Abstract
Context
The landscape heterogeneity hypothesis states that increased heterogeneity in agricultural landscapes will promote biodiversity. However, this hypothesis does not detail which components of landscape heterogeneity (compositional or configurational) most affect biodiversity and how these compare to the effects of surrounding agricultural land-use.
Objectives
Our objectives were to: (1) assess the influence of the components of structural landscape heterogeneity on taxonomic diversity; and (2) compare the effects of landscape heterogeneity to those of different types of agricultural land-use in the same landscape across different taxonomic groups.
Methods
We identified a priori independent gradients of compositional and configurational landscape heterogeneity within an agricultural mosaic of north-eastern Swaziland. We tested how bird, dung beetle, ant and meso-carnivore richness and diversity responded to compositional and configurational heterogeneity and agricultural land-use across five different spatial scales.
Results
Compositional heterogeneity best explained species richness in each taxonomic group. Bird and ant richness were both positively correlated with compositional heterogeneity, whilst dung beetle richness was negatively correlated. Commercial agriculture positively influenced bird species richness and ant diversity, but had a negative influence on dung beetle richness. There was no effect of either component of heterogeneity on the combined taxonomic diversity or richness at any spatial scale.
Conclusions
Our results suggest that increasing landscape compositional heterogeneity and limiting the negative effects of intensive commercial agriculture will foster diversity across a greater number of taxonomic groups in agricultural mosaics. This will require the implementation of different strategies across landscapes to balance the contrasting influences of compositional heterogeneity and land-use. Strategies that couple large patches of core habitat across broader scales with landscape structural heterogeneity at finer scales could best benefit biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural landscapes are typically characterised as heterogeneous mosaics comprised of ‘natural’ habitat patches interspersed among patches of agricultural land-use of varying intensity (Wright et al. 2012). The effect of spatial heterogeneity on biodiversity is a fundamental ecological concept, and there is evidence that heterogeneous agricultural landscapes positively influence biodiversity (Benton et al. 2003; Fuller et al. 2004; Fahrig et al. 2011). However, especially in the developing world, heterogeneous agricultural landscapes are being increasingly developed for intensive agriculture (Kastner et al. 2012), leading to landscape simplification and biotic homogenisation (Gámez-Virués et al. 2015). The influence of landscape heterogeneity on biodiversity in agricultural landscapes is thus an important consideration in determining land-use strategies best suited for conservation.
Landscape heterogeneity, however, is an ambiguous term that can capture different forms of spatial variation operating at different scales (e.g., Kotliar and Wiens 1990; Li and Reynolds 1995). Here we operationalize heterogeneity as the variability of the landscape over space (Li and Reynolds 1995). In agricultural landscapes, heterogeneity is typically quantified across all land-cover types, because it is the interplay of variation between agriculture and other land-cover that is hypothesized to alter biodiversity (Fuller et al. 2004; Fahrig et al. 2011). While landscape heterogeneity effects on faunal diversity across all cover types have been demonstrated (Fahrig et al. 2011; Perović et al. 2015), the landscape heterogeneity that surrounds patches of natural habitat may provide similar benefits for organisms. These benefits can arise through several mechanisms, such as providing a wider range of resources or through altering the connectivity of the landscapes (Dunning et al. 1992; Malanson and Cramer 1999; Tubelis et al. 2004; Steckel et al. 2014).
While there is increasing evidence that landscape heterogeneity influences biodiversity, several gaps in our knowledge remain. First, it is poorly understood which components of landscape heterogeneity most influence biodiversity. Two components of landscape heterogeneity are formally recognised: (i) compositional heterogeneity, e.g., the diversity of land-cover types; and (ii) configurational heterogeneity, which reflects arrangement of land-cover across the landscape (Duelli 1997; Gustafson 1998; Fahrig et al. 2011). Isolating the role of compositional and configurational heterogeneity is important for the conservation of biodiversity. Each component emphasizes different conservation strategies, due to the different ways that composition (e.g., landscape complementation and supplementation) (Dunning et al. 1992) and configuration (e.g., dispersal limitation, edge effects) (Tscharntke et al. 2012) drive changes in biodiversity. Second, disentangling the effect of the components of landscape heterogeneity on biodiversity from the impact of the surrounding agricultural land-use is critical, particularly across agricultural land-uses of varying intensity. There is evidence that the diversity of cover types at the landscape scale i.e., higher landscape compositional diversity, is critical for maintaining diverse communities (Gámez-Virués et al. 2015). In contrast, a number of studies have demonstrated that agriculture reduces biodiversity and that large contiguous areas of native vegetation are required to maintain populations of native species (Schulze et al. 2004; Phalan et al. 2011; Kleijn et al. 2011). Thus, the amount and type of agriculture in the landscape may have a greater influence on biodiversity than heterogeneity itself.
The influence of landscape heterogeneity and agricultural land-use on faunal diversity is also likely to vary across taxonomic groups and spatial scales. Different taxonomic groups interact with environmental variation at different scales, based on their inherent dispersal abilities, resource requirements, and life-histories (Henle et al. 2004; Maciejewski and Cumming 2016). Investigating the effects of landscape heterogeneity and agricultural land-use on multiple taxonomic groups at different spatial scales is thus central in determining generalizable principles of the influence of landscape patterns on biodiversity in agricultural landscapes.
Our objectives were to: (1) assess the relative influence of the components of structural landscape heterogeneity, composition or configuration, on faunal diversity in savanna patches within an agricultural landscape and determine the spatial scale(s) of relevance; (2) disentangle the influence of structural landscape heterogeneity on biodiversity from the influence of variation in different agricultural land-uses i.e., intensive commercial agriculture versus subsistence agriculture; and (3) test the applicability of managing landscape heterogeneity as a general strategy in promoting diversity across different taxonomic groups. To address these objectives, we investigated biodiversity responses of multiple taxonomic groups in savanna patches embedded across a large gradient of agricultural intensification in southern Africa. We chose birds, dung beetles, ants and meso-carnivores as taxonomic groups representing a range of dispersal abilities, habitat requirements, life cycles and trophic positions. We expected that the diversity of the different taxonomic groups would display differential responses to compositional and configurational landscape heterogeneity. Configurational heterogeneity is thought to strongly affect species in terms of limiting dispersal (Tscharntke et al. 2012). Therefore, we predicted that configurational heterogeneity would influence the diversity of less mobile taxa i.e., ants and dung beetles (Roslin 2000; Ness et al. 2004). In contrast, we predicted that more mobile, dispersive taxa i.e., birds and meso-carnivores (Ferguson et al. 1983; Hockey et al. 2005), would be most influenced by compositional heterogeneity and the breadth of resources available in the complementary habitats (Dunning et al. 1992). Finally, we predicted that intensive commercial agriculture would have a large, negative influence on faunal diversity.
Methods
Study area
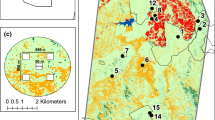
Our study was conducted in the Lowveld region of northeastern Swaziland (Fig. 1a, b), located within the Maputaland-Pondoland-Albany (MPA) biodiversity hotspot recognized for its high biodiversity and endemism (Perera et al. 2011). The region experiences mild, dry winters (8–26 °C; 0–50 mm) and hot, wet summers (15–33 °C; 200–500 mm) (Goudie and Price Williams 1983). The dominant vegetation type in the region is savanna, with fine-leaved Vachellia and Senegalia tree species on basaltic soils in the east and dense stands of broad-leafed Spirostachys africana and Combretum spp. on granitic soils in the west (Goudie and Price Williams 1983). Northeastern Swaziland is an important conservation region with 2–3% of land under formal protection, including both government- and privately-owned protected areas (Monadjem et al. 2003). Commercial agriculture in the region is almost exclusively intensive sugarcane monocultures (Esterhuizen 2015). Finally, a number of rural settlements surrounded by subsistence rain-fed agriculture occur across the region (Bailey et al. 2016). Our study area included approximately 30 km × 100 km of tropical savanna in the Lowveld region, and captured variation across the savanna vegetation type and competing land-uses.
a Map of southern Africa showing the location of Swaziland in green. b Map of Swaziland demonstrating the extent of the study area. c Major land-cover classes and the spatial arrangement of the sampling landscapes within the study area, relative to protected areas. Land-cover classes include Commercial Agriculture in red, Subsistence Agriculture in orange, Savanna in light green, Woodland in dark green and Water in blue. Buffers at 1000-m, 1500-m, 2-km, 3-km, 4-km are shown for each sampling landscape. d A typical 2-km radius study landscape demonstrating the placement of the five 50-m × 50-m survey plots in a 550-m × 550-m savanna sampling grid
Sampling landscape selection
Land-cover mapping
To quantify landscape heterogeneity in our study area, we needed a classification system that delineated the dominant and ecologically distinct land cover-types. The best publicly available dataset was the GlobeLand30 product (Jun et al. 2014), which is a 30 m × 30 m resolution land-cover dataset comprising eight land-cover classes of relevance to our study region: Cultivated, Forest, Grassland, Shrubland, Water, Wetland, Urban and Bare (Fig. S1). However, the dataset does not distinguish between different types of agricultural land-use, with no distinction made between intensive commercial (sugarcane) and subsistence agriculture (rain fed small-holder agriculture) (Fig. S1). This distinction is highly relevant for our study region. Additionally, the dataset did not distinguish savanna, but rather classifies “natural” vegetation as grassland or forest (Fig. S1). Our study region falls distinctly within the savanna biome of southern Africa, and savanna is the dominant vegetation cover. Due to these thematic issues and the lack of other fine-grained land-cover datasets with good accuracy (Jacobson et al. 2015), we developed a land-cover dataset from current satellite imagery that matched the extent of the study region and distinguished between cover classes that we deemed relevant in our landscapes (sensu Wulder and Coops 2014).
We used Google Earth Engine (GEE) (Google Earth Engine Team 2015) to perform supervised pixel-based classification on Landsat 8 annual TOA-corrected percentile composite imagery (Goldblatt et al. 2016). We used a classification and regression tree (CART) classifier to predict the occurrence of the land-cover types across the study area at a ~ 50 m × 50 m resolution (Gislason et al. 2006; Goldblatt et al. 2016). To train the classifier we provided user-defined points and polygons of known land-cover. Our final training dataset consisted of over 3600 training points and 155 training polygons from five land-cover classes: Commercial Agriculture, Subsistence Agriculture, Savanna, Woodland, and Open Water (Fig. 1c). The woodland land-cover category included woody gullies and riparian zones, but did not reflect bush encroached savanna. The correct classification of training and test datasets for each land-cover class was 80.78% for commercial agriculture, 67.92% for subsistence agriculture, 87.04% for savanna, 99.22% for water and 71.65% for woodland. The overall validity of the classification was 79.15%, and rivals that of the accuracy of the GlobalLand30 at 83.5% (Jun et al. 2014).
The two datasets were qualitatively quite similar, and both show overlap in the extent of comparable land-cover classes (Fig S1). Additionally, despite a difference between the GlobalLand30 and GEE classifications in both the spatial resolution (30 m vs. 50 m) and thematic resolution (8 vs. 5 cover classes), across multiple spatial scales for our selected study sites, ~ 30% of 35 calculated landscape metrics of seven different types were significantly correlated (r p > 0.50; p value < 0.04) (Table S1).
Sampling across composition and configuration gradients
To test the effects of the different components of landscape heterogeneity on faunal diversity in savannas, we stratified our sampling sites to capture independent gradients of compositional and configurational heterogeneity (Pasher et al. 2013). We used a moving window analysis to quantify compositional and configurational heterogeneity within a 2-km radius from each cell in the land-cover dataset for stratification purposes. We chose this radius for stratifying our sampling as being sufficiently broad-scale to capture variation in responses amongst the more mobile taxa, but still sufficiently fine-scale to be relevant for less mobile taxa (see Ekroos et al. 2016). Within the 2-km buffer we calculated: the Shannon diversity index of land-cover types (SHDI), land-cover richness (LCR), total length of edge between land-cover classes (TE), total number of patches (NP), large patch index (LPI), patch cohesion (COHESION) and patch division (DIVISION) (Supplementary material). To represent compositional heterogeneity, we chose the commonly used SHDI and LCR indices, and to represent configurational heterogeneity we used the remaining landscape metrics, which represented both edge effect and connectivity processes (Cushman et al. 2008; Fahrig et al. 2011; Schindler et al. 2013). We used principal components analysis (PCA) to derive a single descriptive orthogonal principal component representing compositional heterogeneity and a single orthogonal principal component representing configurational heterogeneity. All cells were then ranked based on their PCA value for compositional and configurational heterogeneity.
With these rankings, we used stratified sampling in a partially factorial manner to sample savanna habitats embedded across gradients of compositional and configurational heterogeneity. To do so, we first identified all savanna locations at least 550 m x 550 m in size (see Biodiversity Sampling below). From this sample, we randomly chose sampling locations (subject to land access approval) that were in the upper and lower quartiles of compositional and configurational heterogeneity (< 25 and > 75% for each combination) and supplemented this factorial sampling with locations of moderate heterogeneity (25–75% quantile). This stratification ensured that we sampled landscapes characterized by (1) both high, (2) both low, (3) high and low, and (4) moderate compositional and configurational heterogeneity. These “treatments” were longitudinally stratified by creating three blocks longitudinally across the study site to control for potential variation across our extensive ~ 3000 km2 study area. Our final selection included 15 sampled landscapes based on a 2-km radius, one replicate of each “treatment” in each longitudinal block, with an additional landscape added for birds (Fig. 1c). Only two sampling landscapes had < 1 km minimum distance between them (sites 14 and 15 Fig. 1c), and was due to logistical constraints of land access. While this design maximized the independence of sampling compositional and configurational gradients in the region, there was still a weak correlation between the compositional and configurational components in these sampled landscapes (r p = 0.48; p-value = 0.06) (Fig. S2). The moving window analysis and subsequent landscape stratification was conducted in R statistical software V 3.2.4 (R Core Team 2015) with functions from the SDMtools package (VanDerWal et al. 2014).
Biodiversity sampling
In each sampling landscape, we surveyed faunal diversity within a 550 m × 550 m grid centered in savanna. Each grid was composed of five 50 m × 50 m plots, with one plot located in the center of the grid and one at each of the four corners (Fig. 1d), totaling 75 plots across 15 landscapes (80 plots across 16 landscapes for birds). The center point of each plot was marked with a GPS. All sampling took place during the dry season between June and July of 2016. Although sampling throughout the year would have been preferable, the biodiversity sampled was representative of the communities (Fig. S3).
We surveyed birds using 10 min point counts, where every species seen or heard within a 50 m radius of the center of the plot was counted and identified. Attention was paid to avoid double counting the same individuals. Point counts were conducted by experienced observers (MDS, CR and AM), occurred between dawn and 3.5 h after sunrise, and were not conducted under rainy or windy conditions. We repeated point counts on each plot over four consecutive days, resulting in 320 point counts for the study. Each plot was surveyed once daily and we randomized the sampling order of the plots and alternated observers between plots on consecutive days, to further reduce bias. Raptors, waterbirds and birds transiting through the plots were excluded from the analysis, as these birds are selecting habitat at a larger scale than the plot.
Dung beetles and ants were collected by pitfall trapping. We placed nine pitfall traps 20 m apart in a 3 × 3 grid, with the middle pitfall trap located on the center coordinate of the plot. Each pitfall trap was constructed from a clear plastic cup (9.5 cm diameter and 12 cm depth) sunk into the ground with the cup lip flush (Brown and Matthews 2016). Bait was rolled into porous fabric and suspended over the pitfall on a piece of wire. Five of the pitfall traps were baited with fresh cow dung, while the other four were baited with a 50/50 mixture of cow dung and chicken liver to attract detritivorous dung beetles and ants (Tshikae et al. 2013). We added approximately 20 ml of dilute detergent to each cup as a non-polar solution to trap insects falling into the pitfall traps (Tshikae et al. 2013). A total of 675 pitfall traps were distributed throughout the sampling landscapes. We kept pitfall traps active for 4 days, and emptied and rebaited after 48 h. We counted and sorted all dung beetles and ants into morpho-species under a dissecting microscope. The classification of individuals into morpho-species was confirmed by experts (Francois Roets and CNM).
To survey meso-carnivores we used motion-sensor camera traps (Truth Cam 35, Primos, Springfield, USA). We defined meso-carnivores as any mammalian predator in the order Carnivora with a mass < 15 kg (Prugh et al. 2009). We placed three cameras on each plot; two were situated near the centre along separate game trails and not baited, the third was positioned in a far corner of the plot and baited with a chicken neck. The bait was wrapped in chicken wire and secured to the ground with metal stakes, and replaced every second day or more frequently when the bait was removed by an animal. The cameras were set to record for four consecutive days, resulting in 900 trap nights from the placement of 225 cameras across the entire study. We examined the photographs to identify and determine the presence of meso-carnivore species in each plot.
To compare the effects of landscape heterogeneity on faunal diversity in savanna, we calculated the species richness per plot for each taxonomic group. We examined species rarefaction curves for each taxonomic group (Fig. S3), which indicated that birds and meso-carnivores were slightly under-sampled. We thus estimated species richness for these two taxonomic groups with a first-order jackknife estimator. We also calculated Shannon’s diversity index per plot for birds, beetles and ants for which we had the relative abundance, to test for effects on community evenness (Table 1). The Hellinger transformation was applied to abundance data prior to analysis (Borcard et al. 2011). All diversity metrics were calculated with functions from the vegan package (Oksanen et al. 2016) in R statistical software V 3.2.4 (R Core Team 2015).
Landscape metrics
The interpretation of the effects of principal components of configurational and compositional heterogeneity is not ecologically intuitive across different scales. To aid interpretation, we chose to model the effects of individual landscape metrics on faunal diversity. We used Shannon diversity index of land-cover types (SHDI) as the single metric of compositional heterogeneity, as it is commonly used to represent compositional landscape diversity (Cushman et al. 2008). We calculated SHDI at five different spatial scales, with buffer radii of 1-, 1.5-, 2-, 3- and 4-km centered on each landscape (Table 1; Fig. 1c). We then determined which of the previously considered configurational heterogeneity metrics (TE, NP, LPI, COHESION or DIVISION) was the least correlated with SHDI and again we calculated these for each spatial scale. The total number of patches (NP) was consistently the least correlated metric across all spatial scales (r p = 0.67–0.48) (Table S2), and was thus chosen to represent configurational heterogeneity (Table 1). Finally, to contrast the effects of landscape heterogeneity on faunal diversity with the influence of variation in agricultural land-use in the same landscape, we determined the amount of agriculture in the buffer. Specifically, we calculated the percentage cover of two different agricultural land-use types i.e., commercial sugarcane agriculture (AG) and subsistence agriculture (SUB) at each spatial scale by dividing the total area under each agricultural land-use by the total area of the buffer. All landscape metric calculations were performed in Fragstats software V 4.2 using the eight neighbor rule for delineating patches (McGarigal et al. 2012).
Data analysis
We used mixed effects models to test the response of each taxon to the fixed effects of compositional (SHDI) and configurational (NP) heterogeneity and agricultural land-use i.e., percent cover of commercial (AG) and subsistence (SUB) agriculture at each scale. Each of the 75 sampling plots (80 for birds) was treated as a sampling unit and we included sampling grid as a random effect to account for spatial dependence within the sampling grids. For birds, beetles and ants, the metrics of both richness and diversity were approximated by a normal distribution, so we fitted linear mixed effects models (LMM). To model species richness for meso-carnivores, we used a negative binomial distribution with a log link function, as the response variable was non-normal and over-dispersed when fitted to a Poisson distribution. For each response variable (richness and diversity) per taxonomic group we tested a candidate set of 21 models comprising the fixed effect of either SHDI, NP, AG or SUB at each spatial scale (1-, 1.5-, 2-, 3- and 4-km) against a null model (Table S3; Fig. S4). Spatial autocorrelation between the sampling grids was found to be a concern in our dataset for all taxa (Moran’s I = 0.28–0.68; p-value < 0.001), except for birds (Moran’s I = 0.12; p-value = 0.09). We applied a Matérn correlation matrix to the random effect of sampling grid to all models (including birds), which removed the spatial autocorrelation from the model residuals (Royle and Wikle 2005). We conducted our generalized mixed effects models in R statistical software V 3.2.4 (R Core Team 2015) with the nlme package (Pinheiro et al. 2017) and used the spaMM package (Rousset and Ferdy 2014) to fit the autocorrelation structure. All predictors were scaled and centered so that model estimates were comparable.
We compared all models within each candidate set using the Akaike’s Information Criterion corrected for small sample size (AICc) (Burnham and Anderson 2002). We considered the model with the lowest AICc score as the best model, but considered all models that fell within < 2 AICc of the best model as competing models. If the best model fell within < 2 AICc of the null model, then these models were considered to have predictive power similar to the null (Burnham and Anderson 2002). The parameters of competing models were considered significant if their 95% confidence interval did not overlap zero (Table S3). Additionally, we tested the additive and interactive effects for the fixed effects of SHDI and NP for spatial scales determined most appropriate from the model selection. No additive or interactive model showed an improvement on the AICc score of the original best model for that scale, and we thus excluded them from our analysis for simplicity.
Finally, to look for a generalizable trend across multiple taxonomic groups we tested the effects of compositional (SHDI) and configurational (NP) heterogeneity and agricultural land-use (AG and SUB) across all taxa combined at each scale. We fitted linear mixed effects models as species richness and Shannon diversity (H’) approximated a normal distribution and included sampling grid corrected for spatial autocorrelation as a random effect. We included taxonomic group as an additional random intercept (an additive effect accounting for inherent differences in richness and diversity between taxa) and as a random coefficient (emphasizing that the effect of a particular landscape variable can differ between taxa) (Hurst et al. 2013). The inclusion of a random coefficient allows for both marginal (overall) and conditional (taxon-specific) predictions about the response of faunal diversity to landscape heterogeneity (Hurst et al. 2013). For each response variable (richness and diversity), we tested the same 21 candidate models comprising the fixed effect of either SHDI, NP, AG or SUB at each spatial scale (1–4 km) against a null model. Meso-carnivores were not included in the combined model, as they did not show a response in the individual models and the response variable was highly non-normally distributed. Again, we used a comparison of the AICc values, as described above, to determine the best models for both species richness and diversity, and profiled the 95% confidence intervals to determine parameter significance (Table S4).
Results
We recorded a total of 165 species in the four taxonomic groups, with birds being the most species-rich group, including 86 species and accounting for 52% of all recorded species (Table 1). Ants displayed the next highest species richness at 43 morpho-species, followed by dung beetles with 30 recorded species and meso-carnivores with six recorded species. Ants and dung beetles tended to occur in patchy distributions with large numbers on certain plots, affecting community evenness and hence lower values of diversity (Table 1).
Responses to landscape heterogeneity and land-use
All best models of species richness for birds, dung beetles and ants included an exclusive and significant effect of compositional heterogeneity (Shannon diversity index of land-cover types, SHDI) (Table 2; Fig. 2). A significant positive response to increasing SHDI occurred for species richness of birds and ants, and the best fitting model for both taxa was at the 1-km scale (Fig. 2c, d). Additionally, a significant response to increasing SHDI was demonstrated by birds at the 1.5-km scale and by ants at both the 1.5- and 2-km scales. Species richness of dung beetles showed a significant negative response to increasing SHDI, with the best fitting model at the 1.5-km scale (Fig. 2b), and a significant competing model at the 2-km scale. Only in one model did configurational heterogeneity (number of patches, NP) demonstrate a significant effect on species richness (ants), and was positively correlated with ant richness at the 1-km scale. The percentage cover of commercial agriculture (AG) at the 1-km scale was a competing model explaining bird richness and demonstrated a significant positive effect. The percentage cover AG at the 3-km scale was a competing model explaining dung beetle richness, and had a significant negative effect. Species richness of meso-carnivores was not significantly affected by landscape heterogeneity or the amount of agricultural land-use across all spatial scales, and no model showed an improvement of ≥ 2 AICc units on the null (Table 2). The best model explaining species richness of all taxa combined was the null model. The conditional estimates of species richness in these models were highly varied in magnitude and extent, and indicated a tendency for taxonomic groups to vary in their response.
a Map of Swaziland demonstrating the location of the study area and the extent of the savanna land-cover class in green. Protected areas boundaries are shown in black and all other land-cover classes are represented in white. b Heatmap of predicted dung beetle species richness in relation to increasing Shannon landscape diversity (SHDI) at the 1.5-km scale for all savanna pixels. c Heatmap of predicted bird species richness in relation to increasing Shannon landscape diversity (SHDI) at the 1-km scale for all savanna pixels. d Heatmap of predicted ant species richness in relation to increasing Shannon landscape diversity (SHDI) at the 1-km scale for all savanna pixels. *Predictions of species richness are based on the best model for each taxonomic group and extrapolated across savanna pixels only. Note the contrasting effects of SHDI on different taxonomic groups
For Shannon diversity of birds, SHDI at the 1-km scale was again the best model and demonstrated a significant positive effect (Table 3). Shannon diversity of ants was positively and significantly affected by the percentage cover of commercial agriculture (AG) at the 2-, 1- and 1.5-km scales, with the best model at the 2-km scale. The competing model set explaining Shannon diversity of dung beetles contained the null model. The best model explaining Shannon diversity of all taxa combined was again the null model.
Discussion
Compositional heterogeneity was consistently the best predictor of faunal diversity metrics; however, the magnitude, direction and scale(s) of influence varied between the taxonomic groups. We also found that commercial sugarcane influenced measures of bird and ant diversity. There was no effect of landscape heterogeneity on combined taxonomic diversity; these differences in response to landscape structure contradict promoting landscape heterogeneity as a general strategy for diversity conservation in agricultural landscapes.
An important caveat in describing the effects of landscape heterogeneity on faunal diversity is the inter-related nature of the compositional and configurational components and the landscape metrics used to describe them. In our study area, Shannon diversity of land-cover types was correlated with total amount of edge, large patch index and patch cohesion (Table S1). This pattern suggests that what we perceive as a response of biodiversity to compositional heterogeneity may be a more complex response to the type, amount and connectivity of habitat. Thus, despite establishing a priori a near independent gradient of compositional and configurational heterogeneity in our study landscape, it was still difficult to reliably test for a dominant effect of either component. This pattern is not limited to our study landscape and has been demonstrated before in both real and simulated landscapes (Griffith et al. 2000; Fortin et al. 2003; Proulx and Fahrig 2010).
Additionally, having used a land-cover dataset with only five land-cover classes, the gradient of compositional heterogeneity was limited. For example, the broad classifications of savanna and subsistence farming overlook inherent compositional heterogeneity within these cover types. Savannas are dynamic ecosystems where vegetation structure and composition are strongly affected by drivers such as fire and herbivory (Archibald et al. 2005; Asner et al. 2009). In recent years changes in these key drivers have been linked to severe bush encroachment in the region, which is negatively affecting species diversity and may have contributed to some of our observed biodiversity patterns (Roques et al. 2001; Sirami and Monadjem 2012). A dataset that delineates these broad land-cover classifications into multiple ecologically distinct land-covers and captures the dynamic heterogeneity of the savanna land-cover class may be helpful. However, given the correlation between our land-cover dataset and public access datasets, we are confident that the effects of landscape composition on taxonomic diversity should be consistent despite the few cover classes.
Responses to landscape heterogeneity and land-use
Bird and ant richness responded positively to increasing compositional heterogeneity between the 1- and 2-km scales. A greater breadth of resources made available through landscape complementarity at these scales (Dunning et al. 1992) was likely more influential on their richness than the shape and arrangement of those resources. More specifically, birds and ants appeared to benefit from having commercial agriculture in the landscape, as higher compositional heterogeneity was positively correlated with the amount of sugarcane at these scales (Table S5). This was further supported by a positive response of bird richness to increasing amounts of commercial agriculture at the 1-km scale. For birds this may be related to food availability in the margins of agricultural fields and increased water availability from irrigation (Wilson et al. 1999). Ants may benefit from altered soil properties, resulting from increased nutrient rich runoff from irrigation (Cerdà et al. 2009). However, at the scales considered significant (1–2 km) the amount of sugarcane in the landscape was never greater than 23% of total area. Thus, whilst having intensive commercial agriculture as a component of a landscape appears to positively modify resource availability for several taxonomic groups, it is likely that as the area under intensive cropping increases and landscapes become more homogenous these benefits may no longer be realized (Hurst et al. 2014).
Ant richness was also positively affected by landscape configurational heterogeneity (number of patches) at the 1-km scale. This fits with our prediction that higher configurational heterogeneity should positively affect taxonomic groups with lower dispersal abilities (Tscharntke et al. 2012). In landscapes with high levels of configurational landscape heterogeneity the spatial proximity between patches decreases, enabling colonization of neighbouring patches and the coexistence of many species (Holling 1973; Perović et al. 2015). For less mobile taxa, it is probable that the benefits of landscape compositional heterogeneity may only be realised if the landscape is equally configurationally diverse.
Dung beetle richness decreased with increasing compositional heterogeneity at 1.5- and 2-km scales. This relationship likely occurred because in our landscapes increasing compositional heterogeneity was correlated with increased land under sugarcane farming (Table S5), which is generally not suitable for large herbivorous dung producers. This inference was also supported by a significant negative response to the amount of sugarcane farming at the 3-km scale. Dung beetle communities thrive in areas with abundant and diverse herbivores (Nichols et al. 2008, 2009), a situation commonly found in African savannas (Du Toit and Cumming 1999). Thus, for certain taxonomic groups increasing compositional heterogeneity may adversely affect diversity by decreasing core habitat and the resources it supports (Fahrig 2013).
Bird species diversity was positively influenced by landscape compositional heterogeneity at the 1-km scale. Additionally, ant diversity responded positively to increasing amounts of intensive commercial agriculture at scales between 1 and 2 km. Diverse biological communities can promote resilience through mechanisms of redundancy and complementarity (Tscharntke et al. 2005). Hence, resource complementarity facilitated by moderate amounts of commercial agriculture may positively influence ecosystem resilience by promoting diversity of certain key taxonomic groups.
Finally, meso-carnivore richness showed no significant response to landscape metrics at any scale. Some meso-carnivores species have been shown to benefit from agriculture and human settlement due to an increased prey base (DeVault et al. 2011; Schuette et al. 2013). In our study system, meso-carnivores may have been able to disperse easily throughout the relatively undeveloped landscape. Thus, meso-carnivores may simply not respond at the scales we considered. Alternatively, their diversity could have been influenced by other processes such as meso-predator release (i.e., the removal of top-predators) or differences in vegetation structure (Chalfoun et al. 2002). The lack of an observed response may also stem from low statistical power associated with the observed low total species richness of this taxonomic group.
Compositional heterogeneity was generally a better predictor of faunal diversity than the type and amount of agricultural land-use. Compositional heterogeneity, however, is related to variation in the underlying land-uses in the landscape, and is perhaps a more useful measure of the influence of landscape structure on biodiversity as it encompasses variation in the amount of multiple land-uses at the same time. The strong influence of compositional heterogeneity in our study suggests that taxonomic diversity is indeed responding to variation among multiple competing land-uses, and hints that mechanisms such as resource complementation and supplementation may be driving biodiversity patterns in our system.
Conservation implications
To maximise taxonomic diversity in agricultural mosaics, there is a need to determine how best to integrate compositional diversity of land-uses at different spatial scales. Land-use conservation strategies need to be multi-scaled when considering multiple taxa (Ekroos et al. 2016), and perhaps require the implementation of different strategies at different scales to balance the contrasting influences of compositional heterogeneity. These strategies may include the integration of moderate amounts of agriculture amongst other land-covers to bolster resource complementarity and the preservation of small patches of habitat to facilitate colonisation at finer scales. However, at larger scales (~ 2-km) it may be necessary to have landscapes that support core areas of natural habitat, ensuring the persistence of large patches of savanna habitat for species that are sensitive intensive agriculture (i.e., herbivores, dung beetles). Not only may such multi-scale strategies better capture taxonomic diversity, they have the potential benefit of preserving different ecological processes that are known to increase population persistence across multiple scales (Ekroos et al. 2016).
Conclusions
Our results highlight that the effects of landscape heterogeneity can vary among functionally diverse taxonomic groups and can operate at different scales. Scale-dependent and taxon-dependent responses like these may provide challenges for implementing conservation strategies aimed at promoting biodiversity in agricultural landscapes, yet understanding this complexity may help guide more effective conservation biodiversity in working landscapes.
References
Archibald S, Bond WJ, Stock WD, Fairbanks DHK (2005) Shaping the landscape: fire-grazer interactions in an African savanna. Ecol Appl 15:96–109
Asner GP, Levick SR, Kennedy-Bowdoin T, Knapp DE, Emerson R, Jacobson J, Colgan MS, Martin RE (2009) Large-scale impacts of herbivores on the structural diversity of African savannas. Proc Natl Acad Sci USA 106:4947–4952
Bailey KM, McCleery RA, Binford MW, Zweig C (2016) Land-cover change within and around protected areas in a biodiversity hotspot. J Land Use Sci 11:154–176
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer Science & Business Media, Berlin
Brown GR, Matthews IM (2016) A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol Evol 6:3953–3964
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media, New York
Cerdà A, Jurgensen M, Bodi M (2009) Effects of ants on water and soil losses from organically-managed citrus orchards in eastern Spain. Biologia (Bratisl) 64:527–531
Chalfoun AD, Thompson FR, Ratnaswamy MJ (2002) Nest predators and fragmentation: a review and meta-analysis. Conserv Biol 16:306–318
Cushman SA, McGarigal K, Neel MC (2008) Parsimony in landscape metrics: Strength, universality, and consistency. Ecol Indic 8:691–703
DeVault TL, Olson ZH, Beasley JC, Rhodes OE (2011) Mesopredators dominate competition for carrion in an agricultural landscape. Basic Appl Ecol 12:268–274
Du Toit JT, Cumming DHM (1999) Functional significance of ungulate diversity in African savannas and the ecological implications of the spread of pastoralism. Biodivers Conserv 8:1643–1661
Duelli P (1997) Biodiversity evaluation in agricultural landscapes: an approach at two different scales. Agric Ecosyst Environ 62:81–91
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Ekroos J, Ödman AM, Andersson GKS, Birkhofer K, Herbertsson L, Klatt BK, Olsson O, Olsson PA, Persson AS, Prentice HC, Rundlöf M, Smith HG (2016) Sparing land for biodiversity at multiple spatial scales. Front Ecol Evol 3:1–11
Esterhuizen D (2015) The supply and demand of sugar in Swaziland. United states department of agriculture foriegn agricultural service GAIN report
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Ferguson JWH, Nel JAJ, de Wet MJ (1983) Social organization and movement patterns of Black-backed jackals Canis mesomelas in South Africa. J Zool 199:487–502
Fortin M-J, Boots B, Csillag F, Remmel TK (2003) On the role of spatial stochastic models in understanding landscape indices in ecology. Oikos 102:203–212
Fuller RJ, Hinsley SA, Swetnam RD (2004) The relevance of non-farmland habitats, uncropped areas and habitat diversity to the conservation of farmland birds. Ibis (Lond 1859) 146:22–31
Gámez-Virués S, Perović DJ, Gossner MM, Börschig C, Blüthgen N, De Jong H, Simons NK, Klein AM, Krauss J, Maier G, Scherber C (2015) Landscape simplification filters species traits and drives biotic homogenization. Nat Commun 6:8568
Gislason PO, Benediktsson JA, Sveinsson JR (2006) Random Forests for land cover classification. Pattern Recognit Lett 27:294–300
Goldblatt R, You W, Hanson G, Khandelwal A (2016) detecting the boundaries of urban areas in india: a dataset for pixel-based image classification in Google Earth Engine. Remote Sens 8:634
Google Earth Engine Team (2015) Google Earth Engine: a planetary-scale geospatial analysis platform
Goudie AS, Price Williams D (1983) The Atlas of Swaziland. Swaziland National Trust Commission, Mbabane
Griffith JA, Martinko EA, Price KP (2000) Landscape structure analysis of Kansas at three scales. Landsc Urban Plan 52:45–61
Gustafson EJ (1998) Quantifying landscape spatial pattern: what is the state of the art? Ecosystems 1:143–156
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Hockey P, Dean W, Ryan P (2005) Roberts birds of Southern Africa. John Voelcker Bird Book Fund, Cape Town
Holling C (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Hurst ZM, McCleery RA, Collier BA, Fletcher RJ Jr, Silvy NJ, Taylor PJ, Monadjem A (2013) Dynamic edge effects in small mammal communities across a cconservation-agricultural interface in Swaziland. PLoS One 8:e74520
Hurst ZM, McCleery RA, Collier BA, Silvy NJ, Taylor PJ, Monadjem A (2014) Linking changes in small mammal communities to ecosystem functions in an agricultural landscape. Mamm Biol-Zeitschrift für Säugetierkd 79:17–23
Jacobson A, Dhanota J, Godfrey J, Jacobson H, Rossman Z, Stanish A, Walker H, Riggio J (2015) A novel approach to mapping land conversion using Google Earth with an application to East Africa. Environ Model Softw 72:1–9
Jun C, Ban Y, Li S (2014) China: open access to Earth land-cover map. Nature 514:434
Kastner T, Rivas MJI, Koch W, Nonhebel S (2012) Global changes in diets and the consequences for land requirements for food. Proc Natl Acad Sci 109:6868–6872
Kleijn D, Rundlöf M, Scheper J, Smith HG, Tscharntke T (2011) Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol Evol 26:474–481
Kotliar NB, Wiens JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59:253–260
Li H, Reynolds JF (1995) On definition and quantification of heterogeneity. Oikos 73:280–284
Maciejewski K, Cumming GS (2016) Multi-scale network analysis shows scale-dependency of significance of individual protected areas for connectivity. Landsc Ecol 31:761–774
Malanson GP, Cramer BE (1999) Landscape heterogeneity, connectivity, and critical landscapes for conservation. Divers Distrib 5:27–39
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS: spatial pattern analysis program for categorical and continuous maps. University of Massachusetts, Amherst
Monadjem A, Boycott RC, Parker V, Culverwell J (2003) Threatened vertebrates of Swaziland. In: Swaziland red data book: fishes, amphibians, reptiles, birds and mammals. Ministry of Tourism, Environment and Communications, Mbabane
Ness JH, Bronstein JL, Andersen AN, Holland JN (2004) Ant body size predicts dispersal distance of ant-adapted seeds: implications of small-ant invasions. Ecology 85:1244–1250
Nichols E, Gardner TA, Peres CA, Spector S (2009) Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118:481–487
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME, Network TS (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MH, Wagner H (2016) Vegan: community ecology package. R package V2.4-4
Pasher J, Mitchell SW, King DJ, Fahrig L, Smith AC, Lindsay KE (2013) Optimizing landscape selection for estimating relative effects of landscape variables on ecological responses. Landsc Ecol 28:371–383
Perera SJ, Ratnayake-Perera D, Procheş Ş (2011) Vertebrate distributions indicate a greater Maputaland-Pondoland-Albany region of endemism. S Afr J Sci 107:52–66
Perović D, Gámez-Virués S, Börschig C, Klein AM, Krauss J, Steckel J, Rothenwöhrer C, Erasmi S, Tscharntke T, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Phalan B, Onial M, Balmford A, Green RE (2011) Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333:1289–1291
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: linear and nonlinear mixed effects models. R package version 3.1-131
Proulx R, Fahrig L (2010) Detecting human-driven deviations from trajectories in landscape composition and configuration. Landsc Ecol 25:1479–1487
Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS (2009) The rise of the mesopredator. Bioscience 59:779–791
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roques KG, O’Connor TG, Watkinson AR (2001) Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J Appl Ecol 38:268–280
Roslin T (2000) Dung beetle movements at two spatial scales. Oikos 91:323–335
Rousset F, Ferdy J-B (2014) Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography (Cop) 37:781–790
Royle JA, Wikle CK (2005) Efficient statistical mapping of avian count data. Environ Ecol Stat 12:225–243
Schindler S, von Wehrden H, Poirazidis K, Wrbka T, Kati V (2013) Multiscale performance of landscape metrics as indicators of species richness of plants, insects and vertebrates. Ecol Indic 31:41–48
Schuette P, Wagner AP, Wagner ME, Creel S (2013) Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Conserv 158:301–312
Schulze CH, Waltert M, Kessler PJ, Pitopang R, Veddeler D, Mühlenberg M, Gradstein SR, Leuschner C, Steffan-Dewenter I, Tscharntke T (2004) Biodiversity indicator groups of tropical land-use systems: comparing plants, birds, and insects. Ecol Appl 14:1321–1333
Sirami C, Monadjem A (2012) Changes in bird communities in Swaziland savannas between 1998 and 2008 owing to shrub encroachment. Divers Distrib 18:390–400
Steckel J, Westphal C, Peters MK, Bellach M, Rothenwoehrer C, Erasmi S, Scherber C, Tscharntke T, Steffan-Dewenter I (2014) Landscape composition and configuration differently affect trap-nesting bees, wasps and their antagonists. Biol Conserv 172:56–64
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol Lett 8:857–874
Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L, Batary P, Bengtsson J, Clough Y, Crist TO, Dormann CF, Ewers RM (2012) Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biol Rev Camb Philos Soc 87:661–685
Tshikae BP, Davis ALV, Scholtz CH (2013) Does an aridity and trophic resource gradient drive patterns of dung beetle food selection across the Botswana Kalahari? Ecol Entomol 38:83–95
Tubelis DP, Cowling A, Donnelly C (2004) Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biol Conserv 118:353–364
VanDerWal J, Falconi L, Januchowski S, Shoo L, Storlie C (2014) SDMTools: species distribution modelling tools: tools for processing data associated with species distribution modelling exercises. R package version 1.1-221
Wilson JD, Morris AJ, Arroyo BE, Clark SC, Bradbury RB (1999) A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric Ecosyst Environ 75:13–30
Wright HL, Lake IR, Dolman PM (2012) Agriculture-a key element for conservation in the developing world. Conserv Lett 5:11–19
Wulder MA, Coops NC (2014) Satellites: make Earth observations open access. Nature 513:30–31
Acknowledgements
We are grateful to all the field assistants who helped with the collection of the data and to the land owners who granted permission to work on their properties. We also acknowledge considerable support from All Out Africa and the Savanna Research Centre. This research was funded by an NSF ISE Grant (No. 1459882) and the College of Agriculture and Life Science at the University of Florida.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10980_2017_595_MOESM1_ESM.docx
Descriptions of landscape metrics. Table S1: Correlations between landscape metrics from land-cover datasets. Table S2: Correlations between landscape metrics. Table S3: Model selection results for each taxon. Table S4: Model selection results for all taxa combined. Table S5: Correlation between landscape diversity and the amount of different land covers. Figure S1: Comparison between land-cover datasets. Figure S2: Correlation plot of heterogeneity components. Figure S3: Species accumulation curves. Figure S4: Graphical model selection. Supplementary material 1 (DOCX 682 kb)
Rights and permissions
About this article
Cite this article
Reynolds, C., Fletcher, R.J., Carneiro, C.M. et al. Inconsistent effects of landscape heterogeneity and land-use on animal diversity in an agricultural mosaic: a multi-scale and multi-taxon investigation. Landscape Ecol 33, 241–255 (2018). https://doi.org/10.1007/s10980-017-0595-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-017-0595-7