Core Team 2016).
Linear models
We attempted to run linear mixed-effects models incorporating the repeated measures of bee communities at each site, but we were unable to achieve convergence in a substantial portion of models. Thus, we used linear models to analyze the effects of local management, landscape metrics, and all local × landscape interactions (but not landscape × landscape interactions). Because local management was represented as a single categorical factor, this meant that we included seven two-way interactions in our set of candidate models.
Richness and abundance
For bee abundance, we used the mean per-sample abundance in each site, which we natural-log-transformed to better meet model assumptions. Because of the dominance of four species of Lasioglossum in our dataset, we also assessed abundance of all bees not including those species to assess potential differences in drivers of abundance. For species richness, because our sampling was not perfectly balanced, and because the probability of species detection increases with sampling effort, we used the iNEXT package (Chao and Jost 2015) to construct rarefaction curves of species richness, bootstrapping 50 times to estimate site species accumulation at the third sample.
Multicollinearity and spatial autocorrelation
We assessed multicollinearity among various landscape metrics with variance inflation factors (e.g. Zuur et al. 2010), using the “fmsb” package for R (Nakazawa 2017) and a stepwise approach to eliminate metrics above a threshold VIF of 5, to confirm that the set of best models did not include collinear explanatory variables. VIF cutoff values are typically five or 10 (Craney and Surles 2002), and we used the more stringent value of five in our analyses. We assessed spatial autocorrelation in abundance and diversity among plots within each sampling year using Moran’s I, calculated in the “ape” package for R (Paradis et al. 2004).
Model selection
We compared candidate models using automated AIC (Akaike’s information criterion) model selection with the “MuMIn” package for R (Barto 2016). AIC model selection balances model fit with model complexity (e.g. Goodenough et al. 2012). We included the full set of (non-collinear) candidate models at each landscape buffer radius in the selection process, to select not only the best set of explanatory variables, but also the best performing buffer radius.
Model assumptions
After model selection, we assessed if the best models met key statistical assumptions, including multivariate normality of errors, homoscedasticity, and linear relationships using diagnostic plots (“plot.lm” in base R).
Results
Overview
In total, we sampled 5758 bee specimens representing 128 species: 1480 specimens (82 species) in Alabama, 1756 specimens (76 species) in Florida, and 2522 specimens (78 species) in Georgia. Overall, the four most abundant species were Lasioglossum floridanum, Lasioglossum reticulatum, Lasioglossum nymphale, and Lasioglossum puteulanum, which together represented almost 42% of all sampled bee specimens (Table 3). All Lasioglossum species combined (not just the four most abundant) represented nearly 61% of specimens. After the four most abundant species, the next most common Lasioglossum (L. pectorale) was represented by fewer than half the number of individuals (156) relative to L. puteulanum. The most common non-Lasioglossum species was Mellisodes communis with 353 individuals (6.1% of all sampled specimens).
Model assumptions
Our best set of models met all the key assumptions for linear models, including linearity, homoscedasticity, normality of errors, lack of spatial autocorrelation (Moran’s I). Best models with (raw) mean abundance did not meet several model assumptions, but best models with logged mean abundance performed well. Our set of explanatory variables at buffer radii < 5 km lacked collinearity (defined as VIFs < 5), but a single variable, mean core patch area, increased VIFs above this threshold with a 5-km buffer radius.
Buffer radius
The best models (within two delta-AICc values of the best model) for overall bee abundance used a 1-km buffer, while the best models for richness and for abundance with dominant Lasioglossum removed used a 2-km buffer. For species richness, one model at the 5-km buffer radius was within two delta-AICc points of the best model (at 2-km), but included mean core patch area; that variable, however, was highly collinear with other explanatory variables at that radius, as determined by VIFs. When mean core patch area was removed, the resulting model was no longer in the set of best models. Thus, we retained the 2-km buffer radius only for bee species richness.
Bee abundance
The two best models for overall bee abundance (Table 4) both included three sets of explanatory variables: (1) local management (Fig. 2); (2) several landscape metrics, both compositional (land cover richness and land cover Shannon diversity) and configurational (mean core patch area, mean shape index, and aggregation index) (see Online Resource 1 for more detail); and (3) an interaction between local management and mean core patch area (Fig. 3). The second-ranked model differed only in including an additional explanatory variable, % tree cover. In terms of main effects, nearly all of the landscape metrics surprisingly showed negative relationships with bee abundance, with the sole exception of land-cover richness, which was positively related (Table 4, Online Resource 1).
By contrast, when assessing abundance with the four dominant Lasioglossum species removed, there was a very different single best model (Table 4). First, that model was at a different buffer radius (2-km) than the model for overall bee abundance (1-km). Second, the best model did not include any metrics of landscape configuration (as compared to overall bee abundance, which included three such metrics; Online Resource 2). Third, while the three metrics of landscape configuration that were included in the best model were shared with either both the two best models for overall bee abundance (land cover richness, land cover Shannon diversity) or one of the models (% tree cover), they differed in all having positive effects on bee abundance, as opposed to negative effects of % tree cover and land cover Shannon diversity in the models for overall bee abundance (Online Resource 2). Fourth, while both responses included local × landscape interactions, without dominant Lasioglossum these were for two compositional metrics (% tree cover and land cover Shannon diversity, Fig. 4) as opposed to a single configurational metric (mean core patch area) in the models for overall bee abundance.
Bee richness
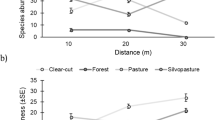
The set of best models included four models for bee species richness (Table 4, Online Resource 3), again all based on a 2 km buffer. All four shared a core set of explanatory variables (and the only variables in the model with the single lowest AIC) including local management (Fig. 2); landscape composition (% tree cover, land cover richness); landscape configuration (mean shape index); and an interaction between local management and % tree cover (Fig. 4). In addition, the three other models each included a single different additional variable: land cover Shannon diversity, aggregation index, and an interaction between local management and mean shape index (Table 4, Fig. 5). In contrast to abundance, in terms of main effects these landscape metrics were nearly all positively related to bee species richness, with only Shannon diversity showing a negative relationship.
Discussion
We examined the effects of local-level forest management practices, landscape context, and their interactions to better understand the potential implications of pine plantation land use on bee communities. Three primary findings emerge from our results. First, we found that both landscape composition and configuration are important for both bee abundance and richness, but that the direction (positive vs. negative) of landscape effects was often contrasting between bee abundance and species richness. Second, these contrasts may be largely explained by the responses of a few highly abundant and closely related taxa in our dataset. Third, there were interactions between the local-level management and the landscape context, which appeared to be driven by differing impacts of landscape heterogeneity based on the quality of local habitats. We discuss each of these findings in more detail below.
Heterogeneity in landscape composition and complexity in landscape configuration can each theoretically have positive and negative effects on biodiversity (Fahrig et al. 2011). In terms of positive effects, more heterogeneous composition can provide a greater diversity of resources for breeding, feeding, and nesting (Benton et al. 2003) and more complex configurations can allow for easier access to a range of resources (Dunning et al. 1992; Flick et al. 2012). By contrast, however, for species that require only a single habitat type or a small number of habitat types to meet their life history requirements, any interruptions in these high-quality habitats—while increasing compositional heterogeneity and configurational complexity—could negatively impact on that biotic group. This is in line with much of the work on the negative effects of habitat fragmentation on biodiversity (Ewers and Didham 2006).
We hypothesized that landscape compositional heterogeneity and configurational complexity would be positively associated with bee richness and abundance. Our study took place in a highly human-modified region, the coastal plain of the southeastern US, which has only ~ 3% of the original land cover (longleaf pine savannah) remaining (Frost 2006). In addition, bees are relatively resilient to land-use change (e.g. Winfree et al. 2007; Brosi et al. 2008), with for example relatively high diversity and abundance found in cities (Hall et al. 2017). This resilience is likely due at least in part to the fact that nearly all bees can forage over relatively large areas; even a central-place foraging bee with only a 200 m flight range (Greenleaf et al. 2007) has a home range of > 12.5 Ha. While previous research on agroecosystems has generally found only weak effects of landscape configuration on wild bee pollinators in agroecosystems (Kennedy et al. 2013), some studies do document such relationships (e.g. Moreira et al. 2015). We expected to find stronger relationships in part because our sample size is among the largest of any study focusing on bees (85 sites), allowing us greater statistical power than some other studies. By contrast, if the only bee taxa left in our highly-modified study region were those that are highly resilient to disturbance, we expected that such relationships would be weaker. Our results could also differ from previous work given our primary sampling focus on tree-covered habitats, in which we found relatively low bee abundance and diversity compared to some studies of more-open habitats (e.g., Brosi et al. 2008, where sampling occurred in pastures).
Contrasting drivers for bee abundance and species richness
Metrics of both landscape composition and configuration are included in our best models of both overall bee abundance and richness. All three of the examined landscape composition metrics (% tree cover, land cover richness, land cover Shannon diversity) as well as two configuration metrics (aggregation index and mean shape index) were included in the set of best models for both overall bee abundance and bee species richness. In addition, for overall abundance one of the best models also included another configuration metric, mean core patch area. The majority of literature on bee communities and land use does not distinguish between the effects of landscape composition and configuration (Lennartson 2002; Hadley and Betts 2012; but see Moreira et al. 2015). Although landscape composition and configuration are often confounded (Fahrig 2003), it is important to separate composition and configuration to understand how to best manage landscape elements—including forest plantations—to conserve biodiversity (Hadley and Betts 2012). An excellent review of studies of landscape effects on bees (Viana et al. 2012) makes it clear that while a multitude of studies have considered landscape composition (particularly the proportion of native or semi-native habitat in a landscape), there have not been enough studies to meaningfully synthesize effects of landscape configuration on bee communities.
While overall bee abundance and richness shared several landscape predictors in their sets of best models, there were two sets of puzzling results. First, there was a consistent contrast in the directional responses between land-cover richness and land-cover Shannon diversity, which in turn are positively related to one another. Second, the response directions with most other landscape variables were largely contrasting between abundance and richness.
Land-cover richness and land-cover Shannon diversity were the only factors with consistent directional responses for both richness and overall abundance; land cover richness was positively related to both, while land cover Shannon diversity was negatively related to both. This is a surprising result because not only are both metrics of heterogeneity in landscape composition, but also because Shannon diversity is a function of two components: richness and evenness. As such these contrasting directional results, with land cover richness positive and land cover Shannon diversity negative, must at some level be driven by a negative relationship with evenness. This could make sense if one or more habitat types provide substantial resources, in combination with other habitat types that provide resources needed only rarely or in small quantities, especially if there were several such distinct habitat types. In such a situation, richness of habitats would typically be beneficial in terms of increasing the probability of presence of those habitats providing such “micro-resources”, while evenness could be detrimental by reducing the quantity of one or more key habitats that provide the bulk of resources.
There were three landscape factors which showed contrasting directional responses when comparing bee species richness and abundance. These included a compositional metric (% tree cover) and two configurational metrics (aggregation index and mean shape index). For each of these metrics, the relationships with overall abundance were negative, and positive with richness. These contrasting responses are counterintuitive because typically abundance and species richness are positively related; if nothing else, by sampling more individuals (greater abundance) there is a greater chance of recording more species.
While the contrasting results for abundance and diversity are puzzling at first glance, parallel results have been reported before, and relate directly to the dynamics we discussed above for land cover (as opposed to bee) diversity and abundance. For example, Meyer et al. (2009), studying hoverflies in an agricultural landscape in Germany, found that in in homogeneous landscapes, hoverfly abundance was high but diversity was low; and similarly, in heterogeneous landscapes, they found higher diversity and lower abundance. Their results were consistent with high dominance and abundance of a few hoverfly species that do well in homogeneous croplands, whereas the less-abundant species may have responded to particular habitat elements in unique ways (reflecting differentiated life-history requirements), thus generating higher diversity but lower overall abundance in heterogeneous landscapes. This finding is highly consistent with our results, given that we also found one highly-abundant group of bees (a few species of Lasioglossum) associated with homogeneous landscapes, as we detail in the next section.
Effects of common Lasioglossum species on abundance measures
The contrasting directional relationships between landscape variables and richness and abundance can be explained in part through the contribution of common Lasioglossum (Dialictus) to our abundance measures. Again, four Lasioglossum (Dialictus) species comprised > 40% of the specimens in our dataset, and when those taxa were removed, the abundance results changed substantially (Table 4), aligning much more closely to the richness models.
The natural history of these four common Lasioglossum (Dialictus) species may partially explain why abundance in these species responds distinctly to local and landscape factors. This is particularly true given that these Lasioglossum species were particularly abundant in clearcut habitats, comprising almost 70% of the specimens found in clearcuts. Lasioglossum is the single most speciose genus of bees on Earth, with more than 1250 described species (Michener 2000). They are primarily ground-nesting, though some species nest in rotting wood (Michener 2000); while the nesting habits of these particular species are not documented, they were common even in clearcuts with woody debris removed (about 50% of our clearcut sites; see Gottlieb et al. 2017), consistent with the idea that they are soil-nesting. The mechanical disturbance of tree harvesting in clearcuts also typically involves substantial soil and non-tree vegetation disturbance, which can open up more nesting sites. In addition to nesting habitat, another life-history dimension which may be important is sociality. While we do not have information on sociality in these particular taxa, approximately 40% of Lasioglossum species are either eusocial or are a member of a predominately eusocial subgenus, of which Dialictus is one; this includes several taxa that are facultatively social (Michener 2000). Social taxa can have very high densities of individuals around nest sites, thus potentially disproportionately contributing to abundance data. If nesting sites are a limiting resource, and particularly if these species are able to quickly colonize newly-open nesting sites in clearcuts (which sociality could contribute to), that could help to explain their high abundance in relatively homogeneous landscapes (those with low % tree cover, land cover Shannon diversity, patch interspersion (aggregation index), and edge-area relationship (shape index)).
Local: landscape interactions
We found interactions between local-level management and landscape context for both bee abundance and species richness, indicating that landscape context has differing effects depending on local management. These interactions are consistent with the hypothesis that in higher-quality local habitats, greater heterogeneity in landscape composition and greater complexity in configuration will be negative for bee communities. This is predicated on the ideas that (1) the local habitat is likely to be a substantial component of the surrounding landscape area; and (2) that for “higher-quality” local habitat, other habitat types, on average, provide a lower resource density for bees (and vice versa for “lower-quality” habitats).
In terms of overall bee abundance, both best models included an interaction between local management and mean core patch area (Fig. 3). The pattern of the interaction is striking, in that there is a positive relationship between core area and bee abundance for the natural reference condition (longleaf pine savannahs), while in the three managed ecosystem types (clearcuts, plantation forests, and cornfields) this relationship is negative, and particularly strongly negative in cornfield systems. This result is consistent with the idea that in lower-quality local habitats, greater landscape complexity leads to better biodiversity outcomes.
For both bee species richness and for abundance without the dominant Lasioglossum, the best models included an interaction between local management and tree cover (Figs. 4a, 5a), and the interaction patterns were qualitatively identical between the two. Specifically, there is a negative trend for the effect of increasing tree cover in managed forests, whereas that effect is positive in other land uses, with a particularly steep slope in corn fields. These relationships are consistent with the idea that in managed forest, adding “more of the same” (tree-covered habitat) is negative for bee communities—likely leading to reduced landscape complexity overall—but that in habitats without tree cover (clearcuts and cornfields), adding tree-covered habitats would increase landscape complexity and thus biodiversity benefits. The exception in this relationship is our natural reference condition (longleaf pine savannah) habitats, where more tree cover increased species richness and abundance (again, excluding dominant Lasioglossum). In this instance, we conjecture that having more of this potentially high-quality habitat available at the landscape level is beneficial for bee biodiversity.
For abundance without the dominant Lasioglossum, the best model also included an interaction between local management and land cover Shannon diversity (Fig. 4b). Here, clearcut habitats are essentially flat with respect to land cover Shannon diversity, managed forests and longleaf pine savannahs have a negative trend, while in cornfields there is a strong positive trend. Again, if cornfields are relatively poor habitats (in terms of providing a range of resources for bees) having more diversity at the landscape level may be most beneficial in those habitats. We suspect that in this case, cornfields are likely the most locally homogeneous land use type, and thus bees in such habitats may benefit most from any increases in complexity at the landscape scale.
Finally, for bee species richness, one model in the set of four best models also included an interaction with mean shape index (Fig. 5b), i.e. edge-area ratio. Here, clearcut and managed forest habitats showed a weakly increasing trend, whereas both cornfield and longleaf pine habitats showed strongly decreasing trends. We are puzzled that bees in cornfields respond in a strong negative way to increasing edge quantity in the landscape, given the previous results we have discussed. One potential explanation for the pattern in cornfield habitats is that if increasing edge allows for easier access to other habitat types, bees may be more likely to disperse to higher-quality habitats nearby.
Study limitations
As in all field studies focused on landscape scales, there are a number of design and analysis trade-offs which present limitations to our work. Among the most important of these are the level of landscape classification, temporal variation, and potential biases in our bee sampling. A key limitation in this category is that we were unable to differentiate between pine plantations and natural forests in our classification of the landscape. A finer classification of the landscape may have allowed us to better understand the impacts of pine plantations on the landscape and the implications that may have for bee diversity. Similarly, we were unable to distinguish between the various types of row crops in our system, which likely have contrasting effects on bee communities. Additionally, our study was unable to account for temporal variation. Another issue was time lags between landscape characterization and sampling. The landscape characterization was based on satellite data from 2011, while our bee sampling took place 2–4 years later. Hopefully, as research continues to move to understand ecological processes on a larger landscape scale and technology continues to advance, more current and finely characterized landscape data will become available. Finally, biases in the sampling of bees may have affected our results. For example, bees in the family Halictidae (which includes the genus Lasioglossum) are known to be sampled more reliably with pan traps than other taxa (Cane et al. 2001). We partially corrected for this bias by running our analyses with and without the four dominant Lasioglossum species. In addition, across the study we used two different sampling methods, with aerial netting in addition to pan traps, and together these methods have been shown to work well to sample bee communities (Westphal et al. 2008).
Future work
Our results underscore several areas in which future work is needed. There continues to be limited research on the effects of landscape context on bee diversity. Future research should address the limitations of our study, and explore the impacts of pine plantations on the landscape and the effects of temporal variation on bee communities. Future studies should explore the effects of landscape context on bee community composition (i.e., not just diversity and abundance), as community composition can significantly impact ecosystem processes (Tilman et al. 1997). To understand how we can increase biofuel cultivation while preserving bee biodiversity, we must consider beta-diversity (Karp et al. 2012). Studies have begun to document decreases in beta-diversity due to land-use intensification (Karp et al. 2012). Future work also should explore how the interactions between local-level management and landscape context impact the community composition and beta-diversity. Diversity is essential to ecosystem resilience (Peterson et al. 1998), and we must understand the impacts of agricultural intensification so that we can best manage forest plantations and the landscape context in which they are placed.
Conclusion
Agricultural intensification is driving major landscape changes (Kareiva et al. 2007) and given the importance of maintaining biodiversity, we must understand how these changes will impact pollinators on both a local and landscape level. It is likely that plantation forestry will expand substantially in the near term in the southeastern United States, to meet ever-increasing demand for wood and wood products, as well as energy demands that may be addressed in the future with cellulosic biofuels cultivation. This landscape transformation will likely significantly impact bee communities. Management policies and guidelines must consider the landscape context in addition to the local-level management, as well as the interactive effects of these different spatial scales. The management of spatial heterogeneity of these biofuel cultivation landscapes is critical to the conservation of bee diversity. Our results indicate that, while adding tree cover in the landscape can be beneficial for bee communities in non-forest land uses, that by contrast as tree cover increases, adding more plantation forests may be detrimental. Bees and other wild pollinators are continuing to decline (Potts et al. 2010; Burkle et al. 2013), and we must halt these declines to ensure stable pollination services in food systems (Garibaldi et al. 2013) and pollination functioning in natural and semi-natural ecosystems (Ollerton et al. 2011).
References
Barto K (2016) MuMIn: multi-model inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn
Bennett AB, Isaacs R (2014) Landscape composition influences pollinators and pollination services in perennial biofuel plantings. Agric Ecosyst Environ 193:1–8
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18(4):182–188
Bourke D, Stanley D, O’rourke E, Thompson R, Carnus T, Dauber J, Emmerson M, Whelan P, Hecq F, Flynn E, Dolan L, Stout J (2014) Response of farmland biodiversity to the introduction of bioenergy crops: effects of local factors and surrounding landscape context. Gcb Bioenergy 6(3):275–289
Bretagnolle V, Gaba S (2015) Weeds for bees? A review. Agron Sustain Dev 35(3):891–909
Brosi BJ, Daily GC, Chamberlain CP, Mills M (2009) Detecting changes in habitat-scale bee foraging in a tropical fragmented landscape using stable isotopes. For Ecol Manage 258(9):1846–1855
Brosi BJ, Daily GC, Ehrlich PR (2007) Bee community shifts with landscape context in a tropical countryside. Ecol Appl 17(2):418–430
Brosi BJ, Daily GC, Shih TM, Oviedo F, Durán G (2008) The effects of forest fragmentation on bee communities in tropical countryside. J Appl Ecol 45(3):773–783
Burkle LA, Marlin JC, Knight TM (2013) Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339(6127):1611–1615
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Verlag, New York
Campbell JW, Miller DA, Martin JA (2016) Switchgrass (Panicum virgatum) intercropping within managed loblolly pine (Pinus taeda) does not affect wild bee communities. Insects 7(4):62. https://doi.org/10.3390/insects7040062
Cane JH (1991) Soils of ground-nesting bees (Hymenoptera: Apoidea): texture, moisture, cell depth and climate. J Kansas Entomol Soc 64:406–413
Cane JH, Minckley R, Kervin L (2001) Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: pitfalls of pan-trapping. J Kansas Entomol Soc 73:208–214
Carroll A, Somerville C (2009) Cellulosic biofuels. Annu Rev Plant Biol 60:165–182
Chao A, Jost L (2015) Estimating diversity and entropy profiles via discovery rates of new species. Methods Ecol Evol 6:873–882
Christensen NL (2000) Vegetation of the southeastern coastal plain. In: Barbour MG, Billings WD (eds) North American terrestrial vegetation, 2nd edn. Cambridge University Press, New York, pp 398–448
Craney TA, Surles JG (2002) Model-dependent variance inflation factor cutoff values. Qual Eng 14(3):391–403
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65(1):169–175
Energy Independence and Security Act of 2007. Public Law 110–140, vol 121 (2007). https://www.congress.gov/bill/110th-congress/house-bill/6. Accessed 10 May 2017
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81(1):117–142. https://doi.org/10.1017/S1464793105006949
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34(1):487–515
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14(2):101–112
FAO (2012) State of the world’s forests. Food and Agriculture Organization of the United Nations
Fargione JE, Cooper TR, Flaspohler DJ, Hill J, Lehman C, McCoy T, McLeod S, Nelson EJ, Oberhauser KS, Tilman D (2009) Bioenergy and wildlife: threats and opportunities for grassland conservation. Bioscience 59(9):767–777
Fletcher RJ, Robertson BA, Evans J, Doran PJ, Alavalapati JR, Schemske DW (2011) Biodiversity conservation in the era of biofuels: risks and opportunities. Front Ecol Environ 9(3):161–168
Flick T, Feagan S, Fahrig L (2012) Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Agr Ecosyst Environ 156:123–133
Frost C (2006) History and future of the longleaf pine ecosystem. In: Jose S, Jokela EJ, Miller DL (eds) The longleaf pine ecosystem: ecology, silviculture and restoration. Springer, New York, pp 9–48
Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O, Bartomeus I, Benjamin F, Boreux V, Cariveau D, Chacoff NP, Dudenhoffer JH, Freitas BM, Ghazoul J, Greenleaf S, Hipolito J, Holzschuh A, Howlett B, Isaacs R, Javorek SK, Kennedy CM, Krewenka KM, Krishnan S, Mandelik Y, Mayfield MM, Motzke I, Munyuli T, Nault BA, Otieno M, Petersen J, Pisanty G, Potts SG, Rader R, Ricketts TH, Rundlof M, Seymour CL, Schuepp C, Szentgyorgyi H, Taki H, Tscharntke T, Vergara CH, Viana BF, Wanger TC, Westphal C, Williams N, Klein AM (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339(6127):1608–1611
Goodenough AE, Hart AG, Stafford R (2012) Regression with empirical variable selection: description of a new method and application to ecological datasets. PLoS ONE 7(3):e34338
Gottlieb IGW, Fletcher RJ Jr, Nunez-Regueiro MM, Ober H, Smith L, Brosi BJ (2017) Alternative biomass strategies for bioenergy: implications for bird communities across the southeastern United States. Glob Change Biol Bioenergy 9:1606–1617
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153(3):589–596
Gruenewald D (2014) Bee community responses in pine systems to future biofuel cultivation in southeastern US. Master’s thesis, Emory University
Hadley AS, Betts MG (2012) The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol Rev 87(3):526–544
Hall DM, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, Ascher JS, Baldock KC, Fowler R, Frankie G, Goulson D (2017) The city as a refuge for insect pollinators. Conserv Biol 31(1):24–29
Hanula JL, Ulyshen MD, Horn S (2016) Conserving pollinators in North American forests: a review. Nat Areas J 36(4):427–439
He HS, DeZonia BE, Mladenoff DJ (2000) An aggregation index (AI) to quantify spatial patterns of landscapes. Landscape Ecol 15(7):591–601
Holzschuh A, Steffan-Dewenter I, Kleijn D, Tscharntke T (2007) Diversity of flower-visiting bees in cereal fields: effects of farming system, landscape composition and regional context. J Appl Ecol 44(1):41–49
Homer CG, Dewitz JA, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold ND, Wickham JD, Megown K (2015) Completion of the 2011 national land cover database for the conterminous United States-representing a decade of land cover change information. Photogram Eng Remote Sens 81(5):345–354
Huang D, Zhou H, Lin L (2011) Biodiesel: an alternative to conventional fuel. Energy Proc 16:1874–1885
IPBES (2016) Summary for policymakers of the assessment report of the intergovernmental science-policy platform on biodiversity and ecosystem services on pollinators, pollination and food production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn
Jaeger JA (2000) Landscape division, splitting index, and effective mesh size: new measures of landscape fragmentation. Landscape Ecol 15(2):115–130
Kareiva P, Watts S, McDonald R, Boucher T (2007) Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316(5833):1866–1869
Karp DS, Rominger AJ, Zook J, Ranganathan J, Ehrlich PR, Daily GC (2012) Intensive agriculture erodes-diversity at large scales. Ecol Lett 15(9):963–970
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Kennedy CM, Lonsdorf E, Neel MC, Williams NM, Ricketts TH, Winfree R, Bommarco R, Brittain C, Burley AL, Cariveau D, Carvalheiro LG (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16(5):584–599
Kline KL, Coleman MD (2010) Woody energy crops in the southeastern United States: two centuries of practitioner experience. Biomass Bioenerg 34(12):1655–1666
Langholtz M, Stokes B, Eaton L (2016) 2016 Billion-ton report: advancing domestic resources for a thriving bioeconomy, vol 1: economic availability of feedstock. US Department of Energy, Oak Ridge, TN
Lennartson T (2002) Extinction thresholds and disrupted plant—pollinator interactions in fragmented plant populations. Ecology 83:3060–3072
McGarigal K, Marks BJ (1995) FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. Gen Tech Rep PNW-GTR-351. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR
Meyer B, Jauker F, Steffan-Dewenter I (2009) Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl Ecol 10:178–186
Michener C (2000) Bees of the World. Johns Hopkins University Press, Baltimore, MD
Moreira EF, Boscolo D, Viana BF (2015) Spatial heterogeneity regulates plant-pollinator networks across multiple landscape scales. PLoS ONE 10(4):e0123628
Nakazawa M (2017) fmsb: functions for medical statistics book with some demographic data. R package version 0.6.0. https://CRAN.R-project.org/package=fmsb
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120(3):321–326
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Peterson G, Allen CR, Holling CS (1998) Ecological resilience, biodiversity, and scale. Ecosystems 1(1):6–18
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rader R, Bartomeus I, Garibaldi LA, Garratt MPD, Howlett BG, Winfree R, Cunningham SA, Mayfield MM, Arthur AD, Andersson GKS, Bommarco R, Brittain C, Carvalheiro LG, Chacoff NP, Entling MH, Foully B, Freitas BM, Gemmill-Herren B, Ghazoul J, Griffin SR, Gross CL, Herbertsson L, Herzog F, Hipólito J, Jaggar S, Jauker F, Klein AM, Kleijn D, Krishnan S, Lemos CQ, Lindström SAM, Mandelik Y, Monteiro VM, Nelson W, Nilsson L, Pattemore DE, de O Pereira N, Pisanty G, Potts SG, Reemer M, Rundlöf M, Sheffield CS, Scheper J, Schüepp C, Smith HG, Stanley DA, Stout JC, Szentgyörgyi H, Taki H, Vergara CH, Viana BF, Woyciechowski M (2016) Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci USA 113(1):146–151
Reynolds C, Fletcher RJ Jr, Carneiro CM, Jennings N, Ke A, LaScaleia MC, Lukhele MB, Mamba ML, Sibiya MD, Austin JD, Magagula CN, Mahlaba T, Monadjem A, Wisely SM, McCleery RA (2018) Inconsistent effects of landscape heterogeneity and land-use on animal diversity in an agricultural mosaic: a multi-scale and multi-taxon investigation. Landscape Ecol 33:241–255
Rundlöf M, Andersson GKS, Bommarco R, Fries I, Hederström V, Herbertsson L, Jonsson O, Klatt BK, Pedersen TR, Yourstone J, Smith HG (2015) Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80
Saunders ME (2016) Resource connectivity for beneficial insects in landscapes dominated by monoculture tree crop plantations. Internat J Ag Sustain 14(1):82–99
Sorda G, Banse M, Kemfert C (2010) An overview of biofuel policies across the world. Energy Policy 38(11):6977–6988
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83(5):1421–1432
Steffan-Dewenter I, Westphal C (2007) The interplay of pollinator diversity, pollination services and landscape change. J Appl Ecol 45(3):737–741
Temple SA (1986) Predicting impacts of habitat fragmentation on forest birds: a comparison of two models. In: Verner J, Morrison ML, Ralph CJ (eds) Wildlife 2000: modeling habitat relationships of terrestrial vertebrates. University of Wisconsin Press, Madison, WI
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277(5330):1300–1302
Timilsina GR, Shrestha A (2010) Biofuels: markets, targets and impacts. Policy research working paper
VanDerWal J, Falconi L, Januchowski S, Shoo L, Storlie C (2014) SDMTools: species distribution modelling tools: tools for processing data associated with species distribution modelling exercises. R package version:1.1-221
Viana BF, Boscolo D, Neto EMGCM, Lopes L, Lopes A, Fereira P, Pigozzo CM, Primo L (2012) How well do we understand landscape effects on pollinators and pollination services? J Pollinat Ecol 7(5):31–41
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SP, Szentgyörgyi H, Tscheulin T, Vaissière BE (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78(4):653–671
Winfree R, Griswold T, Kremen C (2007) Effect of human disturbance on bee communities in a forested ecosystem. Conserv Biol 21(1):213–223
Zuur AF, Leno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14
Acknowledgements
We thank our numerous field technicians and volunteers for their assistance with data collection, and the many landowners and land managers that allowed access to their properties, especially Loncala, Inc., and Resource Management Service LLC. We thank Sam Droege (USGS) and Ismael Hinojosa (Universidad Autónoma de México) for assistance with bee identifications. We also thank the U.S. Department of Agriculture, USDA-National Institute of Food and Agriculture Initiative Grant No. 2012-67009-20090, the University of Florida’s School of Natural Resources and Environment, and Emory University’s SIRE program and Lester fund for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miljanic, A.S., Loy, X., Gruenewald, D.L. et al. Bee communities in forestry production landscapes: interactive effects of local-level management and landscape context. Landscape Ecol 34, 1015–1032 (2019). https://doi.org/10.1007/s10980-018-0651-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-018-0651-y