Abstract
Domestic refrigerators have become an indispensable part of the modern life. Since they are connected to the electric mains and operate throughout the day and year, they consume a lot of energy. Several experimental studies show that the addition of phase change material in the refrigerators at various locations improves the energy efficiency of the refrigerators. In this work, an experimental study was conducted to explore and reveal the improvement in energy efficiency of a small-scale refrigeration system when PCM was applied between the evaporator coil and the insulation. It was observed that the addition of PCM for a thickness of 0.03 m could reduce the heat ingress inside the evaporator by 15–19%. Moreover, the compressor ON cycle time and the temperature fluctuation inside the freezer cabinet were also relatively lesser. The per day energy consumption was reduced by 15.7–17.3% and the improvement in COP was found to be 17.4% with the addition of PCM. The refrigeration system was able to retain its desired temperature level for a period of 5 h even after the power supply was switched OFF. This idea of incorporating PCM between coil and insulation could be extended to household refrigerators so as to reduce the energy consumption. This would come handy in case of power outages which are very common in places having low grid reliability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrical energy is the lifeline for the modern world, and it is one of the important development parameters by which the growth of any country is gauged. The domestic sector is the second biggest consumer of electrical energy, and the energy consumption of this sector depends on many factors such as population, electricity price, installed capacity, gross electricity generation, total subscribership, and household income. The domestic refrigerator in the modern houses accounts for about 15–20% of total domestic electric usage [1] The energy consumption of refrigerators in India is estimated to be 13.2 TWh, and it is predicted to be 65.5 TWh by the year 2030 [2]. It has been estimated that there is one domestic refrigerator for every six people on earth, which corresponds to approximately 6% of the total electrical energy produced [3]. In order to reduce greenhouse gas emissions from these appliances, governments have adapted mandatory energy standards. The manufacturers are also forced to comply with the energy labeling program to regulate the domestic refrigerator market by setting appliance performance targets. Since the refrigerator is connected to the electrical outlet day and night, the improvement in the energy efficiency of the refrigerator assumes importance [4].
The scope of improving the energy efficiency of a refrigerator covers a wide area of research since each part of the refrigeration system has its own technical complexity. The thrust for development is on three main areas characterized by improvement of compressor efficiency, improvement of insulation in refrigerator cabinet and door, and improvement in heat transfer performance of the evaporator and condenser [5].
In the case of conventional refrigerators, reciprocating compressors are used because of their cost effectiveness. But more than 80% of the energy consumed by the refrigerator is spent in the compressor [4]. Experimental studies indicate that the highest exergy destruction takes place in the compressor [6]. For reducing the exergy destruction, the energy consumption has to be reduced [7]. This necessitates the modification of the reciprocating compressor design which is basically an ON/OFF type, and they require higher starting current. The energy consumption is more due to the frequent start-ups of the compressor. The problem could be avoided by using variable-speed compressors and linear compressors, but their higher cost prohibits their use in domestic refrigeration system. These variable-speed compressors are characterized by low starting current, lower noise and vibration, and high COP, and hence, these compressors are energy-efficient [8].
Improving the insulation effect of the refrigerator walls and door is another method to augment the energy efficiency of the refrigeration system. Conventionally, polyurethane foam is used for insulation. If the thermal insulation effect is increased, it would prolong the low temperature inside the compartment, and this reduces the frequency of compressor start-ups resulting in energy savings. Vacuum insulated panels have also been tried out to reduce the heat ingress. Vacuum insulated panels have comparatively better insulation effect than the polyurethane foam, and for the same thickness, the vacuum insulated panel has a thermal resistance which is four times higher than that of polyurethane foam [9]. However, vacuum insulated panels are not commonly used because of the difficulty in fabrication and cost.
It is reported that the above methods of improving the energy efficiency namely changing the compressor design and insulation of the refrigerator have practical limitations in their implementation and are costly.
Improving the efficiency of the heat exchangers (evaporator and condenser) has gained more interest in the recent years. Much work has been done in improving the heat transfer performance of these heat exchangers by way of incorporating liquid-suction line heat exchangers (superheating coil) and using loop heat pipe-based evaporator, along with micro-fin tubes for both evaporator and condenser besides the application of phase change materials [10]. PCMs can be used either on the hot side, which is the condenser, or on the cold side, which is the evaporator in the refrigerators.
Using PCM in the evaporator as a latent heat thermal energy storage system could be a viable proposition for performance improvement of a domestic refrigerator by enhancing its heat transfer [11]. Maltini et al. [12] experimented with sodium chloride–water mixture as a thermal storage system in a household refrigerator and found that it could behave as a mini temperature reservoir and minimized the temperature variations resulting in a better preservation of the food. Based on previous studies, it is reported that an improvement of COP of the order of 2–74% can be achieved by the application of PCM at the evaporator and the improvement depends on factors like ambient temperature, type of PCM, and thermal load [13]. PCMs can be arranged at different locations in the refrigerators, and the direct contact of PCM with a naturally cooled evaporator is advantageous. This arrangement results in higher vapor pressure of the refrigerant and evaporator temperature during the period when the PCM begins to change its phase. This results in a higher refrigerant density, and as a result, cooling capacity increases [14]. Similar results were reported for cases where evaporator coils were immersed in PCM. If the PCM is applied on the inside of the evaporator, the melting point must fall within the thermostat temperature range of operation. When PCM is used around evaporator coils, it is found that the enhancement of the system performance is higher. This is due to the reduction of compressor start/stop frequency and higher system COP because of high evaporation temperature during phase change [13]. Proper phase change temperature could be selected by means of a material with suitable thermodynamic properties [15]. Methods like thermal lattice Boltzmann model (TLBM), entropy minimization method (EGMM), and 3P have been devised to analyze the effect of the enclosure shapes on the performance of PCMs [16]. Numerical simulations using computational fluid dynamics and ANSYS Fluent software have been undertaken to compare the household refrigerator temperature stability with different PCMs incorporated in the storage compartment [3]. Increasing the thermal inertia of a thermal system by the addition of PCM reduces the frequency of ON/OFF cycle of the compressor which leads to a realization of energy savings [17]. In the earlier works, PCMs had been cladded inside the freezer compartment and the improvement in energy efficiency was observed. In few studies, the evaporator coil was submerged inside the PCM before studying the system performance. However, the insulation effect of PCM when it is kept between the evaporator coil and the conventional polyurethane insulation has not been reported in the reviewed literature [18]. Therefore, the present study has been focused on this aspect.
Experimental setup
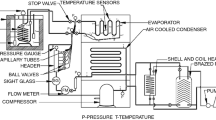
The experimental setup shown in Fig. 1 consists of a hermitically sealed reciprocating compressor, serpentine-type natural convection air-cooled condenser, clinching-type evaporator, and a capillary expansion device. The device was suitably modified to study the effects of using phase change material (PCM) in the evaporator as shown in Fig. 2. The PCMs selected for this application are ammonium bicarbonate solution(PCM1) and sodium and ammonium chloride solution (PCM2) having freezing temperatures of 0 °C and − 16 °C, respectively, which represent the temperature inside the chiller and freezer, respectively. The fore-mentioned PCMs were selected for testing because in the literature reviewed it was found that extensive research work had been carried out with the eutectics when compared with other PCMs based on paraffin and water. The selected PCMs are readily available, and they are used in many practical applications. The properties of the PCMs are given in Table 1.
The freezer compartment of the refrigerator was fabricated, and the encapsulated PCM was kept between the evaporator coil and the polyurethane insulation. Several options like stainless steel, aluminum, galvanized iron, and plastics are available for the encapsulation of the PCM. Here, a stainless steel sheet was used for the encapsulation of PCM. The mass of PCM was calculated to be 1.714 kg and 1.729 kg for ammonium bicarbonate solution and sodium and ammonium chloride solution, respectively, and the thickness of the PCM layer was 0.03 m. The space between the PCM layer and the outer wooden cabinet was filled with polyurethane foam of thickness 0.07 m having a heat transfer coefficient of 0.027 W m−2 K−1. The overall heat transfer coefficient of the composite wall of SS-PCM-SS-insulation—wooden cabinet wall was calculated as 0.3 W m−2 K−1. An electronic thermostat control was provided where the upper and lower temperature threshold limits were set with an accuracy level of approximately ± 0.5 °C to control the temperature inside the evaporator storage space. Compound pressure gauges of range − 2 to 20 bar and an accuracy of ± 0.02 bar are installed in the compressor inlet, compressor outlet, capillary inlet, and capillary outlet. Film-type RTD temperature sensors having sensing range of about − 70 °C to 250 °C with an accuracy of ± 0.1 °C were installed for monitoring the temperatures at various locations both within and outside the freezer compartment, inside the PCM, and inlets and outlets of the compressor and capillary. A wattmeter was also provided to measure the power consumed by the compressor. The whole arrangement was placed inside a temperature-controlled room of dimensions 3.4 m × 3 m × 4 m which was capable of maintaining the desired atmospheric temperature of 32 °C through the use of an air conditioner and a heater. To evaluate the actual refrigeration capacity of the evaporator coil in the modified refrigeration setup, the evaporator space itself was made as a calorimeter by making suitable modifications in the evaporator door by providing a stirrer and an immersion electric heater having a rating of 100 W which in turn was controlled by a dimmerstat. The space was filled with ethylene glycol water (50:50) brine which is considered as a secondary refrigerant. The specifications of the components and the operating conditions used in the experimental setup are given Tables 2 and 3, respectively.
Experimental procedure
The experiments were conducted according to the guidelines mentioned in the literature in the previous works [19]. Prior to the start of the experiment, the refrigeration system was completely leak tested with high-pressure nitrogen and vacuum. After purging the system, 70 g of R134a refrigerant was charged in the system as indicated by the manufacturer. Once the system was found to be working as per the specifications of the manufacturer, the following tests were conducted as per the standard procedure discussed below.
To perform the pull-down test, the system was kept inside the temperature-controlled room and the evaporator compartment door was kept open for 24 h to enable the system attain thermal equilibrium with the surroundings. Once the thermal equilibrium was attained, the door was closed and the system was switched on. During the test, the temperature of the evaporator storage space and the temperature of the PCM inside the encapsulation were measured and the energy consumed was measured by using the energy meter. The pressure and the temperature of the various state points of the system were taken after the equilibrium conditions were reached.
To measure the per day energy consumption, the experimental setup was kept inside the temperature-controlled room at no load condition. Once the system reached its equilibrium state for a particular ambient temperature, the observations were noted down for 24 h. The power and the energy consumed by the compressor were measured by the wattmeter and the energy meter, respectively. The tests were repeated three times, and the average values were taken for the calculations. During the tests, the ambient temperature inside the room was maintained at 32 ± 1 °C.
To calculate the COP at a particular evaporator temperature, the secondary refrigerant calorimeter was used as per the standards [20]. The COP could be found from the equation
The refrigeration system was switched OFF to find the heat infiltration at a particular temperature. The secondary refrigerant (ethyl glycol) gets heated by the infiltration of heat, and the temperature of the ethyl glycol was noted for 1-min intervals. The heat of infiltration could be calculated from the heat transfer equation
Exergy analysis
Exergy of a system, also referred to as the available work or availability at a given state, is equal to the maximum useful work possible that can be realized when operating reversibly between the given state and the reference state. The exergy analysis is a commonly acknowledged tool to enhance the overall performance of refrigeration systems and the related components. This tool helps design engineers to identify locations of exergy destruction and find avenues for improvements in system components. Each component is treated as a control volume to find the irreversibility. The assumptions for the exergy analysis are infinite equilibrium environment surrounding all systems, the changes in the potential and kinetic energies are negligible, the thermos-physical properties have one-dimensional variation, the readings are taken at steady-state condition, and the mass flow rate is constant.
Exergy balance for a control volume in a steady state [21] can be expressed as
where \(\ddot{E}_{\text{i}}\) and \(\ddot{E}_{\text{e}}\) are the exergy transfer rates at control volume inlet and outlet, \(\ddot{E}_{\text{D}}\) is the exergy destruction rate due to irreversibility, \(E_{\text{j}}^{{\text{Q}}}\) the rate of exergy transfer by heat transfer, and \(E^{{\text{w}}}\) is the rate of exergy transfer by work. The specific exergy in any state [22] is given by
Using the exergy balance Eq. (3), the general form of equation for irreversibility of each components [21] is worked out and given in Table 4.
The total irreversibility in the system is the sum of the irreversibilities, in different components of the system [21] and is given by
The exergy efficiency [23] could be expressed by
Total equivalent warming impact (TEWI) is a measure of the global warming impact of equipment based on the total related emissions of greenhouse gases during the operation of the equipment and the disposal of the operating fluids at the end of life. TEWI takes into account both direct emissions and indirect emissions produced because of the energy consumption in operating the equipment. TEWI is calculated in units of mass in kg of carbon dioxide equivalent (CO2-e). TEWI is calculated as the summation of the refrigerant released from the equipment during its lifetime, including the refrigerant lost during the time of decommissioning the equipment and the impact of CO2 emissions from fossil fuels at the generation source to operate the equipment throughout its lifetime.
The TEWI can be calculated from the method [24] mentioned below based on the data given in Table 5:
where GWP = global warming potential of refrigerant, relative to CO2 (GWP of CO2 = 1), Lannual = leakage rate per annum (kg), n (years) = system operating life, m (kg) = refrigerant charge, Eannual (kW h per annum) = energy consumption per year, β (kgCO2 per kW h) = indirect emission factor.
Uncertainty analysis
The influence of uncertainty in measuring instruments on evaporator capacity, compressor power, and COP has been calculated from standard procedure discussed in previous studies [25] and obtained as ± 2.6%, ± 1.9%, and ± 2.9%, respectively. Since these values are in the acceptable limits, the validity of the experimental method has been confirmed.
Results and discussion
Based on the observations from the experimental study, the performance parameters of a refrigeration system such as pull-down time, compressor work, per day energy consumption, COP, and TEWI with the application of two PCMs of 0 °C and − 16 °C freezing points have been studied and discussed in this section. The tests were conducted in a temperature-controlled room at 32 °C ambient condition.
The temperature variations during the pull down for the cutoff temperature setting of 0 °C have been plotted in Fig. 3. The cut-in and cutoff temperatures set in the thermostat are − 3 °C and 2 °C, respectively. It could be seen that the time taken for pull down was 39 min. Since the evaporator is not loaded with PCM, the heat inside the evaporator space is transferred by free convection. This amounts to sensible cooling, and the heat is removed quickly from the evaporator. The average time for which the compressor is in the OFF condition and ON condition has been observed as 21 min and 10 min, respectively.
When PCM 1 is loaded in the evaporator, the pull-down time has been observed as 92 min. This is because of the additional thermal inertia of the system brought about by the PCM in the evaporator. When the system is switched ON, the heat from the evaporator space and the PCM simultaneously gets removed by convection. This accounts for the sensible cooling. When the temperature inside the evaporator reaches the phase change temperature of the PCM, freezing of the PCM starts. The latent heat of the PCM gets removed without much change in temperature. During the cooling process, the sensible heat of the space and the sensible and latent heat of the PCM have been removed. Because of the removal of latent heat, the PCM starts to change its phase from liquid to solid at 0 °C. When the temperature in the evaporator reaches − 3 °C, the compressor is stopped by the thermostat.
Because of the PCM, major portion of the heat in the evaporator space is stored in the PCM when the compressor is not running and this heat is extracted by the refrigerant when the system is in ON cycle. When the PCM starts to freeze, it prevents the intrusion of surrounding heat into the evaporator space as the PCM layer acts as an insulator during phase change. As a result, the temperature inside the evaporator space remains more or less stable for a longer span of time. Moreover, the average compressor ON/OFF cycle time is observed to be 49 min during which the compressor remains ON for 14 min and OFF for 35 min.
Figure 4 shows the variation of evaporator temperature with respect to time when the evaporator temperature was maintained at − 16 °C. The operating range of the thermostat was set between − 18 and − 13 °C. It could be inferred that the time taken for pull down is 87 min and the average compressor OFF timing and ON timing are 24 min and 13 min, respectively, in the absence of PCM. The ratio of the compressor OFF timing to ON timing is 1.85. When the evaporator was loaded with the sodium and ammonium chloride eutectic solution, the pull-down time has been increased to 335 min.
This is because of the high energy storage density and latent heat of the PCM. The average compressor ON and OFF timings are found to be 86 min and 194 min, respectively. The ratio of the compressor OFF to ON timing is 2.26. Even though there is an increase of 84.8% in the average ON cycle timing because of the impact of PCM, it is offset by the increase of 97.6% in the OFF cycle timing. Since there is a total increase in the compressor OFF timing over a complete day, there is an energy saving when the refrigerator is run for a prolonged period of time which had been reported in the previous studies [26]. It can be seen that there is a longer period of lull than the period for which the compressor is in operation. This is because of the increased thermal inertia in the system brought about by the inclusion of PCM with high latent heat.
Figure 5 shows the per day energy consumption of the refrigeration system when there is no PCM and when it is loaded with PCM 1 and PCM 2. The per day energy consumption is measured as 1.96 kW h when there is no PCM, and the evaporator temperature has been set at 0 °C. The per day energy consumption of the refrigerator varies for various months of a year. When the evaporator was not loaded with PCM 2 and when the temperature was brought down to − 16 °C, the energy consumed during pull down was observed as 0.237 kW h. An increase of 3.03% in the energy consumption was observed during the pull down.
During the pull-down time, the heat has to be removed both from the evaporator space and the PCM by sensible cooling and solidification starts when the temperature reaches the freezing temperature of the PCM. PCM gets charged and the latent heat has to be removed during the solidification of the PCM. The per day energy consumption is 1.65 kW h on an average when PCM 1 is loaded in the system with the evaporator temperature at 0 °C. The reduction in energy consumption is found to be 15.73%. The per day energy consumption is 1.91 kW h and 2.31 kW h when the evaporator temperature is set at − 16 °C and the freezer cabin is loaded with and without PCM 2, respectively. Due to the impact of PCM 2, 17.31% reduction in energy consumption has been observed.
The power consumption pattern of the compressor during first few ON/OFF cycles has been recorded and shown in Fig. 6. High starting power is observed in the beginning of the pull down, and after few cycles, it becomes uniform. It is found that during the start, the compressor power surges to 300 W and within few seconds settles down to 120 W. It has been noted that the maximum power reached during each ON cycle gradually reduces over a period of time. This is because of the high temperature prevailing in the freezer cabin when the system starts from the ambient temperature and high suction pressure. When the temperature in the evaporator reduces, the vapor pressure of the refrigerant also reduces which results in a reduction in the work done by the compressor. The presence of PCM in the evaporator prolongs the compressor OFF time because of its high latent heat.
To find the heat infiltration into the evaporator cabinet, the system was switched OFF at − 16 °C. The temperature of the cabinet was measured, and the values are plotted in Fig. 7. The secondary refrigerant (ethyl glycol) was allowed to get heated by the infiltrating heat, and the temperature was noted down every 10 min. The chart was plotted for a time span of 24 h.
Initially, the heat infiltration into the evaporator cabinet was observed with no PCM inside. It was observed that there was an average temperature rise of 2 °C for every hour. The temperature after 24 h was found to be 16.9 °C. When the PCM was loaded in the system, an average temperature rise of 0.4 °C was observed. This is because of the reduction in heat transfer when the PCM is in the solid state. There was a change of 1 °C over a time span of 4 h. The temperature glide of 1 °C is due to its zeotropic nature. The heat infiltration into the cabinet was calculated based on Eq. (2) and the values are plotted in Fig. 8.
Figure 9 shows the variation of COP with the calorimeter temperature for both PCMs. Ammonium bicarbonate solution (PCM1) has a freezing point of 0 °C which will be suitable for the chiller part of the refrigerator, and sodium and ammonium chloride solution (PCM2) has a freezing point of − 16 °C that will be suitable for the freezer part of the refrigerator. The graphs have been constructed for a narrow temperature range closer to their respective freezing points which are of interest, and the characteristics of both PCMs have been incorporated on a common graph.
The improvement in COP is estimated in the range of 10.1–17.4% when PCM 1 is loaded in the system. It is observed that the COP increases with the increase in the evaporator temperature. It is also observed that 1813 kJ of energy could be retained in the cooling coil.
The variation of irreversibility of the refrigeration system with the evaporator temperature has been studied and depicted in Fig. 10. Normally, the increase in the evaporator temperature increases the suction pressure, the consequence of which is a higher pressure ratio and lower efficiency in the compressor, and as a result, the compressor work also increases.
Since the irreversibility increases with increase in operating temperature and pressure, the increase in irreversibility in the freezer cabin temperature has been observed in the figure. The irreversibility of the refrigeration system is reduced by 11.2–14.1% and 16.02–19.5%, respectively, when PCM 1 and PCM 2 are used. The irreversibility of the refrigeration system is the lowest when PCM 2 is used. This is because of the low discharge pressure of the compressor.
Figure 11 depicts the change of exergy efficiency with the calorimeter temperature. It could be found that the exergy efficiency reduces with increase in evaporator temperature. This is because of the increase in irreversibility of the various components of the system with the increase in evaporator temperature. Compared to the conventional system, the improvement in exergy efficiency of the system with PCM 1 and PCM 2 are 14.6–16.9% and 19.01–22.5%, respectively. During charging, the exergy is transferred to the PCM, in which part the exergy is stored and the useful effect is the exergy change of the PCM. During discharging, the exergy output from the PCM becomes available and is gained by the evaporator.
It is observed that the exergy efficiency decreases with the increase in condensing temperature. Obviously, the irreversibility of the whole condensation unit increases with increase in condensing temperature.
Figure 12 shows the total equivalent warming impact of the refrigeration system. When the system operates at 0 °C, TEWI for the system with and without PCM has been estimated as 7962 kgCO2 and 6721 kgCO2, respectively. It is observed that the application of PCM reduces the TEWI by 15.58%. Similarly, the application of PCM 2 reduces the TEWI from 9084 kgCO2 to 7661 kgCO2, i.e., 15.76%. The reduction of TEWI in both the cases is due to the decrease in the indirect effect from the reduction in energy consumption.
Conclusions
The performance of a small-scale refrigeration system with two types of phase change material, PCM 1 and PCM 2, in the evaporator compartment has been investigated, and based on the observations, the following conclusions are drawn.
-
1.
The application of PCM inside the evaporator cabin can reduce the heat infiltration and increase the OFF cycle time.
-
2.
PCM 1 and PCM 2 having freezing points 0 °C and − 16 °C can enhance the COP of the conventional system by 10.1% and 17.4%, respectively.
-
3.
The application of PCM between the evaporator coil and wall of the freezer compartment could reduce the compressor power and per day energy consumption. PCM 1 and PCM 2 can reduce the per day energy consumption by 15.75% and 17.31%, respectively, when the system operates at 32 °C ambient conditions with no load.
-
4.
The irreversibility of the system with PCM 1 and PCM 2 is reduced by 11.2–14.1% and 16.02–19.5%, respectively, for the tested conditions.
-
5.
The improvement in exergy efficiency of the proposed arrangement of PCM 1 and PCM 2 is 14.6–16.9% and 19.01–22.5%, respectively, for the tested conditions.
-
6.
The use of PCM can reduce the indirect emission of global warming gases, and therefore, the reduction in TEWI of the proposed system is estimated as 15.58% and 15.76%, respectively, when PCM 1 and PCM 2 are used.
The proposed arrangement of PCM between the coil and the wall could extend the preservation time of food products in household refrigerators during power outages which is very common in rural areas of developing countries. If this method is adopted in the commercial refrigerators, this leads to savings in energy consumption. However, the cost of modification and the payback has to be taken into account by the manufacturer to find the commercial viability of the concept.
References
Liu D-Y, Chang W-R, Lin J-Y. Performance comparison with effect of door opening on variable and fixed frequency refrigerators/freezers. Appl Therm Eng. 2004;24:2281–92.
McNeil, Michael A. Progress towards managing residential electricity demand: impacts of standards and labeling for refrigerators and air conditioners in India. In: 5th International conference on energy efficiency on domestic appliances and lighting. EEDAL, Berlin, Germany; 2009.
Marques AC, Davies GF, Evans JA, Maidment GG, Wood ID. Theoretical modelling and experimental investigation of a thermal energy storage refrigerator. Energy. 2013;55:457–65.
Geppert J, Stamminger R. Analysis of effecting factors on domestic refrigerator’s energy consumption in use. Energy Convers Manag. 2013;76:794–800.
Cheng WL, Mei BJ, Liu YN, Huang YH, Yuan XD. A novel household refrigerator with a shape-stabilized PCM (phase change material)heat storage condensers—an experimental investigation. Energy. 2011;36(10):5797–804.
Joybari MM, Hatamipour MS, Rahimi A, Modarres FG. Exergy analysis and optimization of R 600a as a replacement of R134a in a domestic refrigerator system. Int J Refrig. 2013;36(4):1233–42.
Ekren O, Celik S, Noble B, Krauss R. Performance evaluation of a variable speed DC compressor. Int J Refrig. 2013;36(3):745–57.
Park YC. Transient analysis of a variable speed rotary compressor. Energy Convers Manag. 2010;51(2):277–87.
Hammond E, Evans J. Application of vacuum insulated panels in the cold chain-analysis of viability. Int J Refrig. 2014;47:58–65.
Khan MIH, Afroz HM. Effect of phase change material on a household refrigerator: Asian. J Appl Sci. 2013;6(2):56–67.
Roth P. Energy saving on the high pressure side of a refrigerating plant. KI. 2008;03:30–5.
Maltini E, Cortella G, Stecchini M, Deltorre M, Pittia P, Spaziani M, Mansutti G. Design and performances of a constant temperature compartment for domestic refrigerator. In: Proceedings of the 9th international congress on engineering and food, Montpellier, France. 7–11 March 2004.
Mastani M, Joybari FH, Moffat J, Sra P. Heat and cold storage using phase change materials in domestic refrigeration systems-The state-of-the-art review. Energy Build. 2015;106:111–24.
Rahman R, Hossain MA, Das SK, Hasan A. Performance improvement of a domestic refrigerator by using PCM (phase change material). Glob J Res Eng. 2014;13(10):17–22.
Zalba B, Jose Marin M, Luisa Cabeza F, Mehling H. Review on thermal energy storage with phase change materials, heat transfer analysis and applications. Appl Therm Eng. 2003;23:251–83.
Saidur R, Masjuki HH, Choudhury IA. Role of ambient temperature, door opening, thermostat setting position and their combined effect on refrigerator-freezer energy consumption. Energy Convers Manag. 2012;43:845–54.
Yehya A, Naji H. A novel technique to analyze the effect of enclosure shape on the performance of phase-change materials. Energy Procedia. 2015;75:2131–6.
Ahmed M, Meade O, Medina MA. Reducing heat transfer across the insulated walls of refrigerated truck trailers by the application of phase change materials. Energy Convers Manag. 2010;51(3):383–92.
Azzouz K, Leducqa D, Gobinb D. Performance enhancement of a household refrigerator by addition of latent heat storage. Int J Refrig. 2008;31:892–901.
Saji Raveendran P, Joseph Sekhar S. Experimental studies on domestic refrigeration system with brazed plate heat exchanger as condenser. J Mech Sci Technol. 2016;30(6):2865–71.
Bayrakci HC, Ozgur AE. Energy and exergy analysis of vapour compression refrigeration system using pure hydrocarbon refrigerants. Int J Energy Res. 2009;33:1070–5.
Gupta A, Anand Y, Anand S, Tyagi SK. Thermodynamic optimization and chemical exergy quantification for absorption-based refrigeration system. J Therm Anal Calorim. 2015;122(2):893–905.
Saravanakumar R, Selladurai V. Exergy analysis of a domestic refrigerator using eco-friendly R290/R600a refrigerant mixture as an alternative to R134a. J Therm Anal Calorim. 2013;115(1):933–40.
Davies TW, Caretta O. A low carbon, low TEWI refrigeration system design. Appl Therm Eng. 2004;24:1119–28.
Holman JP. Experimental methods for engineers. 7th ed. New York: McGraw Hill Publishers; 2000.
Ezan MA, Doganay EO, Yavuz FE, HakkTavman I. A numerical study on the usage of phase change material (PCM) to prolong compressor off period in a beverage cooler. Energy Convers Manag. 2017;142:95–106.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antony Forster Raj, M., Joseph Sekhar, S. Investigation of energy and exergy performance on a small-scale refrigeration system with PCMs inserted between coil and wall of the evaporator cabin. J Therm Anal Calorim 136, 355–365 (2019). https://doi.org/10.1007/s10973-018-7785-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7785-7